Abstract
It has been hypothesized that the destruction of lung tissue observed in smokers with chronic obstructive pulmonary disease and emphysema is mediated by neutrophils recruited to the lungs by smoke exposure. This study investigated the role of the chemokine receptor CXCR2 in mediating neutrophilic inflammation in the lungs of mice acutely exposed to cigarette smoke. Exposure to dilute mainstream cigarette smoke for 1 hour, twice per day for 3 days induced acute inflammation in the lungs of C57BL/6 mice, with increased neutrophils and neutrophil chemotactic CXC chemokines MIP-2 and KC. Treatment with SCH-N, an orally active small molecule inhibitor of CXCR2, reduced the influx of neutrophils into the bronchoalveolar lavage (BAL) fluid. Histologic changes were seen, with drug treatment reducing perivascular inflammation and the number of tissue neutrophils. β-glucuronidase activity was reduced in the BAL fluid of mice treated with SCH-N, indicating that the reduction in neutrophils was associated with a reduction in tissue damaging enzymes. Interestingly, while MIP-2 and KC were significantly elevated in the BAL fluid of smoke exposed mice, they were further elevated in mice exposed to smoke and treated with drug. The increase in MIP-2 and KC with drug treatment may be due to the decrease in lung neutrophils which either are not present to bind these chemokines or which fail to provide a feedback signal to other cells that produce these chemokines. Overall, these results demonstrate that inhibiting CXCR2 reduces neutrophilic inflammation and associated lung tissue damage due to acute cigarette smoke exposure.
Keywords: neutrophil chemokines, emphysema, COPD, MIP-2, KC
Introduction
In 2002, an estimated 24 million Americans were afflicted with chronic obstructive pulmonary disease (COPD; emphysema and chronic bronchitis), including 2.4 million adults with moderate to severe airway obstruction (18). COPD is the fifth leading cause of death worldwide (26). Recent research has focused on the role of inflammation and inflammatory cell mediators in the pathogenesis of COPD (10). Cigarette smoke is a strong inflammatory stimulus that induces pro-inflammatory cytokines such as IL-6 and TNF-α, and recruits activated macrophages and neutrophils to lung tissue (8, 23). It is believed that emphysema results from an imbalance between proteases produced by smoke-recruited inflammatory cells and the anti-protease defenses of the lung (1, 6, 32). Instillation of neutrophil elastase into the lungs of experimental animals has long been a model of emphysema (15). Recent work has confirmed that while macrophage proteases play a key role in cigarette smoke-induced inflammation (6), neutrophils are also required for tissue breakdown following cigarette smoke exposure in an animal model (8, 9).
Recruitment of neutrophils to inflammatory sites is mediated by a number of factors including adhesion molecules, multifunctional pro-inflammatory cytokines, and chemokines of the CXC family. The predominant neutrophil chemokine in humans is IL-8, while mice lack IL-8 but have two neutrophilic CXC chemokines, MIP-2 and KC (13). The importance of these chemokines in promoting inflammation has been investigated in vitro and in vivo. Bronchoalveolar lavage fluid (BAL) from COPD patients contain increased neutrophils, TNF-α and IL-8 (25, 29), while we and others have shown that cigarette smoke extract stimulates human lung cells (epithelial cells, fibroblasts, and macrophages) to release IL-8 (3, 19, 20, 30). In mice, MIP-2 is essential for neutrophil recruitment to the lungs following inflammatory injury (22).
The CXC chemokines signal through a family of CXC receptors on inflammatory cells. In humans, IL-8 binds two receptors, CXCR1 and CXCR2, both of which are expressed on neutrophils (24). Mice lack a CXCR1 homologue; MIP-2 and KC signal exclusively through CXCR2 (4). The CXC chemokines and their receptors are attractive targets for therapeutic intervention in inflammatory lung diseases such as COPD. It has previously been reported that blockade of the MIP-2:CXCR2 interaction inhibits neutrophil recruitment in several mouse models of inflammation (14, 17, 21, 37). However, the role of the CXC chemokines and CXCR2 in cigarette smoke-mediated lung inflammation has not previously been investigated.
In this report we investigated the role of CXCR2 in cigarette smoke-induced inflammation using a CXCR2 inhibitor. SCH-N inhibits the binding of CXC chemokines to the human CXCR1 and CXCR2 receptors as well as the murine CXCR2 receptor, and has an IC50 of 3 nM in a mouse neutrophil chemotaxis assay. SCH-N inhibits neutrophilic inflammation in mice exposed acutely to cigarette smoke. Treatment with SCH-N reduced neutrophils in bronchoalveolar lavage (BAL) fluid and tissue neutrophils by 50%. β-glucuronidase activity was significantly reduced as well, indicating that inhibition of neutrophilic influx ameliorated the tissue damage associated with acute cigarette smoke exposure. Thus, CXCR2 plays a key role in the acute inflammatory response to cigarette smoke.
