Abstract
Objectives
To examine the effectiveness of four interventions on the rate and magnitude of muscle damage recovery, as measured by creatine kinase (CK).
Methods
23 elite male rugby players were monitored transdermally before, immediately after, 36 hours after, and 84 hours after competitive rugby matches. Players were randomly assigned to complete one of four post‐match strategies: contrast water therapy (CWT), compression garment (GAR), low intensity active exercise (ACT), and passive recovery (PAS).
Results
Significant increases in CK activity in transdermal exudate were observed as a result of the rugby match (p<0.01). The magnitude of recovery in the PAS intervention was significantly worse than in the ACT, CWT, and GAR interventions at the 36 and 84 hour time points (p<0.05).
Conclusions
An enhanced rate and magnitude of recovery was observed in the ACT, CWT, and GAR treatment groups when compared with the PAS group. Low impact exercise immediately post‐competition, wearing compression garments, or carrying out contrast water therapy enhanced CK clearance more than passive recovery in young male athletes.
Keywords: creatine kinase, contrast water therapy, active recovery, passive recovery, compression garments
Elite athletes are dependent on consistent high level performance for their livelihoods. As a result, athletes and their management will seek any advantage when training and preparing for competition. Optimising recovery from training and performance may benefit the subsequent training and performance of elite athletes over a period of time (for example, the competition season). In addition, Friden and colleagues1 have suggested that damage to a particular muscle may predispose the muscle to more significant injury in the future.
A frequently investigated aspect of recovery is the physiological response to high intensity eccentric loading, leading to delayed muscle soreness and impairment.2,3,4 The presence of muscle proteins and enzymes in the blood is a commonly reported indicator of muscle damage.5,6,7,8 The response of creatine kinase (CK) to exercise has received research attention, as strenuous exercise that damages skeletal muscle cell structure results in an increase in blood CK activity.9 It is thought that CK travels from damaged muscle tissue into the interstitial fluid before entering the circulation through the lymphatics.10 Volfinger et al11 described a slow transit time through the lymphatic system when they reported that CK injected intramuscularly did not appear in the blood until several hours after the injection.
Structural damage to muscle cells, as indicated by raised CK levels, has been reported after competitive rugby matches.12,13 Rugby Union (rugby) is an intermittent high intensity team sport involving a diverse range of athletes with different skills and anthropometric requirements, yet with quite similar training backgrounds. Rugby consists of aerobic and anaerobic exercise as well as direct impact with opposing players. It is reported that the incidence of injuries in rugby is high compared with other sports.14 Furthermore, an increased incidence of injuries in rugby has been reported at higher levels of play,15 and as a result of professionalism.16,17 The mechanism of increased CK associated with rugby matches is probably only partially explained by the high intensity running exercise as described by other researchers.6,18,19 It is probable that damage caused to muscle by the direct impact between opposing players is the main cause of CK elevation. Zuliani etal20 showed that the repeated trauma associated with a boxing bout led to a significantly higher CK concentration than a shadow boxing bout with an identical physical effort. Large increases in blood CK activity have also been reported in high contact intermittent sports such as American football21 and Australian Rules league football.22
Research has indicated that muscle damage caused by high intensity eccentric exercise has been associated with decrements in muscle function and performance during exercise.4 Rodenburg et al23 reported a significant correlation between maximal voluntary contraction (MVC) torque and blood levels of CK. As a result, various methods of optimising recovery have been investigated. These include compression garments,24 contrast water therapy,25,26 and active recovery.13 It is unclear from the literature to date whether reductions in muscle function and performance following a rugby match can be minimised by various recovery strategies. The purpose of this study was to investigate the effectiveness of four recovery interventions on CK recovery profiles in professional rugby players following competitive matches. It was hypothesised that an active recovery strategy would promote a superior reduction in CK levels than other recovery strategies.
Methods
Subjects
Twenty three elite male rugby players (mean (SD): age, 25 (3) years; height, 184.5 (9.0) cm; body mass, 99.2 (10.1) kg; sum of eight skinfolds, 87.7 (22.4) mm; bench press one repetition maximum (1RM), 131.5 (12.3) kg; 40 metre sprint time, 5.23 (0.27) s) volunteered to participate in the study. Before the study, participants attended a presentation outlining its purpose and the procedures involved. The participants were informed that their involvement was purely voluntary and that they could withdraw from at any time without consequence. Written informed consent was obtained and ethical approval was provided by the Waikato Institute of Technology ethics committee. Participants were well trained professional athletes selected to represent their province in the 2003 New Zealand National Provincial Championship (NPC).
