Abstract
Objective
The aim of this study was to determine the influence of intensive aerobic running on some muscle contractile characteristics and the dynamics of their recovery during a 2 hour period afterwards.
Methods
Seven well trained runners performed a 6 km run at anaerobic threshold (VOBLA). Knee torque during single twitch, low and high frequency electrical stimulation (ES), maximum voluntary knee extension, and muscle activation level test of the quadriceps femoris muscles were measured before and immediately after the run, and at several time points during a 120 minute interval that followed the run.
Results
After exercise, the mean (SE) maximum twitch torque (TTW) and torque at ES with 20 Hz (low frequency ES; TF20) dropped by 14.1 (5.1)% (p<0.05) and 20.6 (7.9)% (p<0.05) respectively, while torque at stimulation with 100 Hz (high frequency ES; TF100), maximum isometric knee extension torque (maximum voluntary contraction torque; TMVC), and activation level did not change significantly. Twitch contraction time was shortened by 8 (2)% (p<0.05). Ten minutes after the run, TTW was 40% higher than immediately after the run and 10% (p<0.05) higher than before the run. TF20, TF100, and TMVC remained lower for 60 minutes (p<0.05) than before the run.
Conclusions
A 6 km continuous run at VOBLA caused peripheral fatigue by impairing excitation–contraction coupling. Twitch torque recovered very quickly. However, the process of torque restoration at maximum isometric knee extension torque and at high and low frequency ES took much longer.
Keywords: Fatigue, continuous running, electrical stimulation
All prolonged and sufficient intensive muscle activities cause fatigue, which reduces the capacity of the neuromuscular system to produce force.1,2 Causes of fatigue can be central or peripheral. Peripheral fatigue can be classified into high and low frequency fatigue (LFF).2,3,4,5
Most fatigue research in cyclic dynamic workloads has been directed at studying prolonged (>30 minutes) lower or moderate intensity workloads (60–75% VO2max).6,7,8,9 In the development of competition efficiency for middle and long distance runners, aerobic workloads of higher intensity (>80% VO2max) either in continuous (tempo runs) or interval mode play a very important role.10 Compared with lower intensity cyclic workloads, intensive running requires activation of larger motor units with increased recruitment of fast oxidative and glycolytic muscle fibres, and the increase of the intensity of chemical processes in the muscle. Increased metabolite concentration inhibits the activity of certain enzymes such as the sodium–potassium, calcium, and myosin ATPases, which exert a direct influence on the contractile ability of the muscle.11,12
It is well known that impairment of performance resulting from muscle fatigue differs according to the types of contraction involved, the muscular groups tested, and the exercise duration and intensity. Depending on these variables, strength loss with fatigue can originate from several sites from the motor cortex through to contractile elements.5,6,7,8,13,14,15,16. LFF has been observed in activities of long duration,7,8,16 while after maximum intensity exercise, the type of peripheral fatigue depends strongly on contraction type; stretch shortening cycle (SSC) exercise promotes high frequency fatigue.15 A 6 km tempo run at anaerobic threshold (VOBLA) is an SSC exercise, which in trained runners is considered a high intensity event compared with running long or extra‐long distances. Thus, specific impairment of the neuromuscular system can be expected at higher intensity running than at lower intensity dynamic workloads.
The aim of this study was to investigate the differences in muscular contractile characteristics and muscle activation level after intensive continuous 6 km run. At the same time, we also wanted to examine recovery dynamics of these mechanisms during the 2 hour postexercise recovery interval.
METHODS
Sample subjects
Seven well trained middle and long distance runners participated in the study. Their mean (SE) age was 25.3 (1) year. The subjects were informed about the possible risks associated with the experiment and gave their informed consent prior to the experiment. The study was approved by the Slovenian National Committee for Medical Ethics.
Experimental design
All subjects participated in one or two pre‐test measurements to familiarise themselves with the percutaneous electrical stimulation procedures. This was followed by a treadmill test to determine the running anaerobic threshold speed (VOBLA).17 After 5 days, the subjects performed continuous running at a steady pace over 6 km at VOBLA on an athletic track. They performed a warm up before the experimental running task. The measurements were performed before the exercise (after the warm up), after running load (start 60 seconds after completion of the exercise with the last test ending 3 minutes after the completed running load) and after 10, 20, 30, 40, 60, and 120 minutes of recovery.