Materials and Methods
Cigarette Smoke Exposure
Adult female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and housed in the Inhalation Core Facility at the University of Rochester. Mice were placed in individual compartments of a wire cage, which was placed inside a closed plastic box connected to the smoke source. Research cigarettes (1R3F, University of Kentucky) were smoked according to the FTC protocol (1 puff/min of 2 sec duration and 35 ml volume) in a Baumgartner-Jaeger CSM2072i cigarette smoking machine (CH Technologies, Westwood, New Jersey). Mainstream cigarette smoke was diluted with filtered air and directed into the exposure chamber. The smoke exposure (total particulate matter per cubic meter of air, TPM) was monitored in real time with a MicroDust Pro aerosol monitor (Casella CEL, Bedford, UK) and verified daily by gravimetric sampling. The smoke concentration was set at a nominal value of 600 mg/m3 TPM by adjusting the flow rate of the dilution air. The average actual exposure for these experiments was 647 ± 58 mg/m3. Mice received two 1-hour exposures, four hours apart, for three consecutive days, and were sacrificed on the fourth day. Control mice were exposed to filtered air in an identical chamber according to the same schedule. All animal procedures were performed under the supervision of the University Committee on Animal Research.
Drug Treatment
A 5 mg/ml suspension of SCH-N (provided by the Schering Plough Research Institute, Kenilworth, NJ) in vehicle (0.4% hydroxypropyl-methylcellulose) was prepared immediately prior to each administration. Mice received 50μl SCH-N suspension (or vehicle alone), p.o., one hour prior to each smoke exposure. To ensure accurate dosing, drug or vehicle was administered using Hamilton Gastight syringes fitted with Hamilton Repeating Dispensers (Hamilton, Reno, Nevada).
Bronchoalveolar Lavage
Mice were anesthetized with 2,2,2-tribromoethanol (Avertin, 250 mg/kg i.p.) and sacrificed by exsanguination. The heart and lungs were removed en bloc and the lungs were lavaged twice with 0.5 ml PBS. The lavage fluid was centrifuged and the cell-free supernatants frozen for later analysis. The BAL cell pellet was resuspended in PBS and the total cell number was determined by counting on a hemacytometer. Differential cell counts (minimum of 300 cells per slide) were performed on Cytospin-prepared slides (Thermo Shandon, Pittsburgh, PA) stained with Diff-Quik (Dade Behring, Newark, DE).
Analysis of BAL Fluid
Murine IL-6, TNF-α, MIP-2 and KC were measured in BAL samples by commercial ELISA (R&D Systems, Minneapolis, MN). PGE2 was measured by EIA using commercially available reagents (Cayman Chemical, Ann Arbor, MI) as described (16). The limit of detection was 7 pg/ml for IL-6, MIP-2 and KC, 15 pg/ml for TNF-α, and 30 pg/ml for PGE2. To assay β-glucuronidase activity, the liberation of p-nitrophenol from 4-nitrophenol glucuronide (Sigma, St. Louis, MO) was measured at 405 nm in glycine buffer (pH 4.5) as described (33).
Histological Analysis and Immunohistochemistry
Mouse lungs (which had not been lavaged) were fixed by inflation with 10% neutral buffered formalin at a pressure of 15 cm H2O. Tissues were embedded in paraffin, sectioned (4 μm), and stained with hematoxylin and eosin. For enumeration of tissue neutrophils, sections were stained with a rat monoclonal anti-mouse neutrophil antibody (MCA771GA, 1:25 dilution, Serotec, Oxford, UK), developed with NovaRed (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin. Six non-serial sections (3 each from the left and right lung) were prepared for each mouse in each treatment group. The slides were randomized and blinded, 10 high-power fields (500 × 500 μm) per section were counted, and the average number of neutrophils per mm3 of tissue was determined.
Statistical Analysis
All results are reported as the mean ± SEM. The effect of drug treatment of smoke-exposed mice on differential cell counts, lavage cytokines, and tissue neutrophils was assessed by 2-factor ANOVA and the Student’s t-test. A p-value <0.05 was considered significant.
Results
Acute cigarette smoke-induced inflammation is significantly reduced by inhibiting CXCR2
C57BL/6 mice were exposed to cigarette smoke for one hour, twice a day for three days, and sacrificed on the fourth day. This exposure protocol elicits a strong inflammatory response characterized by significant increases in the number of neutrophils, lymphocytes and eosinophils recovered by bronchoalveolar lavage (BAL), with neutrophils comprising up to 50% of total BAL cells (Fig. 1). This acute cigarette smoke exposure protocol did not result in significant increases in BAL macrophages. SCH-N is a novel CXCR2 antagonist that is a potent inhibitor of mouse neutrophil chemotaxis in vitro (IC50 of 3 nM, McHugh and Egan, unpublished data). Pretreatment with SCH-N before each smoke exposure significantly reduced the neutrophil influx into the lungs, with a 50% decrease in the number of neutrophils in BAL (Fig. 1A). SCH-N did not affect the number of lymphocytes or eosinophils in BAL (Fig. 1D, E). Levels of β-glucuronidase, one of several hydrolytic enzymes found in phagocytic cells and thought to play a role in tissue breakdown in inflammatory lung disease (27), were elevated 96% in the BAL of cigarette smoke-exposed mice. β-glucuronidase activity was elevated 71% in SCH-N treated, smoke-exposed mice compared to non-smoke-exposed controls, representing a 26% reduction in smoke exposure-specific activity (Fig. 1F).
