Abstract
Objectives
To detect neuropeptides in human skeletal muscle at rest and after eccentric exercise.
Method
Eight healthy subjects participated in the study. Microdialysis of the distal part of the vastus lateralis of the quadriceps muscle and pain evaluation were performed immediately after eccentric exercise, after two days, and at rest. Calcitonin gene related peptide (CGRP) and neuropeptide Y (NPY), representatives of the sensory and autonomic nervous system, were analysed by radioimmunoassay.
Results
Overall, the measured concentrations were low, some even below the limit of detection. At rest, CGRP was detected in two of seven samples, but after eccentric exercise it was detected in 27 of 30 samples. At rest, all NPY concentrations were below the limit of detection, but after exercise it was found in six of 30 samples.
Conclusion
The significant increase in detectability of CGRP after eccentric exercise may be related to the increased experience of pain. Therefore the occurrence of CGRP after heavy eccentric exercise may be associated with the regulation of delayed onset muscle soreness and possibly also the stimulation of tissue regeneration.
Keywords: microdialysis, neuropeptides, calcitonin gene related peptide, neuropeptide Y, delayed onset muscle soreness
The aetiology and pathogenesis of muscular pain, especially after hard eccentric exercise, are still poorly understood. Accumulated data suggest that the peripheral nervous system plays an important role, not only in pain and inflammation, but also in tissue healing and adaptation through the release of neuronal mediators, the so called neuropeptides. Both sensory and autonomic neuropeptides have been identified in the musculoskeletal system, mainly by immunohistochemical studies in animals,1,2 but few studies have focused on human skeletal muscle.3
The tissue concentration of calcitonin gene related peptide (CGRP), representing the sensory nervous system, has been correlated with pain1,4,5 and arthritis.6 Besides nociceptive actions, CGRP has been shown to act vasodilatory7 and to participate in the proliferation of fibroblasts and endothelial cells as well as growth factor synthesis.8 An adaptive role for CGRP in pain and healing was also suggested in studies of minor orthopaedic trauma, in which raised concentrations of plasma CGRP were found.9 The autonomic neuropeptide Y (NPY) seems to show opposite effects to CGRP—that is, it acts as a vasoconstrictor8 and exerts antinociceptive10 effects.
It is well known that hard eccentric training, if unaccustomed, results in distinct muscle soreness after one to three days, the so called delayed onset muscle soreness (DOMS).11,12 Homonko and Theriault13 showed in rats that, 72 hours after eccentric downhill running, CGRP concentrations had increased in the neurones of the medial gastrocnemius muscle. However, neuropeptides such as CGRP and NPY have been little studied in vivo in human skeletal muscle.14
Microdialysis is a technique that can be used in vivo to measure tissue concentrations of different substances in human tissue. A thin plastic tube with a diameter of about 1 mm is introduced into the muscle with a thin needle. Within the muscle, the tube can be used as a dialysis membrane. Microdialysis has been used to study glucose metabolism in muscle.15 It has even been performed during exercise.16
The aim of this study was to analyse the occurrence of CGRP and NPY in human skeletal muscle in vivo at rest and after hard eccentric exercise. Microdialysis was used to measure local changes in tissue neuropeptides after hard exercise, to elucidate possible mechanisms of muscular pain and tissue recovery.
Methods
Subjects
Eight subjects, four men and four women, participated in the study. The mean age was 28 years (range 20–38); they were all healthy, with no injuries, at the time of study. They were all accustomed to various levels of physical activity but they were not elite athletes. All subjects avoided hard exercise during the week before the investigation and the week before the control investigation. Parallel to this study, the subjects also took part in a study that investigated the effect of sports massage after exercise.