Measurements were performed in the following order: skin temperature on the surface of the vastus lateralis (VL) muscle, taking of a blood sample, and five single twitches of relaxed VL, followed by low and high frequency electrical stimulation test. After 30 seconds' delay, the subjects performed maximum voluntary knee extension (maximum voluntary contraction; MVC) and muscle activation level tests.
Muscle contractile function measurements
Electrical stimulation
During the measurements involving electrical stimulation (ES) and MVC (right leg only), the subjects adopted a supine lying position. The distal part of the lower leg was fixed to the force transducer, which had a constant lever arm to the knee joint axis. The angle at the knee of the fixed leg was 45°. We used a force transducer (MES, Maribor, Slovenia) with a range of 0 to 5000 N.
The self adhering neurostimulation electrodes (50×80 mm; Axelgaard Manufacturing Co., Fallbrook, CA) were placed over the vastus lateralis, vastus medialis (VM), and rectus femoris (RF) muscles. Distal electrodes were placed over the distal part, and the proximal ones over the middle part of the centre of the muscle. The electrodes remained fixed during the entire duration of the experimental procedure.
Each pair of electrodes was connected to its own stimulation channel, which was galvanically separated from the others. On all occasions, constant current square biphasic impulses of 0.3 ms duration were used. A custom made, computer controlled, electrical stimulator was used. Data were sampled at 1 kHz using a 12 bit AD converter (Burr‐Brown, USA) and stored in the computer.
Single twitch test
Five supramaximum stimuli were delivered consecutively (one per second) to the relaxed VL muscle. The current used to elicit a maximum twitch was determined in each individual by increasing the stimulation current until no further increase in tension was observed despite further increments in current. The current at maximum twitch torque was further increased by 50%. This procedure ensured that each twitch was indeed maximum for each individual. The torque signals from the twitch responses were smoothed (moving mean; n = 5) and averaged with a trigger point at stimulus delivery. Maximum twitch torque (TTW), electromechanical delay (EMD), contraction time (CT) and half relaxation time (RT1/2) were calculated. 5,13
Low and high frequency stimulation test
The relaxed VL muscle was stimulated with two connected trains of impulses, of 0.8 s duration with a frequency of 20 Hz (low ES) and 100 Hz (high ES). For the 20 Hz and 100 Hz stimulation procedures, the current was increased to the highest tolerable level determined by the subject and was maintained during all measurements. We obtained the mean torque during the last 50 ms of stimulation of each frequency (TF20 and TF100).15,16
Maximum voluntary knee extension test and muscle activation level test
Subjects tried to achieve their maximum isometric knee extension torque (MVC) and maintained it for 5 seconds. The torque signal was smoothed and analysed for maximum MVC torque (TMVC).
For muscle activation level measurement, the subjects performed maximum voluntary knee extension, and after achieving maximum voluntary activation level, the quadriceps femoris muscle was also electrically stimulated while the subjects maintained their MVC. Electrical stimulation was delivered to VL, VM and RF muscles for 0.8 seconds with a frequency of 100 Hz.6,18 The train of impulses was triggered 3 seconds after the start of the voluntary concentric muscle contraction when the maximum voluntary contraction (maximum voluntary torque) was established.
The activation level (AL) was calculated by dividing the maximum knee extension torque before ES (TMVC) by maximum torque during ES (TMVC + ES) according to the formula: AL = TMVC TMVC+ES−1×100. AL was measured only twice: after the warm up and after the end of exercise.
Skin temperature on the surface of the VL muscle
Using a digital thermometer (Yokogawa EW 2572; Japan) and thermoelements (Cu Constant Type T; Yokogawa, Japan), we measured the surface temperature of the VL (TSVL). Electrodes were placed on the skin of the mid and distal parts of the muscle. Measurements were taken with an accuracy of 0.1°C.