Figure 1. Treatment with SCH-N significantly reduces cigarette smoke-induced inflammation in BAL.
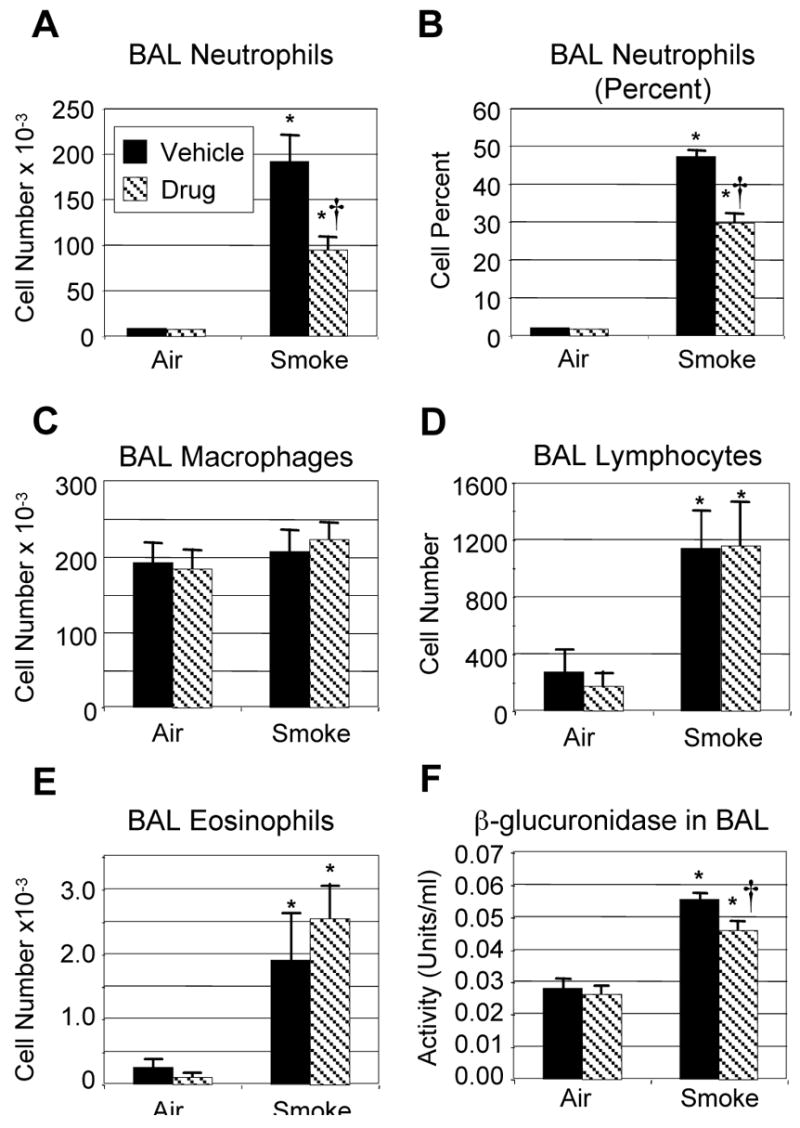
Mice were exposed to filtered air or cigarette smoke as described, and treated with SCH-N (gray bars) or vehicle (black bars). Differential counts were performed on BAL cells and the number of neutrophils (A), the percentage of neutrophils (B), the number of macrophages (C), the number of lymphocytes (D) and the number of eosinophils (E) are reported. Results shown are the combined results for 3 experiments with a total of 12–15 mice per group. (F) β-glucuronidase activity in BAL was measured as described for two experiments with a total of 8 mice per group. The percent inhibition by SCH-N of smoke-induced β-glucuronidase activity was calculated as 100 × ( (smoke+vehicle − control) − (smoke+drug − control))/ (smoke+vehicle − control). *=significant difference from air-exposed controls; †=significant difference from smoke-exposed, vehicle-treated mice (p<0.05).
