Abstract
Context
Critical assessment of recommendations that athletes consume additional sodium during athletic events.
Objective
To evaluate if sodium supplementation is necessary to maintain serum sodium concentrations during prolonged endurance activity and prevent the development of hyponatraemia.
Design
Prospective randomised trial of athletes receiving sodium (620 mg table salt), placebo (596 mg starch), or no supplementation during a triathlon. The sodium and placebo tablets were taken ad libitum, with the suggested range of 1–4 per hour.
Setting
The 2001 Cape Town Ironman triathlon (3.8 km swim, 180 km cycle, 42.2 km run).
Subjects
A total of 413 triathletes completing the Ironman race.
Main outcome measures
Sodium supplementation was not necessary to maintain serum sodium concentrations in athletes completing an Ironman triathlon nor required to prevent hyponatraemia from occurring in athletes who did not ingest supplemental sodium during the race.
Results
Subjects in the sodium supplementation group ingested an additional 3.6 (2.0) g (156 (88) mmol) sodium during the race (all values are mean (SD)). There were no significant differences between the sodium, placebo, and no supplementation groups with regard to age, finishing time, serum sodium concentration before and after the race, weight before the race, weight change during the race, and rectal temperature, systolic and diastolic blood pressure after the race. The sodium supplementation group consumed 14.7 (8.3) tablets, and the placebo group took 15.8 (10.1) tablets (p = 0.55; NS).
Conclusions
Ad libitum sodium supplementation was not necessary to preserve serum sodium concentrations in athletes competing for about 12 hours in an Ironman triathlon. The Institute of Medicine's recommended daily adequate intake of sodium (1.5 g/65 mmol) seems sufficient for a healthy person without further need to supplement during athletic activity.
Keywords: sodium supplementation, exercise, hyponatraemia, Ironman triathlon
Current doctrine advises that athletes ingest 460–920 mg/l (20–40 mmol/l) of sodium (Na+) during exercise.1,2,3,4 The prevailing theory argues that salt ingestion is the sole method by which a fall in serum Na+ concentration ([Na+]) can be prevented during prolonged exercise. This hypothesis is based on the model that predicts that Na+ losses during exercise are large,2,4 especially in “salty sweaters”5 including those with the gene for cystic fibrosis.4,5,6 Failure to replace large Na+ losses is then believed to cause a progressive fall in serum [Na+], leading to the hyponatraemia of exercise and, ultimately, hyponatraemic encephalopathy. It is further argued that this mechanism becomes important during ultradistance events such as the 90 km Comrades Marathon4 and the 226 km Ironman triathlon,5 where total sweat Na+ losses might be as high as 400–650 mmol.4
Guidelines promoting increased Na+ intake during exercise conflict with those recently released by the Institute of Medicine.7 The Institute of Medicine argues that the daily adequate intake of Na+, which is 65 mmol or 1.5 g, is sufficient for physically active people. As the median daily Na+ intake in the United States is 3.1–4.7 g (135–204 mmol) for men and 2.3–3.1 g (100–135 mmol) for women, additional supplementation seems unnecessary in athletes who ingest a Western diet. Furthermore, the body has appropriate defence mechanisms to protect against the development of a Na+ deficit during prolonged exercise. Two immediate defence mechanisms are the release of Na+ from internal body stores8,9,10,11 and contraction of the extracellular fluid volume. Contraction of the extracellular fluid volume by as little as 1 litre (∼7%) would release 140 mmol (∼3 g) of Na+, equivalent to the amount of Na+ present in 7.8 litres of the popular sports drinks Gatorade (USA) and Energade (South Africa) or 28 litres of Powerade (USA).12
Accordingly, to determine the potential role of Na+ supplementation during very prolonged exercise, this study was designed to answer the following questions. (a) Do athletes who ingest additional Na+ during a 226 km Ironman triathlon maintain higher serum [Na+] than those who do not ingest additional Na+? (b) Are athletes who fail to ingest additional Na+ during the Ironman triathlon able to maintain their serum [Na+] within the normal range, or are they at increased risk of developing hyponatraemia? (c) Is Na+ supplementation associated with superior performance in the Ironman triathlon? (d) Does Na+ supplementation reduce the probability that athletes will require medical care after finishing the race?
Methods
A total of 145 triathletes competing in the 2001 Cape Town Ironman triathlon (3.8 km swim, 180 km cycle, and 42.2 km run) volunteered to participate in this study and were randomly assigned to either a control (placebo) or experimental (Na+ supplementation) group. Before participating in this trial, each athlete signed an informed consent form. The study was approved by the Research and Ethics Committee of the Faculty of Health Sciences of the University of Cape Town.