Experimental protocol
Four competition weeks were monitored over the course of the NPC season, with the subjects being randomly assigned to one of four post‐match recovery strategies. Transdermal exudate samples were obtained for CK analysis 3.5 hours before and immediately after four NPC competition games and at 36 and 84 hours post‐match, using a previously described method.27 Sample collection was superimposed on normal training regimens. The unbalanced sample size for each treatment could not be avoided, as some participants were unable to complete their randomly assigned recovery strategy because of injury, non‐selection, or lack of match time.
Recovery interventions
Passive recovery (PAS)
Participants sat on a bench for nine minutes before carrying out their normal post‐match routine. This routine involved showering, rehydrating, snacking, and preparing for a post‐match function.
Active recovery (ACT)
Participants undertook low intensity exercise on an exercycle for seven minutes in the dressing room after the match (80–100 rpm, ∼150W) before carrying out their normal post‐match routine. A seven minute duration was selected because of time and resource limitations.
Contrast water therapy (CWT)
Participants immersed their body to the level of the anterior superior iliac spine in one of two temperature controlled water baths, alternating between one minute in cold water (8–10°C) and two minutes in hot water (40–42°C) for approximately nine minutes. The normal post‐match routine was then carried out.
Compression garment (GAR)
After the match participants undertook their normal post‐match routine before donning a lower body compression garment (Skins®), which was worn until the following morning (approximately 12 hours).
Transdermal sampling
Electrosonophoretic transdermal samples have been reported to show a high correlation with plasma constituents at rest and during exercise and recovery.27 Electrosonophoresis uses a combination of ultrasound (0.35 W/cm2 at the generator) and an electric current (9V) to stimulate a transdermal flux. A mixture of standard ultrasound gel (Aquasonic®, Parker, USA) containing 1% wt/vol of emu oil extract (Emea, NZ), 2% sodium lauryl sulphate, and 1% ethanol was applied to the skin before ultrasound application. Previous work has shown that this mixture facilitates transdermal movement.27,28 The sampling site was the ventral forearm. Collection fluid was circulated through the sampling head at 1.2 ml/min and samples were collected in 1.7 ml graduated microtubes (Invitrogen, Auckland, NZ). Samples were stored at −20°C until analysis.
Creatine kinase analysis
Transdermal exudate samples were divided into two and concentrated between 10‐ and 100‐fold, respectively, using vacuum extraction and nitrogen blow‐off. The samples were then assayed for CK activity using a derivation of the Rosalki method29 employing a Du Series 500 spectrophotometer (Beckman Coulter, Australia Pty). The mean coefficient of variation (CV) for transdermal CK analyses was <10%.
Statistical analysis
Data were analysed using residual maximum likelihood (REML) in GenStat to fully utilise the information on treatment differences from within and between players. Significances were calculated from a REML analysis of log transformed ratios of CK levels using the Wald test. The CK concentration was expressed in international units (IU). Means and standard deviations were calculated for the CK increase and recovery using Microsoft® Excel 9.0.3821 SR‐1. Only subjects who played for more than 20 minutes and who undertook no additional rehabilitation were included in the analysis. Percentage recovery was calculated on the basis of the increase in interstitial CK resulting from the rugby match. Statistical significance was set at p<0.05.
Results
The rugby matches examined in this study produced significant increases (p<0.01) in interstitial creatine kinase concentration ([CK]) from pre‐ to post‐competition, with levels of 1023.0 (308.3) and 2194.0 (833.7) IU, respectively. The sample taken immediately after the completion of the game represented the peak [CK] observed during the recovery period.
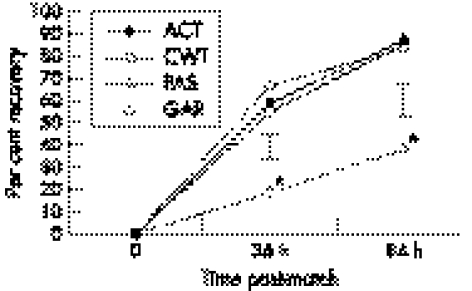
The ACT intervention showed 88.2% recovery after 84 hours (fig 1). Compression garments and CWT produced 84.4% and 85.0% recovery after 84 hours, respectively. The poorest rate of post‐match recovery (39.0%) was observed following the PAS intervention. The PAS recovery was significantly less than the ACT, CWT, and GAR interventions at both the 36 and 84 hour time points (p<0.05). No significant differences in CK recovery profile were observed between the ACT, CWT, or GAR interventions at any time point.