Eccentric exercise
All subjects performed a 10 minute warm up on an ergometer cycle followed by brief gentle stretching of the thigh muscles. They were then seated on a Kin‐Com dynamometer (Kinetic Communicator Exercise System; Chattecx Corp, Chattanooga, Tennessee, USA), with a hip angle of about 100°. The hip and thigh were fastened with a belt, and the distal part of the lower leg was anchored to the lever of the dynamometer. The axis of rotation was through the knee joint. The subjects performed 300 maximal eccentric contractions of the quadriceps muscles of each thigh. Eccentric contractions were performed from an angle of 10° to 90° of knee flexion, at a velocity of 180°/s. Only eccentric contractions were performed because the machine rotated the leg back to the starting position after each contraction. The subjects were able to follow the exercise procedure on the computer screen and thus receive immediate feedback after each contraction. They were also vocally encouraged by the test leader to perform their best during each session. After a few minutes of rest, the same exercise was performed using the other leg. The exercise on the Kin Com dynamometer lasted about 15 minutes for each leg.
Microdialysis
After the exercise, the subjects were allowed to rest for a few minutes and drink some water. The microdialysis was performed as a surgical procedure under strict sterile conditions. Participants were placed supine on a bench, in light clothes, at room temperature (22–24°C). The skin was sterilised with 2 mg/ml chlorhexidin (Klorhexidin: Pharmacia & Upjohn, Täby, Sweden). Thereafter the skin and underlying subcutaneous tissue, a few millimetres deep, was anaesthetised with 1 ml 10 mg/ml prilocain (Citanest; Astra Läkemedel, Södertälje, Sweden). The distal part of the vastus lateralis muscle about 10 cm proximal to the knee joint of both legs was chosen. A 1.4 mm puncture needle was used to introduce a CMA 60 Microdialysis catheter (CMA Microdialysis AB, Solna, Sweden) parallel to the muscle fibres. The shaft is made of polyurethane and has a diameter of 0.9 mm and length of 20 mm. The membrane is made of polyamide with a diameter of 0.6 mm and a length of 30 mm; the molecular mass cut‐off point is 20 kDa. The catheter was connected to a CMA 107 Microdialysis pump, and an infusion speed of 5µl/min was selected. This relatively high infusion speed was selected because we suspected that the neuropeptide concentrations would be low.16 Sterile isotonic perfusion fluid (Apoteksbolaget, Stockholm, Sweden) was used: Na, 142 mmol/l; K, 4.7 mmol/l; Mg, 1.2 mmol/l; Ca, 2.5 mmol/l; Cl, 149 mmol/; dextran, 40 g/l; water. The dialysate was collected into a microvial. An equilibration time of 30 minutes was chosen. We wanted to perform the analyses as soon as possible after the exercise, but not during the first 20 minutes after introduction of the probe when metabolic changes can be found.17 Four 30 minute sampling periods were used. Every dialysis sample of 150 ml was immediately frozen to –70°C. After each microdialysis the catheter was removed and disposed of.
Two days after the exercise, microdialysis was again performed on both legs, but this time the catheter was inserted about 5 cm proximal to the first insertion site. Two weeks after the first microdialysis, one more was performed on one thigh, but with no prior exercise. This sample was used as a control. The subjects were instructed not to perform any strenuous activity between the trials.
To test the ability of CGRP to pass through the microdialysis membrane, a relative recovery test was performed in vitro. Two CGRP standards with concentrations of 250 fmol/ml and 1000 fmol/ml were dialysed at a temperature of 37°C. Two perfusion speeds were used in the relative recovery test, 1 µl/min and 5 µl/min respectively.
Radioimmunoassay
For each neuropeptide analysis in which four samples were collected, the samples from two consecutive 30 minute periods were pooled and independently run to obtain two values, from which a mean value was calculated. The concentrations of CGRP and NPY were expressed as fmol/ml of microdialysate.
CGRP was analysed using antiserum CGRP8 raised in a rabbit against bovine serum albumin‐conjugated rat CGRP. High performance liquid chromatography (HPLC) purified rat 125I‐histidyl‐CGRP was used as radioligand, and rat CGRP as standard. Aliquots (100 μl) of samples or standards were mixed with 100 μl antiserum and incubated for 48 hours at 4°C; 100 µl radiolabelled CGRP was added and the solution was incubated for an additional 24 hours. Free and antibody bound CGRP were separated using 50 ml Sac‐Cel (anti‐rabbit solid phase second antibody coated cellulose suspension; IDS, Bolton, Lancashire, UK). Samples were left for 30 minutes at room temperature; the reaction was then blocked with 1 ml distilled water. Samples were centrifuged at 3000 rpm for 20 minutes at 4°C, and the supernatants were decanted. Pellets were counted in a gamma counter for three minutes. The detection limit of the assay for rat CGRP was 3.9 fmol/ml. Intra‐assay and interassay coefficients of variation were 8% and 14% respectively.