Blood lactate
Blood lactate concentration (LA) was measured using a Contron 640 lactate analyser (Vienna, Austria). A sample of 20 μl blood was taken from the hyperaemic earlobe. The accuracy of the measurement of the lactate concentration in the fresh blood was ±0.1 mmol/l.
Statistical methods
We used analysis of variance for repeated measurements. When it showed statistically significant differences, Bonferroni's t test was employed to test differences between all pairs of time points. To calculate the correlation between changes in parameters after workouts the Pearson correlation coefficient was used. Statistical significance was accepted at p<0.05 (two tailed). All data were expressed as mean (SE).
RESULTS
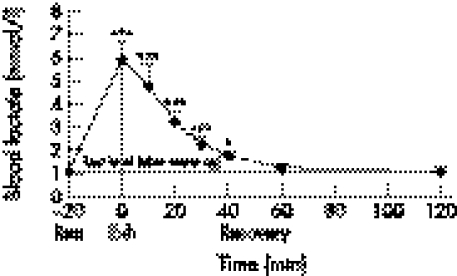
Mean (SE) 6 km run velocity was 4.94 (0.29) m/s (mean (SE) or 56% of their maximum velocity. Blood lactate increased from 1.3 (0.16) mmol/l pre‐exercise to 5.9 (0.76) mmol/l (p<0.001). At the end of the run, heart rate was 196 (3) beats/min. During the run, the temperature on the surface of VL rose from 34.1 (0.3)°C to 35.5 (0.3)°C (p<0.01).
Muscle contractile function
Influences of running workload on the characteristics of stimulated and voluntary muscular contraction are shown in table 1.
Table 1 Parameters of voluntary and electrically stimulated muscle contraction before and after 6 km running load.
| Parameter | Before | After | ||
|---|---|---|---|---|
| TTW (Nm) | 12.8 (1.6) | 11.0 (1.4)* | ||
| EMD (ms) | 47 (0.7) | 44 (0.8)* | ||
| CT (ms) | 92 (2) | 85 (3.1)* | ||
| RT½ (ms) | 53 (2.5) | 52 (1.6) | ||
| TF20 (Nm) | 35.8 (5.5) | 28.5 (5.7)* | ||
| TF100 (Nm) | 56.6 (7.2) | 49.7 (8.3) | ||
| TMVC (Nm) | 165.1 (14.5) | 154.9 (16.1) | ||
| AL (%) | 74 (6) | 75 (7) |
TTW, twitch peak torque; EMD, electromechanical delay; CT, contraction time; RT½, half relaxation time; TF20, torque during 20 Hz ES; TF100, torque during 100 Hz ES; TMVC, maximum torque during explosive isometric MVC; AL, activation level. *p<0.05 compared with the value before the workout.
After the 6 km run, mean TTW decreased by 14.1 (5.1)% (p<0.05). EMD and CT shortened by 6.1 (0.6)% (p < 0.05) and 8 (2)% (p<0.05), respectively, while RT½ did not change. In subjects with a more pronounced rise of the VL muscle surface temperature, we observed a greater shortening of CT (r = −0.82; p = 0.034).
TF20 showed a reduction from 35.8 (5.5) Nm at rest to 28.5 (5.7) Nm (p<0.05) after the 6 km run, whereas the TF100 reduction of 10.6 (3)% was not significant. Reduction of TMVC was also not significant.
The dynamics of the muscle contractile function recovery
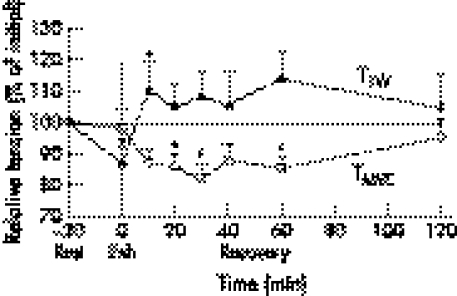
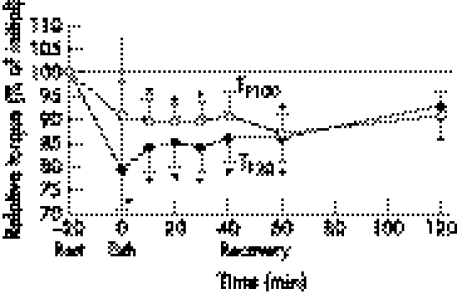
Within 60 minutes of the completion of the run, blood lactate concentration returned to its pre‐exercise value (post‐warm up) (fig 1). TTW recovery was very rapid (fig 2); only 10 minutes after the workload, TTW was 10% (p<0.05) higher than the control value measured immediately after the warm up. Increased TTW (5–14%) (p>0.05) remained elevated for 120 minutes after the exercise. TF20 remained decreased for 60 minutes (p<0.05), and had risen back to the pre‐exercise level at 120 minutes following exercise (fig 3). TMVC and TF100 showed similar recovery dynamics (figs 2 and 3).