Inhibition of CXCR2 reduces airway inflammation and infiltration of lung tissue by neutrophils in cigarette smoke-exposed mice
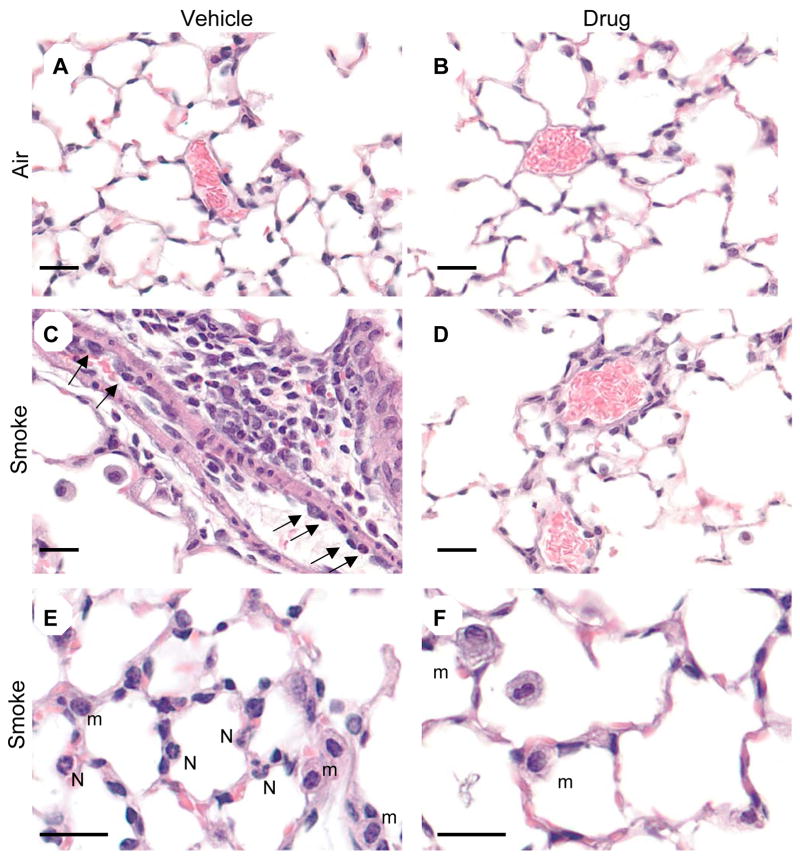
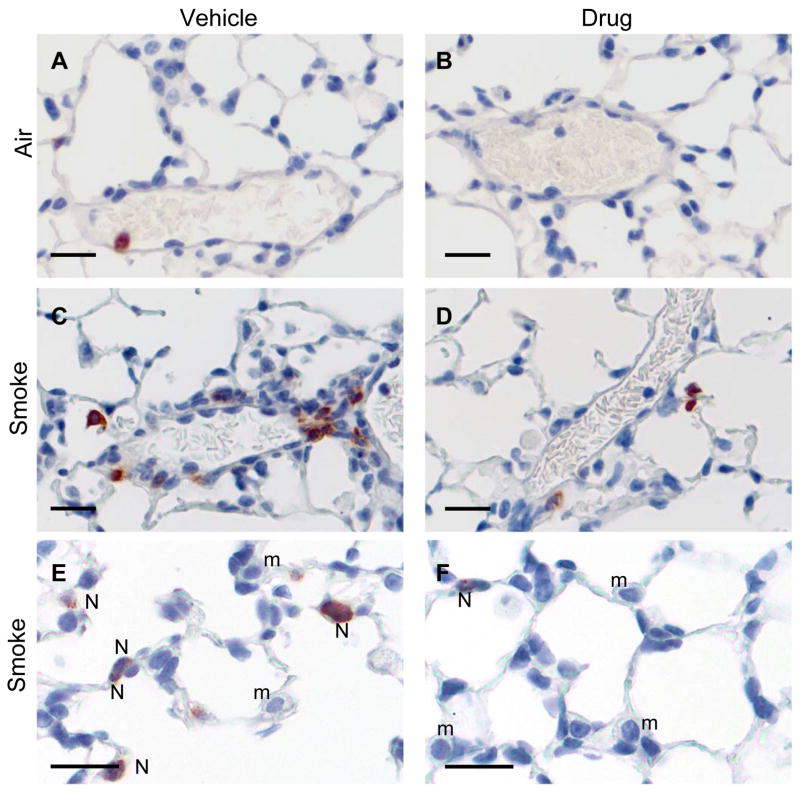
Lungs from mice exposed to air or cigarette smoke and treated with SCH-N were fixed, and sections were stained with hematoxylin and eosin, or with an antibody to mouse neutrophils. Exposure to cigarette smoke induced a significant perivascular inflammation with prominent neutrophils and mononuclear cells (Fig. 2C). Adherent neutrophils were frequently seen in the lumen of pulmonary blood vessels (Fig. 2C), and neutrophils and macrophages were prominent in alveolar capillaries (Fig. 2E). Treatment with SCH-N reduced the size and frequency of perivascular infiltrates, as well as the number of adherent neutrophils inside blood vessels (Fig. 2D). Although macrophages were still prominent in alveolar capillaries, neutrophils were largely absent (Fig. 2E). These observations were confirmed by immunohistochemical staining using an antibody that recognizes a surface protein on mouse neutrophils. Neutrophils are prominent in perivascular infiltrates of smoke-exposed mice treated with vehicle (Fig. 3C) but not SCH-N (Fig. 3D). SCH-N also reduced the appearance of neutrophils trapped in the alveolar capillary bed (Fig. 3E and F). Neutrophils were counted in immunostained sections by a blinded researcher and the number of neutrophils per cubic mm of tissue was determined (Fig. 4). Cigarette smoke exposure induced a 7-fold increase in the number of tissue neutrophils, which was reduced by 45% by treatment with SCH-N.
Figure 2. SCH-N reduces neutrophil accumulation in lung tissue of cigarette smoke-exposed mice.
