Abstract
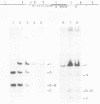
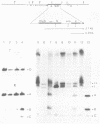
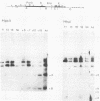
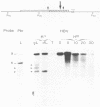
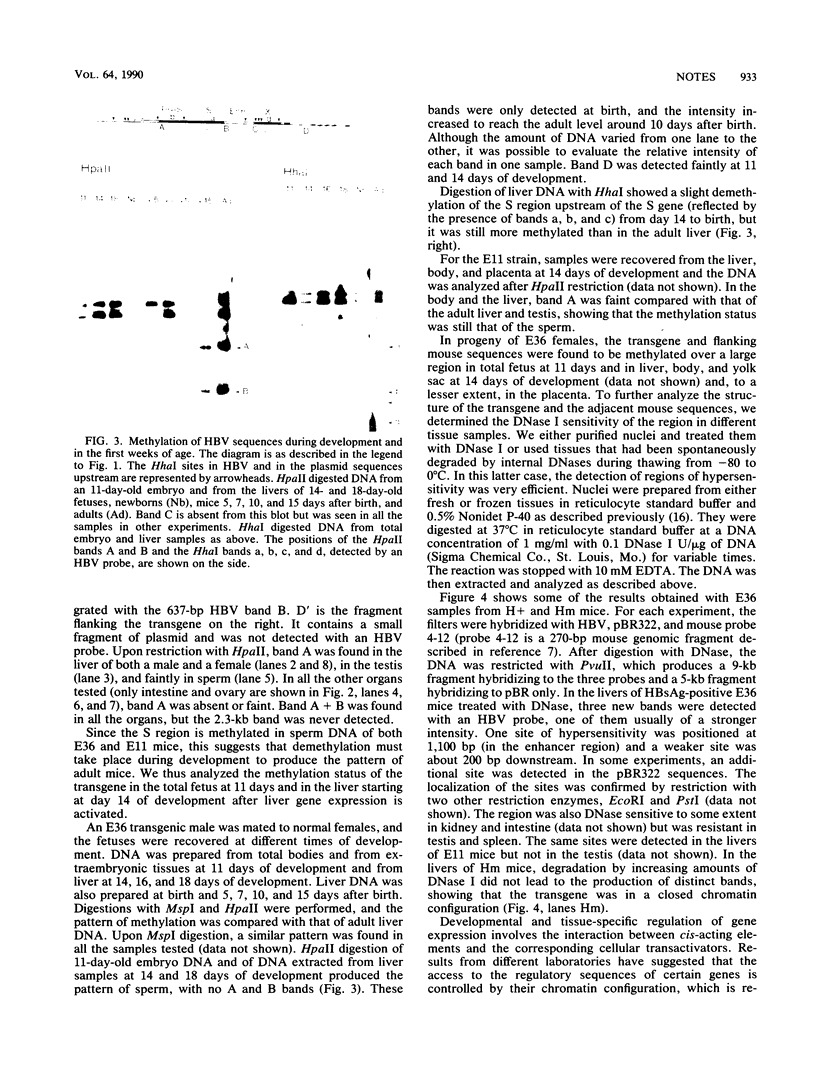
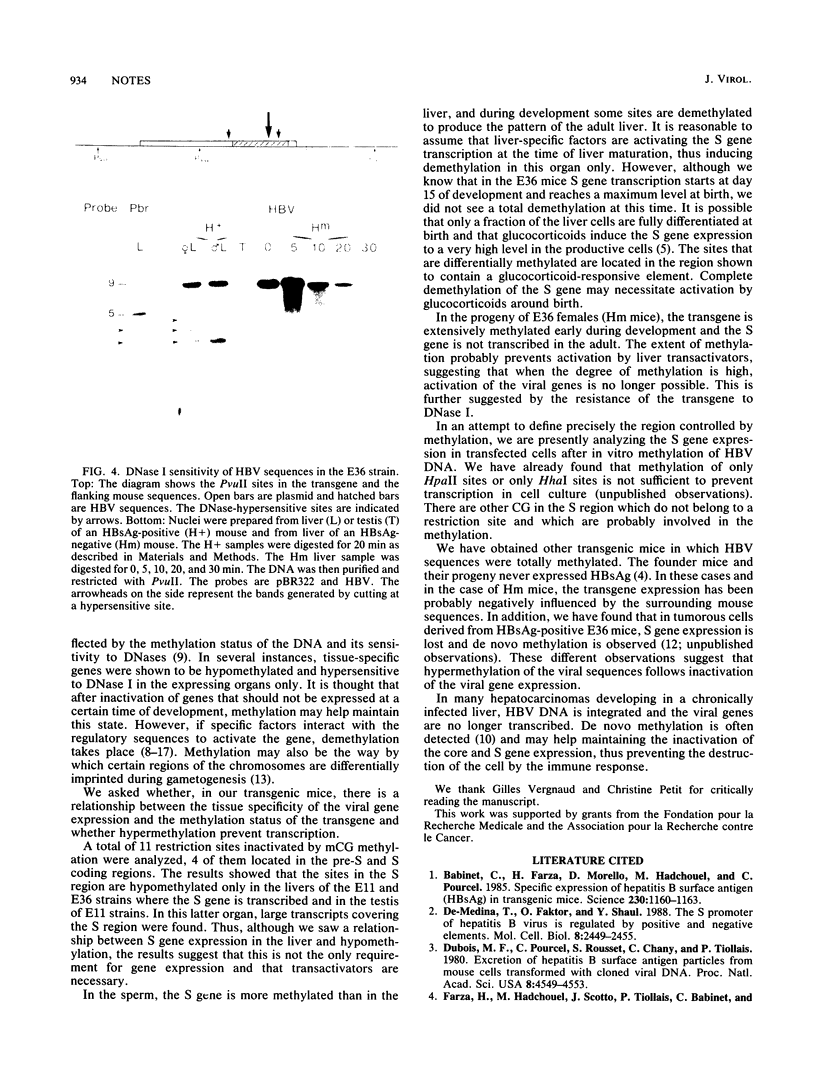
The methylation status of hepatitis B virus (HBV) DNA was investigated in different organs from two strains of transgenic mice (E36 and E11) expressing the hepatitis B surface antigen (HBsAg) gene specifically in the liver. Specific sites in the S gene were shown to be methylated in all the organs of adult mice except in the liver. These sites were methylated in 14-day-old fetal liver and were progressively demethylated during development and after birth. In one strain in which HBsAg expression is lost upon transmission by females, extensive de novo methylation of the transgene was detected in the livers and bodies of 14-day-old fetuses from transgenic females. The extent of methylation was such that activation of the gene was no longer possible. DNase I-hypersensitive sites were detected in the enhancer region of HBV in the liver of HBsAg-positive mice but not in HBsAg-negative progeny of E36 females. These data indicated that in two independent transgenic lines, HBV sequences are reproducibly activated in the developing liver along with cellular liver-specific genes and that transcription is associated with demethylation at specific sites in the S gene and with DNase hypersensitivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babinet C., Farza H., Morello D., Hadchouel M., Pourcel C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science. 1985 Dec 6;230(4730):1160–1163. doi: 10.1126/science.3865370. [DOI] [PubMed] [Google Scholar]
- De-Medina T., Faktor O., Shaul Y. The S promoter of hepatitis B virus is regulated by positive and negative elements. Mol Cell Biol. 1988 Jun;8(6):2449–2455. doi: 10.1128/mcb.8.6.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., Pourcel C., Rousset S., Chany C., Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farza H., Hadchouel M., Scotto J., Tiollais P., Babinet C., Pourcel C. Replication and gene expression of hepatitis B virus in a transgenic mouse that contains the complete viral genome. J Virol. 