Figure 1 The percentage recovery post‐rugby match as examined by interstitial creatine kinase activity at 0, 36, and 84 hours, grouped by recovery strategy. Error bars show the standard error of the differences (SED) at the respective time points. ACT, active recovery; CWT, contrast water therapy; GAR, compression garment; PAS, passive recovery. *Significantly different (p<0.05) from other interventions.
Discussion
The results of this study confirm the findings of other researchers that the exercise and collisions involved in a game of rugby cause a significant increase in [CK].12,13 Different recovery interventions immediately post‐match influenced the rate and magnitude of the decrease in interstitial [CK] in the week following a rugby match. The data suggest that the ACT, GAR, and CWT interventions used in the current study are more effective forms of the post‐match recovery than the PAS intervention. However, the optimal duration and combination of recovery interventions requires further investigation.
It could be suggested that the low impact exercise undertaken by the participants in the ACT protocol increased blood flow, induced changes in blood flow distribution, and improved the range of motion. These responses could enhance the clearance rate of CK from the muscle and be responsible for the favourable temporal pattern observed as a result of active recovery. Owing to the time and resource limitations imposed by sampling in a competitive environment the duration of the ACT protocol was seven minutes. Increasing the duration of the ACT recovery intervention may further modulate the recovery profile observed as a result of this intervention. A study by Suzuki et al13 reported no difference in CK activity between an active and a passive recovery group. However, in that Japanese study the active recovery group carried out one hour of low intensity exercise in water once a day, whereas in the current study the intervention was immediately post‐match.
Contrast water therapy is considered to reduce oedema through a “pumping action” created by the alternating vasoconstrictor and vasodilator response of blood vessels to temperature changes.30 Blood flow changes, reduced muscle spasm, and an influence on the inflammatory response are also physiological effects attributed to CWT.25 In a pilot study conducted at the Waikato Institute of Technology and supervised by NDG, data suggested that CWT led to a reduction in blood CK activity when compared with a passive recovery protocol after a muscle damaging weights session. Muscle function, as measured by isometric force production and jump squat performance, was also improved as a result of CWT in this pilot study. The present study confirmed the effectiveness of CWT in decreasing an indirect marker of muscle damage when compared with a passive intervention.
Compression has long been suggested as an important therapeutic intervention following soft tissue injury as part of the RICE treatment with various modes of ice and compression used to provide pain relief, diminish inflammatory responses, and reduce swelling.31 Intermittent pneumatic compression32 and compression sleeves24 have been shown to be effective in providing some relief from exercise induced muscle damage. Kraemer et al24 reported a decreased perception of soreness, less swelling, and improved recovery of force production compared with a control group after wearing a compressive sleeve 24 hours a day for five days following a muscle damaging weight session. Results from the current study reinforce the importance of compression in reducing the effects of soft tissue injury.
In terms of CK levels, a difference was apparent between the lowest pre‐competition [CK] in two studies investigating CK responses in Japanese rugby players and the current study (Suzuki et al13: 351.6 (131.6) U/l; Takarada12: ∼400 (∼120) U/l; current study: 1023.0 (308.3) U/l). This may indicate that the players studied in the current New Zealand based study have not fully recovered from the serial muscle damage imparted by the rigors of the competitive season. It could also be hypothesised that the physical nature of professional rugby in New Zealand leads to more severe muscle damage than the fast paced game played by Japanese college students. Some evidence for the difference in the levels played may be seen in the average physical characteristics of the subjects (Takarada12: 178.8 cm, 87.4 kg; current study: 184.5 cm, 99.2 kg). On average the players in the current study were 5.7 cm taller and 11.8 kg heavier than their Japanese counterparts. These anthropometric variables suggest that the magnitude of the physical contacts in the New Zealand players would be much greater and could cause a greater degree of soft tissue damage.
The highest reported serum CK levels in these two studies of Japanese rugby players were also much lower than those observed in the current study (Suzuki et al13: 715.4 (438.3) U/l; Takarada12: 1081 (159) U/l; current study: 2194.0 (833.7) U/l. Furthermore in these two Japanese studies the [CK] peaked 24 hours post‐competition whereas the highest observed [CK] in the current study was seen immediately post‐game. This may not represent the peak value in the current study, however, as a subsequent sample was not taken until 36 hours after the match.