Mice were exposed to filtered air or cigarette smoke and treated with SCH-N or vehicle. Lungs were inflated and fixed with formalin, and sections were stained with H&E. Air-exposed mice have normal alveoli and blood vessels (A, B). Smoke-exposed mice exhibit extensive perivascular inflammation with extravasating monocytes and neutrophils (arrows, C) and monocytes and neutrophils in the alveolar capillaries (E). Smoke-exposed mice treated with SCH-N have less perivascular inflammation (D) and greatly reduced numbers of extravasating (D) and capillary neutrophils (F). m, monocyte; N, neutrophil. Bar=20 μm.
Figure 3. Neutrophil immunohistochemistry in smoke-exposed and drug-treated mice.
Lung sections were prepared as described and immunostained with an antibody specific for a neutrophil surface marker (Serotec, Oxford, UK) and counterstained with hematoxylin. Neutrophils are stained red. Very few neutrophils are present in air-exposed mice (A, B). The perivascular infiltrates in smoke-exposed mice contain many neutrophils (C), as do alveolar capillaries (E). Smoke-exposed mice treated with drug have reduced perivascular and capillary neutrophils (D, F). m, monocyte; N, neutrophil. Bar=20 μm.
Figure 4. Treatment with SCH-N significantly reduces neutrophils in lung tissue of smoke-exposed mice.
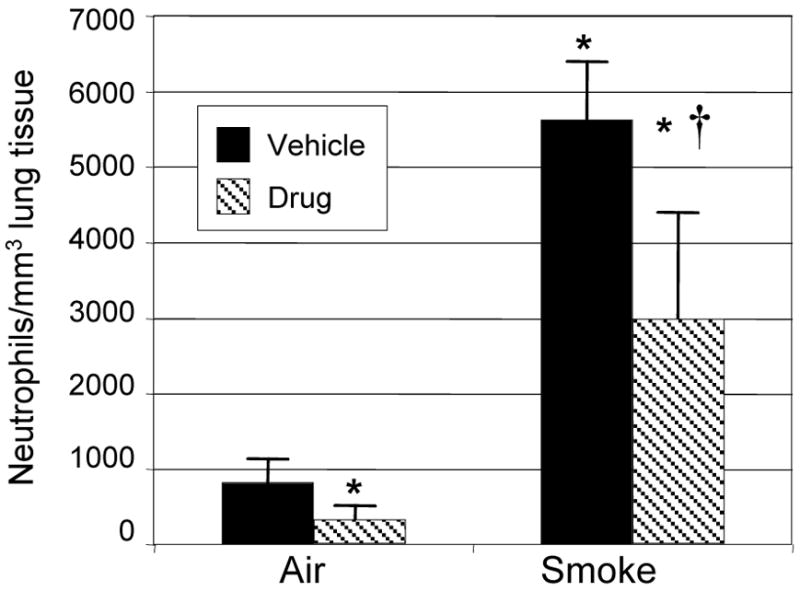
To determine the number of neutrophils in tissue, lung sections (6 per mouse, 3 mice per treatment group) were immunostained to identify neutrophils, 10 random high power fields per section were counted, and the average number of neutrophils per mm3 of tissue was determined. *=significant difference from air-exposed controls; †=significant difference from smoke-exposed, vehicle-treated mice (p<0.05).
Inhibition of CXCR2 does not otherwise alter the inflammatory milieu
Neutrophil migration from the peripheral blood into tissues requires both a proinflammatory milieu in the tissue, with expression of endothelial adhesion molecules, and ligation of the CXCR2 receptor on neutrophils, which induces leukocyte rolling and extravasation (11, 35, 39). We analyzed the levels of the pro-inflammatory cytokines IL-6 and TNF-α, and PGE2, an inflammatory mediator produced by lung fibroblasts that is implicated in neutrophil activation and recruitment, in mice exposed to cigarette smoke with and without SCH-N treatment. Exposure to cigarette smoke resulted in a 10-fold increase in IL-6 and a 2–3 fold increase in PGE2 in BAL fluid (Fig. 5). No differences were observed in mice treated with SCH-N, suggesting that SCH-N does not interfere with neutrophil migration by altering the general inflammatory microenvironment. It should be noted that TNF-α levels were not elevated by cigarette smoke exposure (Fig. 5B), however, these mice were harvested 24 hours after the final smoke exposure, while TNF-α levels peak 2–4 hours after smoke exposure (data not shown).
Figure 5. SCH-N does not alter the level of proinflammatory cytokines.