NPY was determined using rabbit anti‐NPY (human, rat) serum (Phoenix Laboratories, Belmont, CA, USA). HPLC purified porcine 125I‐NPY was used as radioligand, and synthetic porcine NPY was used as standard (SC116; Neosystem, Strasbourg, France). Aliquots (100 µl) of samples or standards were mixed with 500 μl antiserum and incubated for 48 hours at 4°C; 500 µl radiolabelled NPY was added, and the solution was incubated for an additional 24 hours. Free and antibody bound NPY were separated using 50 ml Suspension‐3 (Amersham Biotech, Uppsala, Sweden). Samples were left for 30 minutes at room temperature; the reaction was then blocked with 1.5 ml distilled water. Samples were centrifuged at 3000 rpm for 20 minutes at 4°C, and the supernatants were decanted. Pellets were counted in a gamma counter for three minutes. The detection limit was 3.9 fmol/ml. Intra‐assay and interassay coefficients of variation were 7% and 12% respectively.
Pain assessment
Pain and discomfort in the thighs were assessed while the subjects were taking a few steps in the laboratory. A visual analogue scale (VAS) was used. The scale was labelled with consecutive numbers from 0 to 10, where 0 represented no pain at all and 10 represented the worst imaginable pain.
Statistical analysis
We used Friedman analysis of variance for non‐parametric data to test the detectability of CGRP over time (before and after exercise). Statistica (Statsoft, Tulsa, Oklahoma, USA) software was used.
Results
All eight subjects performed the exercise and were examined with microdialysis. In a few samples, traces of blood were found on visual inspection of the microvials, and these were excluded from the analyses. Overall, the measured concentrations were low, close to the limit of detection.
CGRP
Detectable concentrations of skeletal muscle CGRP were found in most of the samples. However, in the control situation at rest, only two of seven samples (29%) showed detectable concentrations of CGRP (4.01 and 4.12 fmol/ml; table 1). Directly after exercise, all 15 samples (one was excluded because of blood contamination) had detectable concentrations of CGRP (100%). The mean concentration was 4.85 fmol/ml (range 4.13–5.54). Two days after exercise, 12 of the 15 samples (80%) had detectable concentrations of CGRP (mean concentration 5.4 fmol/l (range 3.94–6.21); table 1). The increase in the number of samples with detectable concentrations of CGRP after exercise was significant (p<0.01). The relative recovery of CGRP was 8.3% for the perfusion speed 1 µl/min and 4.4% for the perfusion speed 5 µl/min.
Table 1 Calcitonin gene related peptide detected by microdialysis followed by radioimmunoassay in quadriceps muscle.
| Subject | Control | After | After | Day 3 | Day 3 |
|---|---|---|---|---|---|
| 1 | nd | 4.56 | 4.57 | 4.95 | 5.38 |
| 2 | nd | 4.56 | 4.13 | 4.39 | 5.34 |
| 3 | nd | 4.62 | 4.81 | 5.44 | 5.97 |
| 4 | nd | 4.95 | 4.83 | 5.93 | 5.04 |
| 5 | 4.01 | 5.04 | – | 6.19 | – |
| 6 | 4.12 | 4.77 | 5.03 | 6.21 | 6.05 |
| 7 | – | 5.03 | 5.27 | nd | nd |
| 8 | nd | 5.54 | 5.03 | nd | 3.94 |
Values are expressed as fmol/ml.
Control, At rest, microdialysis on one leg; after, directly after eccentric exercise, microdialysis on both legs; day 3, 48 hours after eccentric exercise, microdialysis on both legs; nd, below detection limit (3.9 fmol/l); –, technical error.