Figure 1 Dynamics of blood lactate concentration after 6 km run. EXH, end of run. *p<0.05; ** p<0.01; *** p<0.001.
Figure 2 Recovery dynamics of twitch torque (TTW) and maximum voluntary torque (TMVC) after 6 km run. EXH, end of run. *p<0.05.
Figure 3 Recovery dynamics of torque during 20 Hz stimulation (TF20), and torque during 100 Hz stimulation (TF100) after 6 km run. EXH, end of run. *p<0.05.
DISCUSSION
After 20 minute of continuous running at anaerobic threshold, blood lactate concentration and skin temperature on the surface of the VL muscle were only moderately elevated. TTW of VL was reduced; however, contraction time was shortened, while relaxation of twitch time remained almost unchanged. This was accompanied with decreased low frequency torque. Maximum TMVC, high frequency stimulation torque, and the level of muscle activation remained unchanged.
The type of fatigue induced by intensive continuous running and its characteristics
Muscle contractile force reduction is a key feature of fatigue. A reduction of 13% for TTW and 21% for TT20, along with an unchanged F100, TMVC, and muscle activation level, indicate that fatigue after 6 km running at anaerobic threshold is mainly due to peripheral mechanisms, which are predominantly characteristic for LFF. It seems that central mechanisms were not greatly impaired as muscle activation levels remained almost constant. Similar behaviour has been observed in cycling7,11 and intermittent isometrics exercises,13 which last up to 1 hour, while in prolonged activities (>2 hours) central fatigue may also be involved, mainly at the spinal level.6
LFF is generally considered to reflect E‐C coupling failure and is primarily linked with the reduced efficiency of the calcium cycle.19 Several studies11,20 provide evidence that calcium cycle efficiency is connected to intracellular pH. A fall in pH can inhibit the release of Ca2+ into the sarcoplasmic reticulum19,21 and reduces Ca2+ affinity for troponin.12 Higher concentrations of ADP, Pi, and H+ reduces Ca2+ ATPase and actomyosin ATPase activity, and consequently the muscle contractile force.8,11,12,22
Our research does not fully allow us to confirm the hypothesis that high blood lactate and therefore a decrease in intracellular pH is the only or the most important factor of TTW reduction. A relatively low blood lactate concentration increase (up to 6 mmol/l), a low and non‐significant correlation between blood lactate change and relative TTW change (r = −0.12; p>0.05), and shortening of all the time parameters of the twitch show that a reduction in TTW is not only due to acidosis but probably also to the shortening of the calcium cycle. CT and RT½ shortening may also explain the torque reduction at low frequency ES; smaller CT and RT½ mean less efficient fusion of twitches at low (subtetanic) stimulation frequency and thus a decrease in TF20.