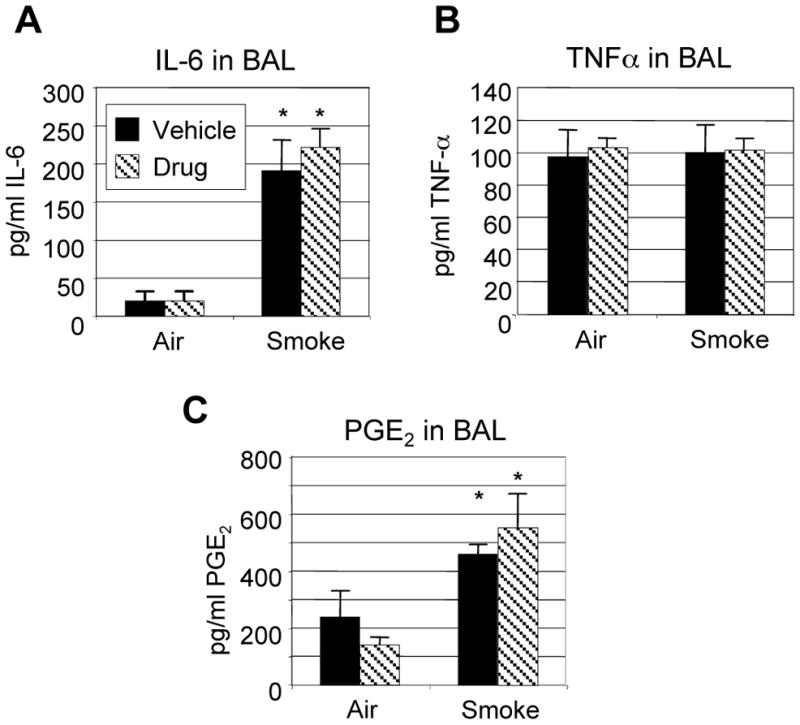
Mice were exposed to filtered air or cigarette smoke as described, and treated with SCH-N (gray bars) or vehicle (black bars). IL-6 (A), TNF-α (B) and PGE2 (C) were measured in BAL fluid by commercially available ELISA and EIA, as described. Results shown are from a representative experiment with 6 mice per group. *=significant difference from air-exposed controls (p<0.05); the differences between smoke-exposed mice treated with vehicle or drug are not significant.
Inhibition of CXCR2 leads to elevated levels of CXC chemokines
IL-8, the human neutrophilic CXC chemokine, can be induced in vivo and in vitro by cigarette smoke (3, 31). To determine whether MIP-2 and KC, the mouse CXC chemokines that bind CXCR2, are induced in vivo by this acute cigarette smoking protocol, these chemokines were measured in BAL fluid from air and smoke-exposed mice. Cigarette smoke exposure induces a 2-fold increase in MIP-2 and a 30-fold increase in KC, which is consistent with cigarette smoke being a strong neutrophilic inflammatory stimulus (Fig. 6). Interestingly, treatment with SCH-N further increases MIP-2 and KC levels 2–3 fold over smoke alone.
Figure 6. Treatment with SCH-N results in elevated levels of CXC chemokines.
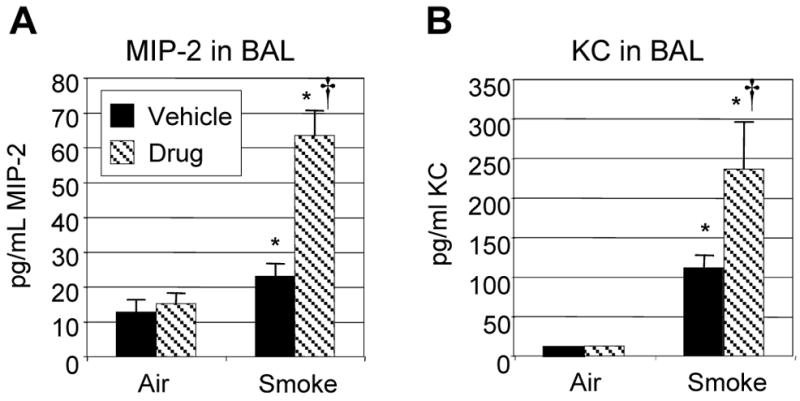
Mice were exposed to filtered air or cigarette smoke as described, and treated with SCH-N (gray bars) or vehicle (black bars). CXC chemokines MIP-2 and KC were measured in BAL fluid by commercially available ELISA. Results shown are combined from 3 experiments with a total of 12–15 mice per group. *=significant difference from air-exposed controls; †=significant difference from smoke-exposed, vehicle-treated mice (p<0.05).
Discussion
This study investigated the role of CXCR2 receptor on neutrophils in acute cigarette smoke-induced inflammation. We have developed a cigarette smoke exposure protocol that results in acute lung inflammation characterized by massive increases in BAL neutrophils (up to 50% of total BAL cells), tissue neutrophils, and production of inflammatory cytokines, prostaglandins, and neutrophil chemokines. SCH-N, a CXCR2 antagonist, inhibited smoke-induced lung neutrophilia by 50%, with reductions in BAL neutrophils and β-glucuronidase, an enzyme associated with tissue breakdown. Perivascular inflammatory infiltrates were smaller, less frequent and contained fewer neutrophils, and fewer neutrophils were observed adherent to blood vessel endothelium. SCH-N did not alter the number of inflammatory lymphocytes or eosinophils recruited by smoke, and did not alter the general proinflammatory milieu, as indicated by measurement of IL-6 and PGE2 in the BAL. It should be noted that while TNF-α has been shown by some to be a key initiator of inflammation following cigarette smoke exposure (7), we did not observe changes in TNF-α with either smoke exposure or drug treatment (Fig. 5B). Since we determined that neutrophilic inflammation of the lungs peaks 24 hours after the final smoke exposure in this model, the mice were sacrificed at this time point for all analyses. However, TNF-α levels peak 2–4 hours after smoke exposure (data not shown), so it is not surprising that we did not observe changes in TNF-α in these mice. However, since IL-6 and PGE2 levels are elevated by smoke exposure and unaffected by SCH-N, we expect that TNF-α would follow the same pattern if mice were sacrificed at an earlier time point.