NPY
In all control samples (at rest), NPY was below the limit of detection (3.9 fmol/l; table 2). However, after exercise, NPY could be detected in some samples: six of 30 samples (20%) showed traces of NPY (mean 8.2 fmol/ml (4.72–13.98); table 2).
Table 2 Neuropeptide Y detected by microdialysis followed by radioimmunoassay in quadriceps muscle.
| Subject | Control | After | After | Day 3 | Day 3 |
|---|---|---|---|---|---|
| 1 | nd | nd | nd | nd | nd |
| 2 | nd | nd | nd | nd | 6.75 |
| 3 | nd | nd | nd | 5.91 | nd |
| 4 | nd | nd | nd | nd | nd |
| 5 | nd | nd | – | 13.98 | – |
| 6 | nd | nd | 4.72 | 10.94 | nd |
| 7 | – | nd | nd | nd | nd |
| 8 | nd | 6.73 | nd | nd | nd |
Values are expressed as fmol/ml.
Control, At rest, microdialysis on one leg; after, directly after eccentric exercise, microdialysis on both legs; day 3, 48 hours after eccentric exercise, microdialysis on both legs; nd, below detection limit (3.9 fmol/l); –, technical error.
Pain assessment
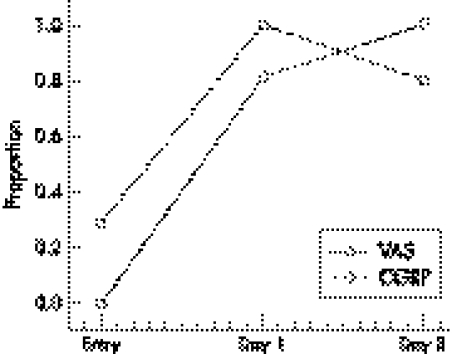
The median value for pain, as assessed by VAS, was 0 (range 0–0) at rest, 1 (range 0–5) directly after exercise, and 2 (range 1–6) after 48 hours. Figure 1 shows the relation between the proportion of subjects with detectable concentrations of CGRP and those reporting pain.
Figure 1 Proportion of subjects with detectable calcitonin gene related peptide (CGRP) and reporting pain. Entry, Before exercise; day 1, one day after exercise; day 3, three days after exercise; CGRP, proportion of subjects with detectable concentrations of CGRP (>3.9 fmol/ml); VAS, proportion of subjects reporting any pain (visual analogue scale >0).
No sex differences in peptide concentrations and VAS scores were observed.
Discussion
This study shows that CGRP can be analysed in human skeletal muscle after eccentric exercise using the microdialysis technique followed by radioimmunoassay. The increased detectability of CGRP observed after hard eccentric exercise was related to increased experience of pain.
In our experimental model, muscle pain was induced by eccentric exercise, which is a well documented model18 that is easy to perform and standardise. The subjects' experience of pain, as measured by VAS, had increased two days after exercise, which is consistent with earlier reports.11,12 The increased pain level, described as DOMS, was related to the finding of increased concentrations of CGRP (fig 1). Further experiments—for example, inhibition of DOMS by treatment with a CGRP antagonist—would greatly support the contention that CGRP is involved in muscle nociception.
Presumably CGRP in human muscle exerts similar actions to those shown in other tissues—that is, nociceptive, as well as vasoregulatory and proinflammatory actions. Microdialysis followed by radioimmunoassay revealed increased concentrations of CGRP after exercise, but the analyses of NPY were inconclusive. Only a few samples gave detectable concentrations of NPY after exercise. The inconsistency in NPY detection may be due to the low microdialysis membrane cut‐off point (20 kDa). The molecular mass of NPY is 4272 Da,19 whereas that of CGRP is somewhat lower (3789 Da).20 However, the low concentrations of NPY confirm earlier studies showing the vasoconstrictive effect of NPY in working muscle.21
CGRP concentration in human muscle is probably lower than in skin, where microdialysis using the same membrane cut‐off point as in the present study has been used to detect CGRP.22 It has been suggested that, in kinetic experiments, in which the main aim is to follow changes in the concentration of tissue molecules over time, it is often not necessary to consider recovery problems.16 However, as the CGRP concentrations were low, we performed an in vitro recovery test, which showed 4–8% recovery depending on the infusion speed. This is a higher recovery than in the microdialysis study on skin, in which the in vitro recovery of CGRP was only 2.6%.22 The relative recovery of NPY in the kind of probes that we used has recently been found to be about 16% at a perfusion speed of 4 µl/min.14
The increase in detectability of CGRP in the present study from mostly below the limit of detection at rest to clearly detectable concentrations after exercise probably represents a local release of CGRP in response to exercise.