Three days before the race, subjects were weighed (Adamlab JPS electronic scales, Brackenfell, South Africa) in racing attire, and a baseline blood sample was obtained from an antecubital vein to measure serum [Na+] (Easylyte PLUS Na/K/Cl analyzer, Bedford, Massachusetts, USA). Participants in the control group were provided with 40 placebo tablets, each filled with 596 mg starch (Cakes pride superfine corn flour). Subjects in the experimental group were given 40 identical looking tablets, each containing 620 mg table salt (244 mg (10.6 mmol) Na+ with 376 mg (10.6 mmol) Cl−). Both the Na+‐containing and placebo tablets were prepared by the researchers. All athletes were asked to ingest tablets ad libitum within a suggested range of 1–4 tablets every hour and were discouraged from taking any other electrolyte‐containing tablets during the race. After the race, all subjects returned their packets so that any remaining tablets could be counted. From this, each subject's intake of Na+ or placebo tablets could be accurately quantified. Food and fluid intake were allowed ad libitum and were not considered in this analysis. All subjects confirmed that they had not ingested electrolyte‐containing tablets other than those provided as part of the trial.
During the race, athletes were provided with access to water or sports drink (Energade; [Na+] = 18 mmol/l) at stations placed every 20 km in the cycling leg and every 2.5 km in the running leg. Seconding stations placed this infrequently are associated with a reduced incidence of hyponatraemia in the Ironman triathlon.13,14
Upon completion of the race, all subjects were immediately weighed, and a venous blood sample obtained for measurement of serum [Na+]. Blood pressure and rectal temperatures were then recorded as previously described.15 Each athlete was then examined clinically for indicators of post‐race fluid status, including the presence or absence of sweating, the ability to form saliva (spit test), measurements of skin turgor (pinch test), and swelling of the hands (observation of the tightness of fit of rings and watches). An appropriate clinical diagnosis was made and recorded for any triathlete requiring medical care after the race.
Athletes in the experimental trial were asked specific questions about perceived exercise intensity during the race and muscle soreness and mental wellbeing after the race, all rated on a scale of 0–10 (table 1).
Table 1 Measurement scales for exercise intensity, muscle soreness, and mental wellbeing.
| Score | Description |
|---|---|
| Exercise intensity | |
| 0 | Rest |
| 1 | Very easy |
| 2 | Easy |
| 3 | Moderate |
| 4 | Somewhat hard |
| 5 | Hard |
| 6 | |
| 7 | Very hard |
| 8 | |
| 9 | Very, very hard |
| 10 | Maximal |
| Muscle soreness | |
| 0 | No soreness |
| 1 | Very little soreness |
| 2 | Little soreness |
| 3 | Moderate soreness |
| 4 | Somewhat intense soreness |
| 5 | Intense soreness |
| 6 | |
| 7 | Very intense soreness |
| 8 | |
| 9 | Very, very intense soreness |
| 10 | Maximal soreness |
| Mental wellbeing | |
| 0 | Best I've ever felt |
| 1 | Felt great |
| 2 | Felt good |
| 3 | Felt OK |
| 4 | Somewhat depressed |
| 5 | Depressed |
| 6 | |
| 7 | Very depressed |
| 8 | |
| 9 | Very, very depressed |
| 10 | Maximally depressed |
Data were analysed using the Statistica 6.0 (Tulsa, Oklahoma, USA) software program. Using the one way analysis of variance technique, we compared the experimental and control groups alone and with a group consisting of all the other triathletes in the race in which the same measurements had been made (n = 299). The Kruskal‐Wallis variation was used to evaluate non‐parametric data. Statistical significance was set at p<0.05.
Results
A total of 590 athletes successfully completed the Ironman triathlon. Air temperature during the race ranged from 15.6°C to 20.9°C with a midday value of 20°C and a mean value of 17.2°C. Mean humidity was 63%, with a maximum of 79% and a minimum of 48%. Sea temperature was 15°C. Average wind speed was 6.4 m/s, with maximum gusts of 22.3 m/s (81 km/h).