It was interesting that SCH-N, a CXCR2 antagonist, increased the levels of the CXC chemokines MIP-2 and KC 2–3 fold over smoke alone. SCH-N should be acting against CXCR2 on circulating neutrophils, while MIP-2 and KC are probably produced by lung resident cells exposed to smoke. One possible explanation is that MIP-2 and KC are regulated in part by a negative feedback mechanism, and that the arrival of neutrophils at the site of inflammation sends a signal that downregulates further chemokine production. Because fewer neutrophils enter the lung with SCH-N treatment, this downregulatory signal is not sent. It is also possible that an autocrine negative feedback loop exists in which cells that produce MIP-2 and KC modulate chemokine production via their own CXCR2 receptors. It has recently been reported that mouse alveolar type 2 cells express both MIP-2 and CXCR2 (38), so it is possible that blockade of CXCR2 blocks an autocrine regulatory mechanism resulting in increased production of MIP-2.
SCH-N is a potent CXCR2 antagonist, inhibiting mouse neutrophil chemotaxis in vitro with an IC50 of 3 nM (data not shown), while inhibiting the neutrophilic inflammatory response to cigarette smoke by 50%. It should be noted that in mice, neutrophil migration can also be induced by the CC chemokine MIP-1α acting via the CCR1 receptor (34, 36). Cigarette smoke induces MIP-1α in rat alveolar macrophages in vitro (5), so it is likely that the partial inhibition seen in this study is due to redundancy in neutrophil chemotaxis in mice (28, 40).
Neutrophilic inflammation is a key factor in chronic bronchitis and emphysema. Disease progression is associated with a switch from a T-cell mediated inflammation in healthy smokers and patients with mild COPD to neutrophilic inflammation in severely ill patients (10), who also have higher levels of the human neutrophil CXC chemokine IL-8 (25, 29). Neutrophils have also been shown to be a key effector cell of matrix breakdown in an animal model (8, 9). Due to the fact that neutrophil recruitment in humans requires activation of the CXCR1 or CXCR2 receptors by CXC chemokines, these interactions have been explored as potential therapeutic targets (2, 12). In mouse models of inflammation, neutrophil recruitment can be blocked by treatment with antibodies to MIP-2 and KC, CXCR2 antibodies (mice lack CXCR1), and CXCR2 antagonist peptides derived from CXCR2 ligands (14, 17, 21, 37). This study demonstrates that CXCR2 plays a key role in cigarette smoke-induced inflammation, and that inhibiting chemokine binding to CXCR2 can significantly reduce neutrophilic infiltration and tissue damage resulting from acute exposure to cigarette smoke. Further experiments will be needed to determine whether long-term blockade of CXCR2 can reduce the severity of emphysema-like changes in mouse models of chronic cigarette smoke exposure.
Acknowledgments
SCH-N was provided by the Chemical Research Department of Schering-Plough Research Institute. R. W. Egan is currently at Inflammation Discovery Research, Millennium Pharmaceuticals, 35 Landsdowne Street, Cambridge, MA.
Grants
This research was supported in part by K08HL04492, HL075432, NIEHS Center Grant P30ES01247 and EPA R827354.
Disclosures
This research was funded in part by the Schering-Plough Research Institute.
References
- 1.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Cytokine modulators as novel therapies for airway disease. Eur Respir J Suppl. 2001;34:67s–77s. doi: 10.1183/09031936.01.00229901. [DOI] [PubMed] [Google Scholar]
- 3.Beisswenger C, Platz J, Seifart C, Vogelmeier C, Bals R. Exposure of differentiated airway epithelial cells to volatile smoke in vitro. Respiration. 2004;71:402–409. doi: 10.1159/000079647. [DOI] [PubMed] [Google Scholar]
- 4.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 5.Chong IW, Lin SR, Hwang JJ, Huang MS, Wang TH, Hung JY, Paulauskis JD. Expression and regulation of the macrophage inflammatory protein-1 alpha gene by nicotine in rat alveolar macrophages. Eur Cytokine Netw. 2002;13:242–249. [PubMed] [Google Scholar]
- 6.Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167:1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- 7.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2004;170:492–498. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- 8.Churg A, Zay K, Shay S, Xie C, Shapiro SD, Hendricks R, Wright JL. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27:368–374. doi: 10.1165/rcmb.4791. [DOI] [PubMed] [Google Scholar]
- 9.Dhami R, Gilks B, Xie C, Zay K, Wright JL, Churg A. Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by alpha1-antitrypsin. Am J Respir Cell Mol Biol. 2000;22:244–252. doi: 10.1165/ajrcmb.22.2.3809. [DOI] [PubMed] [Google Scholar]
- 10.Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–1167. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 11.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 12.Donnelly LE, Rogers DF. Therapy for chronic obstructive pulmonary disease in the 21st century. Drugs. 2003;63:1973–1998. doi: 10.2165/00003495-200363190-00002. [DOI] [PubMed] [Google Scholar]