Homonko and Theriault13 showed increased concentrations of CGRP in the motor neurones of medial gastrocnemius muscles in rats 72 hours after eccentric downhill running. Other studies on minor orthopaedic trauma, such as sprains and muscle injuries, have shown raised concentrations of CGRP in plasma,9 and after tendon rupture in the rat there is extensive nerve ingrowth into the tendon, which is normally devoid of nerves, expressing CGRP.23 Therefore these studies indicate that the peripheral nervous system initially reacts to exercise and injury by releasing regulatory neuronal mediators such as CGRP.
CGRP is known to be a potent vasodilator and has also been suggested to exert trophic effects—that is, proliferation of fibroblasts and endothelial cells in response to stress. Our detection of CGRP after eccentric exercise, which is known to cause cleavage of contractile proteins,24 can therefore be assumed to reflect tissue regeneration.
CGRP is also implicated in nociception. It is known that peripheral release of CGRP is involved in nerve sensitisation, and that CGRP is essential for secondary hyperalgesia.4,6 In our study we observed increased concentrations of CGRP after eccentric exercise together with increased experience of pain. Thus CGRP may be involved in the regulation of DOMS after heavy exercise while simultaneously stimulating tissue regeneration.
What is already known on this topic
Tissue concentrations of CGRP have been correlated with pain
Raised concentrations of CGRP have been found in plasma after minor orthopaedic trauma and in the motor neurones of medial gastrocnemius muscles in rat after eccentric exercise
What this study adds
CGRP can be detected in vivo in human muscle by microdialysis
CGRP concentrations are increased after eccentric exercise
Unaccustomed eccentric exercise results in DOMS in a process that is not yet fully understood. Several mechanisms seem to be involved. The exercise is followed by discomfort and pain in the muscles, which seems to act in parallel with adaptive processes involving resynthesis of muscle proteins. Neuropeptides have been found to be involved in the regulation of several physiological functions in peripheral tissue. Increased neuropeptidergic release after eccentric exercise may offer an interesting adaptive process, which may involve regulation of both pain and tissue regeneration. Our experimental protocol, including microdialysis, could be used in future studies on other sympathetic neurotransmitters and sensory neuropeptides such as glutamate and substance P.
Acknowledgements
This study was supported by the Swedish Sports Research Council (CIF grant 83/00) and the Karolinska Institute foundations.
Abbreviations
CGRP - calcitonin gene related peptide
DOMS - delayed onset muscle soreness
HPLC - high performance liquid chromatography
NPY - neuropeptide Y
VAS - visual analogue scale
Footnotes
Competing interests: none declared
All subjects gave their informed consent. The ethics committee at the Karolinska Institutet, Stockholm approved the study (99–251).