Of the 145 athletes who volunteered for the study, 114 ingested the supplemental tablets and had complete data collected before and after the race. Of these 114 athletes, 53 were in the experimental group and 61 in the control group. Another 299 triathletes, who were voluntarily participating in other medical research, were included as a third comparison group. This comparison group had identical measurements obtained during this triathlon15 but did not ingest either placebo or Na+ supplements during the race. Only 8–10% of the participants were female: four (8%) in the experimental, six (10%) in the placebo, and 26 (9%) in the comparison group. The small number of female competitors precluded further evaluation of the effect of sex on serum [Na+] and fluid balance during the race.
There were no significant differences between the three groups for age, finishing time, [Na+] before and after the race, weight before the race, weight change during the race, percentage dehydration during the race, rectal temperature and systolic and diastolic blood pressures after the race or in the prevalence of medical diagnoses (table 2). Mean serum [Na+] had not changed after the race in any of the groups. Clinical measures of fluid status after the race were also not significantly different between groups.
Table 2 Measured variables in three groups (experimental, placebo, and comparison) of triathletes completing the 2001 South African Ironman triathlon.
| Variable | Experimental (n = 53) | Placebo (n = 61) | Comparison (n = 299) | Average (n = 413) | p Value |
|---|---|---|---|---|---|
| Age (years) | 33.4 (7.4) | 33.9 (7.3) | 35.3 (8.1) | 35.0 (8.0) | 0.09 |
| Finish time (min) | 758.3 (87.6) | 762.0 (100.7) | 741.3 (96.7) | 745.4 (96.4) | 0.14 |
| Pre‐race [Na+] (mmol/l) | 140.6 (1.7) | 140.7 (1.7) | 140.5 (1.7) | 140.5 (1.7) | 0.67 |
| Post‐race [Na+] (mmol/l) | 141.5 (2.7) | 140.5 (3.5) | 140.9 (2.7) | 140.9 (2.8) | 0.15 |
| Pre‐race weight (kg) | 78.2 (9.6) | 75.7 (10.2) | 76.4 (10.5) | 76.5 (10.4) | 0.36 |
| Weight change (kg) | −2.9 (1.3) | −3.0 (1.7) | −3.1 (1.3) | −3.1 (1.4) | 0.36 |
| Dehydration during race (%) | −3.6 (1.4) | −3.9 (2.1) | −4.0 (1.6) | −3.9 (1.7) | 0.16 |
| Post‐race rectal temperature (°C) | 37.1 (0.6) | 37.4 (0.7) | 37.2 (0.9) | 37.2 (0.9) | 0.46 |
| Post‐race systolic blood pressure (mm Hg) | 112 (13) | 109 (14) | 112 (17) | 112 (16) | 0.91 |
| Post‐race diastolic blood pressure (mm Hg) | 72 (9) | 73 (10) | 73 (10) | 73 (10) | 0.59 |
Values are mean (SD).
The experimental group ingested 14.7 (8.3) salt tablets (156 (88) mmol Na+), and the number of starch tablets consumed by the placebo group was 15.8 (10.1) (p = 0.55; NS) (mean (SD) values). Measures of exercise intensity, muscle soreness, and mental wellbeing were also not significantly different between the control and experimental groups (table 3).
Table 3 Subjective assessment of physical condition at the end of the race in the experimental and control groups.
| Variable | Experimental | Control | Average | p Value |
|---|---|---|---|---|
| Exercise intensity | 6.3 (2.1) | 6.5 (2.0) | 6.4 (2.1) | 0.59 |
| Muscle soreness | 3.9 (2.1) | 4.3 (2.2) | 4.1 (2.1) | 0.40 |
| Mental wellbeing | 1.8 (1.3) | 1.9 (1.2) | 1.9 (1.3) | 0.62 |
| Tablets consumed | 14.7 (8.3) | 15.8 (10.1) | 15.2 (9.1) | 0.55 |
Values are mean (SD).
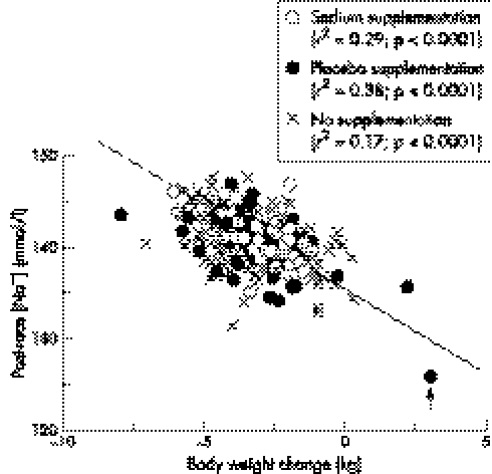
A significant inverse correlation was noted between the post‐race serum [Na+] and the body weight change in all groups (fig 1) so that serum [Na+] increased with increasing body weight loss during exercise. The slope of this relation was not different between groups. The athlete who developed hyponatraemic encephalopathy during the race is identified (arrow).