Another important factor for twitch time shortening may be increased muscle temperature.23,24 Temperature on the surface of the muscle increased by 1.4°C (p<0.01) during the 6 km intensive run. There is a difference between skin temperature on muscle surface and that inside the muscle, because skin and the underlying fascia are good insulators. Differences increase with production temperature inside the muscle.25 It is possible to conclude that the core temperature during the 6 km run causes an increase in muscle temperature, which in turn improves muscular contraction. The rise in muscle temperature was probably the most important cause of shortening the twitch time parameters as evidenced by the high correlation between CT and skin temperature on surface VL in both this study and that of Bishop.25
Reduced TTW and TT20 can also be a result of structural changes in SR caused by the prolonged intensive running workload, which represents an SSC type of exercise. It is well known that that muscular damage can exist after eccentric contractions are performed. One factor causing fatigue in SSC workloads of submaximum and maximum intensity (double leg takeoff) is a higher concentration of creatine kinase, which shows that this type of workload induces damage in certain structural elements of the active muscle.6,16
It is possible to conclude that the differences of neuromuscular function after intensive 6 km run at VOBLA are more common to submaximum intensive SSC exercise16 than to changes after very long (>2 hours) cyclic activities.6
What is already known on this topic
Impairment of performance resulting from muscle fatigue differs according to the types of contraction involved, the muscular groups tested, and the exercise duration and intensity
In long lasting activities, low frequency fatigue is dominant, while after maximum intensity exercise, the type of peripheral fatigue depends strongly on contraction type; stretch shortening cycle exercise promotes high frequency fatigue
What this study adds
A 6‐km continuous run at VOBLA caused only peripheral low frequency fatigue, while changes at high frequency electrical stimulation were statistically not significant
The differences in neuromuscular function after an intensive 6 km run are more common to submaximum intensive stretch shortening cycle exercise than to changes after very long (<2 hours) cyclic activities
The dynamics of the recovery of muscle contractile characteristics
Despite moderate blood lactate concentration after the 6 km run, the restoration of TTW was rapid and complete. In the 10 minutes following the workload, blood lactate value decreased by 1.1 mmol/l (19%), while TTW during this time increased by 24% and exceeded the pre‐exercise value by 10%. Other studies have also observed rapid twitch force restoration in conditions of moderate or even high blood lactate.6,20,26 Post‐exercise twitch force restoration and its potentiation may be connected to rapid restoration of muscle creatine phosphate.7,22
In contrast, the process of force regeneration at MVC and tetani stimulation muscle contractions took much longer, which is in accordance with earlier research.7,20 Sahlin and Ren20 found that muscle endurance was still 25% lower at 4 minutes after exhausting exercise than it was before the exercise.
CONCLUSION
A 6 km continuous run at VOBLA caused only peripheral fatigue. The most significant sign was low frequency fatigue, while changes at high frequency electrical stimulation were statistically not significant. These changes showed on impaired excitation–contraction coupling as the most prominent site of fatigue in the observed exercise.
TTW recovery was very rapid; post‐tetanic potentiation lasted for 120 minutes after the exercise. Recovery of TMVC, TF20, and TF100 were not complete 1 hour of the completion of the running test.
Acknowledgements
This study was supported by the Slovenian Ministry of Science and Technology (grant nos. L5‐6570‐0587 and L5‐5330‐0587).
Abbreviations
AL - activation level
CT - contraction time
EMD - electromechanical delay
ES - electrical stimulation
LFF - low frequency fatigue
MVC - maximum voluntary contraction (measured here as maximum isometric knee extension)
RF - rectus femoris
RT½ - half relaxation time
SSC - stretch shortening cycle
TF20 - torque at ES with 20 Hz
TF100 - torque at stimulation with 100 Hz