What is known about this topic
Structural damage to muscle cells has been reported after competitive rugby matches
Muscle damage caused by high intensity eccentric exercise is associated with decrements in muscle function and performance during exercise
Various methods of optimising recovery have been proposed
What this study adds
Interstitial creatine kinase activity was significantly increased in elite male rugby players as a result of competing in a rugby match
Low impact exercise immediately post‐competition, wearing compression garments, or carrying out contrast water therapy all enhanced CK clearance more than passive recovery in young male rugby players
An alternative explanation for the greater [CK] observed in the current study is sampling differences. The current study sampled interstitial fluid, whereas the two Japanese studies looked at the CK activity in blood. As a result of muscle damage, CK leaks from the muscle cells into the interstitial fluid before entering the blood stream through the lymphatic system.5,10 Therefore, it is conceivable that the [CK] in the interstitial fluid is higher than [CK] in the blood owing to partitioning effects. Indeed, it is possible that CK in the interstitial fluid undergoes metabolism before entering circulation which could further decrease the [CK] observed in the blood. Methodological differences may also explain the discrepancy between the [CK] peaks observed in the current study and the studies of Takarada12 and Suzuki et al.13
Furthermore, the time lag observed by researchers investigating impaired muscle function as a result of eccentric exercise (for example, Sorichter et al,7 Clarkson and Nosaka33) might be an artefact of methodological limitations. Monitoring interstitial fluid may describe the physiological milieu experienced by the muscle cells more accurately. As such, the time course of muscle functional impairment may be more closely related to enzymatic leakage from the cells than previously proposed.33,34,35,36 Future work should investigate the relation between interstitial and venous [CK].
Conclusions
The results of this study indicate that interstitial CK activity was significantly increased in elite male rugby players as a result of competing in a rugby match. The highest observed CK levels were recorded immediately after the match and decreased in an intervention specific profile over the experimental time period. Undertaking low impact exercise immediately post‐competition, wearing compression garments, or carrying out contrast water therapy promoted better physiological recovery than passive recovery in young male athletes.
Abbreviations
ACT - active recovery
CK - creatine kinase
CWT - contrast water therapy
GAR - compression garment
NPC - National Provincial Championship
PAS - passive recovery
Footnotes
Competing interests: none declared
References
- 1.Friden J, Sjostrom M, Ekblom B. A morphological study of delayed onset muscle soreness. Experientia 198137506–507. [DOI] [PubMed] [Google Scholar]
- 2.Byrne C, Twist C, Eston R. Neuromuscular function after exercise‐induced muscle damage: Theoretical and applied implications. Sports Med 20043449–69. [DOI] [PubMed] [Google Scholar]
- 3.Cheung K, Hume P A, Maxwell L. Delayed Onset Muscle Soreness. Sports Med 200333145–164. [DOI] [PubMed] [Google Scholar]
- 4.Connolly D A J, Sayers S P, McHugh M P. Treatment and Prevention of Delayed Onset Muscle Soreness. J Strength Cond Res 200317197–208. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers G G, Ball T E, Liston L. Creatine kinase levels are elevated during 2‐a‐day practices in collegiate football players. J Athl Train 200237151–156. [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson D, Nicholas C W, Williams C. Muscular soreness following prolonged intermittent high‐intensity shuttle running. J Sports Sci 199917387–395. [DOI] [PubMed] [Google Scholar]
- 7.Sorichter S, Mair J, Koller A.et al Skeletal troponin I as a marker of exercise‐induced muscle damage. J Appl Physiol 1997831076–1082. [DOI] [PubMed] [Google Scholar]
- 8.Tiidus P M, Ianuzzo C D. Effects of intensity and duration of muscular exercise on delayed soreness and serum enzyme activities. Med Sci Sports Exerc 198315461–465. [PubMed] [Google Scholar]
- 9.Epstein Y. Clinical significance of serum creatine phosphokinase activity levels following exercise. Isr J Med Sci 199531698–699. [PubMed] [Google Scholar]
- 10.Hortobagyi T, Denahan T. Variability in creatine kinase: methodological, exercise, and clinically related factors. Int J Sports Med 19891069–80. [DOI] [PubMed] [Google Scholar]