Figure 1 Post‐race [Na+] versus body weight change in 413 athletes completing the 2001 South African Ironman triathlon in the sodium, placebo, and no supplement groups. Note that the only athlete in this trial to develop hyponatraemic encephalopathy (arrow) was also the athlete who gained the most weight (3.8 kg) during the race.
Discussion
The first important finding of this study was that triathletes who ingested either placebo or salt tablets ad libitum, or who ate and drank as they would normally, all maintained their serum [Na+] within the normal range while exercising for a mean duration of ∼12.5 hours (table 2; fig 1). The sole exception (arrowed in fig 1) was one triathlete in the placebo group who gained the most weight during exercise because he drank to excess, ultimately developing hyponatraemic encephalopathy, for which he required hospital treatment for 12 hours.14
In this study, subjects in the experimental group ingested a mean of 156 mmol Na+ (3.6 g) in addition to any Na+ they might have imbibed in either sports drinks or foods consumed during the race. This is equivalent to the Na+ present in 9 litres of Gatorade or Energade or 32 litres of Powerade. If, in addition, subjects in the experimental group had ingested another 7.5 litres of sports drinks (as seems probable as this equates to a fluid intake rate of 600 ml/h for 12.75 hours), their total Na+ intake would have been only ∼297 mmol during the Ironman race. This combined intake is substantially less than the calculated sweat Na+ loss of 400–650 mmol postulated to occur during even only nine hours of prolonged exercise.4 Yet hyponatraemia did not occur, even though it was highly probable that most of these athletes developed an acute whole body Na+ deficit, which may have been substantial in most of the athletes we studied.
Our finding that Na+ supplementation did not alter the response of the serum [Na+] during prolonged exercise supports the findings from our previous laboratory studies during which subjects drank sufficiently to replace either 50% or 100% of the weight they lost during three hours of laboratory exercise,16,17 as well as our earlier uncontrolled field study of competitors in the 2000 South African Ironman triathlon.18 The absence of an adequate control group in our previous Ironman triathlon study limited the scientific strength of that particular finding.
We can reasonably conclude from these studies that additional Na+ supplementation is unnecessary during prolonged endurance exercise to maintain the serum [Na+] within the normal range.
Historical evidence confirms that when athletes were advised not to drink during exercise19 and hence did not ingest any Na+ during prolonged exercise, they completed prolonged exercise with raised serum [Na+].20,21,22 For example, in 1990 we showed that only one of 101 competitors in the 1986 South African 186 km ultradistance triathlon developed asymptomatic hyponatraemia (lowest serum [Na+] = 131 mmol/l), whereas the mean serum [Na+] after the race was 142 mmol/l compared with 143 mmol/l before. This was despite the fact that the only fluids available during the race were water and Coca‐Cola, which has a very low [Na+] (3.4 mmol/l).23 Opportunities for fluid replacement during that event were quite limited.
However, despite this evidence to the contrary, two studies are often quoted to support the notion that Na+ ingestion during exercise is essential if a progressive fall in serum [Na+] is to be prevented. Both studies contain important logical flaws, not least because they encouraged trial subjects to drink to excess during exercise.
The goal of the study of Vrijens and Rehrer24 was for athletes to drink sufficiently to ensure that they did not lose weight during two hours of laboratory exercise. Normal fluid balance during exercise requires that some weight must be lost due to (a) the release of stored water consequent to glycogenolysis, and (b) irreversible loss of fuel through substrate oxidation.25,26,27 As a result, athletes in any trial who do not lose weight during exercise must complete the trial in a mild state of overhydration. That study therefore evaluated the effect of Na+ supplementation on serum [Na+] in subjects encouraged to overdrink during prolonged exercise. The data show that the response of the serum [Na+] to overdrinking was determined by the renal response to exercise, so that those athletes who passed the most urine during exercise were best able to maintain their serum [Na+]. This is compatible with the conclusion that serum [Na+] is far more sensitive to changes in total body water than to Na+ balance during prolonged exercise28 and with the explanation that acute hyponatraemia is always due to altered renal function in which the rate of free water clearance fails to match the rate of free water ingestion, whether at rest or during exercise.29 Finally only four of 10 subjects completed all trials in that study, further limiting the validity of these findings.