References
- 1.Ackermann P W, Li J, Finn A.et al Autonomic innervation of tendons, ligaments and joint capsules. A morphologic and quantitative study in the rat. J Orthop Res 200119372–378. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann P W, Finn A, Ahmed M. Sensory neuropeptidergic pattern in tendon, ligament and joint capsule. A study in the rat. Neuroreport 1999102055–2060. [DOI] [PubMed] [Google Scholar]
- 3.Ljung B O, Forsgren S, Friden J. Substance P, and calcitonin gene‐related peptide expression at the extensor carpi radialis brevis muscle origin: implications for the etiology of tennis elbow. J Orthop Res 199917554–559. [DOI] [PubMed] [Google Scholar]
- 4.Schafers M, Sorkin L S, Sommer C. Intramuscular injection of tumor necrosis factor‐alpha induces muscle hyperalgesia in rats. Pain 2003104579–588. [DOI] [PubMed] [Google Scholar]
- 5.Carleson J, Lundeberg T, Appelgren B. Muscle and brain changes of calcitonin gene‐related peptide in experimentally induced unilateral rat masseter myositis. J Orofac Pain 200418246–252. [PubMed] [Google Scholar]
- 6.Zhang L, Hoff A O, Wimalawansa S J.et al Arthritic calcitonin/alpha calcitonin gene‐related peptide knockout mice have reduced nociceptive hypersensitivity. Pain 200189265–273. [DOI] [PubMed] [Google Scholar]
- 7.Brain S D, Williams T J, Tippins J R.et al Calcitonin gene‐related peptide is a potent vasodilator. Nature 198531354–56. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann P, Mazurkiewicz J, Holtmann G.et al Capsaicin‐sensitive nerve fibres induce epithelial cell proliferation, inflammatory cell immigration and transforming growth factor‐alpha expression in the rat colonic mucosa in vivo. Scand J Gastroenterol 200237414–422. [DOI] [PubMed] [Google Scholar]
- 9.Onuoha G N, Alpar E K. Calcitonin gene‐related peptide and other neuropeptides in the plasma of patients with soft tissue injury. Life Sci 1999651351–1358. [DOI] [PubMed] [Google Scholar]
- 10.Naveilhan P, Hassani H, Lucas G.et al Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature 2001409513–517. [DOI] [PubMed] [Google Scholar]
- 11.Friden J, Lieber R L. Eccentric exercise‐induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand 2001171321–326. [DOI] [PubMed] [Google Scholar]
- 12.Friden J, Sjostrom M, Ekblom B. A morphological study of delayed muscle soreness. Experientia 198137506–507. [DOI] [PubMed] [Google Scholar]
- 13.Homonko D A, Theriault E. Downhill running preferentially increases CGRP in fast glycolytic muscle fibers. J Appl Physiol 2000891928–1936. [DOI] [PubMed] [Google Scholar]
- 14.Ernberg M M, Alstergren P J. Microdialysis of neuropeptide Y in human muscle tissue. J Neurosci Methods 2004132185–190. [DOI] [PubMed] [Google Scholar]
- 15.Henriksson J. Microdialysis of skeletal muscle at rest. Proc Nutr Soc 199958919–923. [DOI] [PubMed] [Google Scholar]
- 16.Arner P. Microdialysis: use in human exercise studies. Proc Nutr Soc 199958913–917. [DOI] [PubMed] [Google Scholar]
- 17.Bangsbo J. Vasoactive substances in the interstitium of contracting skeletal muscle examined by microdialysis. Proc Nutr Soc 199958925–933. [DOI] [PubMed] [Google Scholar]
- 18.MacIntyre D L, Reid W D, Lyster D M.et al Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol 1996801006–1013. [DOI] [PubMed] [Google Scholar]
- 19.Stanley B G, Magdalin W, Seirafi A.et al Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides 199213581–587. [DOI] [PubMed] [Google Scholar]
- 20.Feuerstein G, Willette R, Aiyar N. Clinical perspectives of calcitonin gene related peptide pharmacology. Can J Physiol Pharmacol 1995731070–1074. [DOI] [PubMed] [Google Scholar]
- 21.Buckwalter J B, Hamann J J, Kluess H A.et al Vasoconstriction in exercising skeletal muscles: a potential role for neuropeptide Y? Am J Physiol Heart Circ Physiol 2004287H144–H149. [DOI] [PubMed] [Google Scholar]
- 22.Schmelz M, Luz O, Averbeck B.et al Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. Neurosci Lett 1997230117–120. [DOI] [PubMed] [Google Scholar]
- 23.Ackermann P W, Li J, Lundeberg T.et al Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J Orthop Res 200321432–441. [DOI] [PubMed] [Google Scholar]
- 24.MacIntyre D L, Sorichter S, Mair J.et al Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol 200184180–186. [DOI] [PubMed] [Google Scholar]