- 11.Volfinger L, Lassourd V, Michaux J M.et al Kinetic evaluation of muscle damage during exercise by calculation of amount of creatine kinase released. Am J Physiol 1994266R434–R441. [DOI] [PubMed] [Google Scholar]
- 12.Takarada Y. Evaluation of muscle damage after a rugby match with special reference to tackle plays. Br J Sports Med 200337416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki M, Umeda T, Nakaji S.et al Effect of incorporating low intensity exercise into the recovery period after a rugby match. Br J Sports Med 200438436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junge A, Cheung K, Edwards T.et al Injuries in youth amateur soccer and rugby players‐comparison of incidence and characteristics. Br J Sports Med 200438168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee A J, Garraway W M. Epidemiological comparison of injuries in school and senior club rugby. Br J Sports Med 199630213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bathgate A, Best J P, Craig G.et al A prospective study of injuries to elite Australian rugby union players. Br J Sports Med 200236265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garraway W M, Lee A J, Hutton S J.et al Impact of professionalism on injuries in rugby union. Br J Sports Med 20003348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obminski Z, Aniol‐Strzyzewska K, Burkhard‐Jagodzinska K.et al Endocrine and metabolic responses to an indoor rowing competition in male and female elite athletes. Biol Sport 199714145–153. [Google Scholar]
- 19.Ohkuwa T, Saito M, Miyamura M. Plasma LDH and CK activities after 400m sprinting by well‐trained sprint runners. Eur J Appl Physiol 198452296–299. [DOI] [PubMed] [Google Scholar]
- 20.Zuliani U, Bonetti A, Franchini D.et al Effect of boxing on some metabolic indices of muscular contraction. Int J Sports Med 19856234–236. [DOI] [PubMed] [Google Scholar]
- 21.Garry J P, McShane M D. Postcompetition elevation of muscle enzyme levels in professional football players. Medscape General Medicine 20002(1) [PubMed] [Google Scholar]
- 22.Pohl A P, O'Halloran M W, Pannall P R. Biochemical and physiological changes in football players. Med J Aust 19811467–470. [DOI] [PubMed] [Google Scholar]
- 23.Rodenburg J B, Bar P R, De Boer R W. Relations between muscle soreness and biochemical and functional outcomes of eccentric exercise. J Appl Physiol 1993742976–2983. [DOI] [PubMed] [Google Scholar]
- 24.Kraemer W J, Bush J A, Wickham R B.et al Influence of compression therapy on symptoms following soft tissue injury from maximal eccentric exercise. J Orthop Sports Phys Ther 200131282–290. [DOI] [PubMed] [Google Scholar]
- 25.Myrer W, Draper D O, Durrant E. Whirlpool contrast therapy. J Athl Train 199429318–322. [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins D, Kaminski T W. Contrast water therapy does not cause fluctuations in human gastrocnemius intramuscular temperature. J Athl Train 199833336–340. [PMC free article] [PubMed] [Google Scholar]
- 27.Cook C J. Rapid noninvasive measurement of hormones in transdermal exudate and saliva. Physiol Behav 200275169–181. [DOI] [PubMed] [Google Scholar]
- 28.Kost J, Mitragotri S, Gabbay R A.et al Transdermal monitoring of glucose and other analytes using ultrasound. Nat Med 20006347–350. [DOI] [PubMed] [Google Scholar]
- 29.Rosalki S. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med 196769696. [PubMed] [Google Scholar]
- 30.Rivenburgh D W. Physical modalities in the treatment of tendon injuries. Clin Sports Med 199211645–659. [PubMed] [Google Scholar]
- 31.Swenson C, Sward L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports 19966193–200. [DOI] [PubMed] [Google Scholar]
- 32.Chleboun G S, Howell J N, Baker H L.et al Intermittent pneumatic compression effect on eccentric exercise‐induced swelling, stiffness, and strength loss. Arch Phys Med Rehabil 199576744–749. [DOI] [PubMed] [Google Scholar]
- 33.Clarkson P M, Nosaka K. Muscle function after exercise‐induced muscle damage and rapid adaptation. Med Sci Sports Exerc 199224512–520. [PubMed] [Google Scholar]
- 34.Byrne C, Eston R. The effect of exercise‐induced muscle damage on isometric and dynamic knee extensor strength and vertical jump performance. J Sports Sci 200220417–425. [DOI] [PubMed] [Google Scholar]
- 35.Margaritis I, Tessier F, Verdera F.et al Muscle enzyme release does not predict muscle function impairment after triathalon. J Sports Med Phys Fitness 199939133–139. [PubMed] [Google Scholar]
- 36.Warren G L, Lowe D A, Armstrong R B. Measurement tools used in the study of eccentric contraction‐induced injury. Sports Med 19992743–59. [DOI] [PubMed] [Google Scholar]