TMVC - maximum voluntary contraction torque (measured here as maximum isometric knee extension torque)
TTW - twitch torque
VL - vastus lateralis
VM - vastus medialis
Footnotes
Competing interests: none
References
- 1.Bigland‐Ritchie B, Johansson R, Lippold O J.et al Contractile speed and EMG changes during fatigue of sustaind maximal voluntary contractions. J Neurophysiol 198350313–323. [DOI] [PubMed] [Google Scholar]
- 2.Gibson H, Edwards R H T. Muscular exercise and fatigue. Sports Med 19852120–132. [DOI] [PubMed] [Google Scholar]
- 3.Jones D A. High‐ and low‐ frequency fatigue revisited. Acta Physiol Scand 1996156265–270. [DOI] [PubMed] [Google Scholar]
- 4.Kirkendall D T. Mechanisms of peripheral fatigue. Med Sci Sports Exer 199022444–449. [PubMed] [Google Scholar]
- 5.Bigland‐Ritchie B, Furbush F, Woods J J. Fatigue of intermittent submaximal voluntary contractions: central and peripheral fatigue. J Appl Physiol 198661421–429. [DOI] [PubMed] [Google Scholar]
- 6.Millet G Y, Lepers R. Alteration of neuromuscular function after prolonged running, cycling and skiing exercise. Sports Med 200434105–116. [DOI] [PubMed] [Google Scholar]
- 7.Booth J, Mc Kenna M J, Ruell P A.et al Impaired calcium pump function does not slow relaxation in human skeletal muscle after prolonged exercise. J Appl Physiol 199783511–521. [DOI] [PubMed] [Google Scholar]
- 8.Davies C T M, White M J. Muscle weakness folloving dynamic exercise in humans. J Appl Physiol 198253236–241. [DOI] [PubMed] [Google Scholar]
- 9.Sahlin K, Seger J Y. Effect of prolonged exercise on the contractile properties of human quadriceps muscle. Eur J Appl Physiol 199571180–186. [DOI] [PubMed] [Google Scholar]
- 10.Billat L V. Interval training for performance: a scientific and empirical practice. Sports Med 20013113–31. [DOI] [PubMed] [Google Scholar]
- 11.Sahlin K. Muscle fatigue and lactic acid accumulation. Acta Physiol Scand 198612883–91. [PubMed] [Google Scholar]
- 12.Hultman E, Spriet L L, Soderlund K. Biochemistry of muscle fatigue. Biomed Biochim Acta 198645(suppl)97–106. [PubMed] [Google Scholar]
- 13.Vollestad N K, Sejersted I, Saugen E. Mechanical behavior of skeletal muscle during intermittent voluntary isometric contractions in humans. J Appl Physiol 1997831557–1565. [DOI] [PubMed] [Google Scholar]
- 14.Linnamo V, Hakkinen K, Komi P V. Neuromuscular fatigue and recovery in maximal compared to explosive strength loading. Eur J Pppl Physiol 199877176–181. [DOI] [PubMed] [Google Scholar]
- 15.Strojnik V, Komi P V. Neuromuscular fatigue ater maximal stretch‐shortening cycle exercise. Eur J Appl Physiol 199884344–350. [DOI] [PubMed] [Google Scholar]
- 16.Strojnik V, Komi P V. Fatigue after submaximal intensive stretch‐shortening cycle exercise. Med Sci Sports Exerc 2000321314–1319. [DOI] [PubMed] [Google Scholar]
- 17.Beaver W L, Wasserman K, Whipp B. Improved detection of lactate threshold during exercise using a log–log transformation. J Appl Physiol 198559321–329. [DOI] [PubMed] [Google Scholar]
- 18.Strojnik V. Muscle activation level during maximal voluntary effort. Eur J Appl Physiol 199572134–141. [DOI] [PubMed] [Google Scholar]
- 19.Fitts R H, Metzger J M. Mechanisms of muscular fatigue. In: Poortmans JR, ed. Principles of exercise byochemistry. Basel: Karger, 1991212–229.
- 20.Sahlin K, Ren J M. Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. J Appl Physiol 198967648–654. [DOI] [PubMed] [Google Scholar]
- 21.Metzger J M, Moss R L. Shortening velocity in skinned single muscle fibres. Biophys J 198752127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid M B, Shoji T, Moody M R.et al Reactive oxygen in skeletal muscle. Extracellular release of free radicals. J Appl Physiol 1992731805–1809. [DOI] [PubMed] [Google Scholar]
- 23.Davies C T, Young K. Effect of temperature on the contractile properties and muscle power of triceps surae in humans. J Appl Physiol 198355191–195. [DOI] [PubMed] [Google Scholar]
- 24.Enoka R M. Acute adaptations. In: Enoka RM, ed. Neuromechanical basis of kinesiology. 2nd ed. Champaign, IL: Human Kinetics, 1994271–302.
- 25.Bishop D. Warm up I (potential mechanisms and the effects of passive warm up on exercise performance. Sports Med 200333439–454. [DOI] [PubMed] [Google Scholar]
- 26.Duchateau J, Hainaut K. Electrical and mechanical failures during sustained and intermittent contractions in humans. J Appl Physiol 198558942–947. [DOI] [PubMed] [Google Scholar]