- 13.Haelens A, Wuyts A, Proost P, Struyf S, Opdenakker G, van Damme J. Leukocyte migration and activation by murine chemokines. Immunobiology. 1996;195:499–521. doi: 10.1016/S0171-2985(96)80019-2. [DOI] [PubMed] [Google Scholar]
- 14.Johnston RA, Mizgerd JP, Shore SA. Cxcr2 Is Essential for Maximal Neutrophil Recruitment and Methacholine Responsiveness after Ozone Exposure. Am J Physiol Lung Cell Mol Physiol. 2004 doi: 10.1152/ajplung.00101.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kimmel EC, Winsett DW, Diamond L. Augmentation of elastase-induced emphysema by cigarette smoke. Description of a model and a review of possible mechanisms. Am Rev Respir Dis. 1985;132:885–893. doi: 10.1164/arrd.1985.132.4.885. [DOI] [PubMed] [Google Scholar]
- 16.Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP. Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(−) human female reproductive tract fibroblasts. Am J Pathol. 2001;159:925–935. doi: 10.1016/S0002-9440(10)61768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]
- 18.Mannino DM. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care. 2003;48:1185–1191. discussion 1191–1183. [PubMed] [Google Scholar]
- 19.Martey CA, Pollock SJ, Turner CK, O’Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase 2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: Implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol. 2004 doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- 20.Masubuchi T, Koyama S, Sato E, Takamizawa A, Kubo K, Sekiguchi M, Nagai S, Izumi T. Smoke extract stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Pathol. 1998;153:1903–1912. doi: 10.1016/S0002-9440(10)65704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- 22.Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol. 1999;163:6086–6094. [PubMed] [Google Scholar]
- 23.Miller LM, Foster WM, Dambach DM, Doebler D, McKinnon M, Killar L, Longphre M. A murine model of cigarette smoke-induced pulmonary inflammation using intranasally administered smoke-conditioned medium. Exp Lung Res. 2002;28:435–455. doi: 10.1080/01902140290096728. [DOI] [PubMed] [Google Scholar]
- 24.Murphy PM. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin Hematol. 1997;34:311–318. [PubMed] [Google Scholar]
- 25.O’Byrne PM, Postma DS. The many faces of airway inflammation. Asthma and chronic obstructive pulmonary disease. Asthma Research Group. Am J Respir Crit Care Med. 1999;159:S41–63. [PubMed] [Google Scholar]
- 26.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Arellano JL, Barrios MN, Martin T, Sanchez ML, Jimenez A, Gonzalez-Buitrago JM. Hydrolytic enzyme of the alveolar macrophage in diffuse pulmonary interstitial disease. Respir Med. 1996;90:159–166. doi: 10.1016/s0954-6111(96)90158-4. [DOI] [PubMed] [Google Scholar]
- 28.Remick DG, Green LB, Newcomb DE, Garg SJ, Bolgos GL, Call DR. CXC chemokine redundancy ensures local neutrophil recruitment during acute inflammation. Am J Pathol. 2001;159:1149–1157. doi: 10.1016/S0002-9440(10)61791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richman-Eisenstat JB, Jorens PG, Hebert CA, Ueki I, Nadel JA. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol. 1993;264:L413–418. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- 30.Sato E, Koyama S, Takamizawa A, Masubuchi T, Kubo K, Robbins RA, Nagai S, Izumi T. Smoke extract stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activities. Am J Physiol. 1999;277:L1149–1157. doi: 10.1152/ajplung.1999.277.6.L1149. [DOI] [PubMed] [Google Scholar]
- 31.Sher ME, Bank S, Greenberg R, Sardinha TC, Weissman S, Bailey B, Gilliland R, Wexner SD. The influence of cigarette smoking on cytokine levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 1999;5:73–78. doi: 10.1097/00054725-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Snider GL, Lucey EC, Stone PJ. Animal models of emphysema. Am Rev Respir Dis. 1986;133:149–169. doi: 10.1164/arrd.1986.133.1.149. [DOI] [PubMed] [Google Scholar]
- 33.Stahl PD, Fishman WH. Measurement of beta-glucuronidase activity. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis, Enzymes. 2. 1984. pp. 246–256. [Google Scholar]
- 34.Standiford TJ, Kunkel SL, Lukacs NW, Greenberger MJ, Danforth JM, Kunkel RG, Strieter RM. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155:1515–1524. [PubMed] [Google Scholar]
- 35.Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med. 1994;42:640–651. [PubMed] [Google Scholar]
- 36.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 37.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun. 2000;68:4289–4296. doi: 10.1128/iai.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am J Respir Cell Mol Biol. 2003;29:661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XW, Liu Q, Wang Y, Thorlacius H. CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br J Pharmacol. 2001;133:413–421. doi: 10.1038/sj.bjp.0704087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang XW, Wang Y, Liu Q, Thorlacius H. Redundant function of macrophage inflammatory protein-2 and KC in tumor necrosis factor-alpha-induced extravasation of neutrophils in vivo. Eur J Pharmacol. 2001;427:277–283. doi: 10.1016/s0014-2999(01)01235-3. [DOI] [PubMed] [Google Scholar]