1988 Nov;62(11):4144–4152. doi: 10.1128/jvi.62.11.4144-4152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farza H., Salmon A. M., Hadchouel M., Moreau J. L., Babinet C., Tiollais P., Pourcel C. Hepatitis B surface antigen gene expression is regulated by sex steroids and glucocorticoids in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1187–1191. doi: 10.1073/pnas.84.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Hadchouel M., Farza H., Simon D., Tiollais P., Pourcel C. Maternal inhibition of hepatitis B surface antigen gene expression in transgenic mice correlates with de novo methylation. Nature. 1987 Oct 1;329(6138):454–456. doi: 10.1038/329454a0. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Pollok B. A., Atchison M. L., Perry R. P. The coupling between enhancer activity and hypomethylation of kappa immunoglobulin genes is developmentally regulated. Mol Cell Biol. 1988 Feb;8(2):930–937. doi: 10.1128/mcb.8.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Integrated hepatitis B virus DNA sequences specifying the major viral core polypeptide are methylated in PLC/PRF/5 cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2534–2538. doi: 10.1073/pnas.80.9.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surani M. A., Barton S. C., Norris M. L. Nuclear transplantation in the mouse: heritable differences between parental genomes after activation of the embryonic genome. Cell. 1986 Apr 11;45(1):127–136. doi: 10.1016/0092-8674(86)90544-1. [DOI] [PubMed] [Google Scholar]
- Tur-Kaspa R., Shaul Y., Moore D. D., Burk R. D., Okret S., Poellinger L., Shafritz D. A. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology. 1988 Dec;167(2):630–633. [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Yisraeli J., Adelstein R. S., Melloul D., Nudel U., Yaffe D., Cedar H. Muscle-specific activation of a methylated chimeric actin gene. Cell. 1986 Aug 1;46(3):409–416. doi: 10.1016/0092-8674(86)90661-6. [DOI] [PubMed] [Google Scholar]