Similarly Twerenbold et al1 studied athletes who drank to excess while running ∼40 km in four hours. As sweat rates were only ∼500 ml/h whereas rates of fluid ingestion were ∼1000 ml/h, subjects gained an average of 2 kg weight during the run. In the presence of this large weight gain, the ingestion of additional [Na+] predictably lessened the fall in serum [Na+] by about 2–3 mmol/l. Yet, despite the ingestion of an additional 118 mmol Na+, the group that ingested the most Na+ still developed hyponatraemia during exercise (mean serum [Na+] after the run = 134 mmol/l). The authors' suggestion that their data prove that all athletes should ingest additional Na+ during exercise is incorrect, as fully argued elsewhere.12 Rather the correct conclusion is that, as the single best predictor of post‐exercise serum Na+ is the change in body mass during exercise (fig 1), avoidance of overhydration is the most important intervention necessary to prevent the development of symptomatic exercise associated hyponatraemia.14,30,31,32
Another line of argument used by the proponents of Na+ supplementation during exercise is that athletes carrying the gene for cystic fibrosis have much higher sweat [Na+] than normal. As a result, these “salty sweaters” are at increased risk of developing hyponatraemia during exercise.4,5 However, the published evidence contradicts this conclusion. Four separate studies show that subjects with cystic fibrosis maintain their serum [Na+] within the normal range during exercise.33,34,35,36 These studies clearly establish that even patients with cystic fibrosis, who excrete a “salty sweat” during exercise, do not need to replace their excessive Na+ losses during exercise to maintain their serum [Na+] within the normal range.
The second important finding of our study is that Na+ supplementation was not associated with any difference in triathlon racing performance. The intervention group finished the race 13 minutes slower than the mean time for all other triathletes (758.3 (87.6) v 745.4 (96.4); p = 0.14, NS) with no significant differences in finishing times between any of the groups (table 2). An absence of association in a cross sectional study does not, of course, exclude the possibility that a carefully controlled prospective trial might reveal such a relation.
What is already known on this topic
Exercise associated hyponatraemia is primarily a condition of dilution rather than depletion
Prevention of this condition requires that athletes avoid overdrinking and weight gain during exercise
What this study adds
Ad libitum sodium supplementation does not preserve serum sodium concentrations in athletes competing for ∼12 hours in an Ironman triathlon
Current recommendations to ingest supplemental sodium during exercise to prevent the development of hyponatraemia are not supported by these findings
Subjective scores for exercise intensity and mental wellbeing were also not significantly different between the groups (table 3), suggesting that Na+ supplementation did not alter the perception of fatigue during the race.
The third important finding was that Na+ supplementation did not alter the probability of requiring medical care after the race. Weight change and percentage weight loss during the race and clinical measures of fluid status, rectal temperatures, and blood pressures after the race did not differ significantly between triathletes in the different groups (table 2). There were also no significant differences in the degree of muscle soreness experienced by triathletes in the different groups (table 3).
Finally, subjects in the experimental and control groups chose to ingest a similar number of supplemental tablets (14.7 (8.3) v 15.8 (10.1) respectively; p = 0.55, NS). This number was substantially less than the recommended intake of 1–4 tablets per hour. Our finding that athletes in the control group did not ingest more tablets than those in the experimental group (who would have been less Na+ deficient because they ingested an additional 156 mmol Na+) suggests that neither group “craved” Na+, as is the normal response in mammals who are Na+ deficient.37,38,39 We can assume that, had a Na+ deficiency been present, (a) the control group would have ingested more tablets than the experimental group, in an attempt to correct their greater Na+ deficit, whereas (b) the experimental group would have ingested closer to the 400–600 mmol Na+ that reasonable calculations suggest is lost during the Ironman triathlon.
Conclusion
Triathletes competing in the 2001 South African Ironman triathlon maintained their serum [Na+] within the normal range whether they drank the Na+‐poor drinks (water or a sports drink with [Na+] = 18 mmol/l) or supplemented with ∼156 mmol Na+. The only athlete to develop hyponatraemic encephalopathy was also the only one to show a substantial weight gain (3.8 kg) during the race.14
Na+ supplementation was not associated with a difference in triathlon performance. Athletes in the placebo, sodium supplementation, and “no” supplementation groups did not differ in their finishing time nor in subjective measures of exercise intensity and mental wellbeing, nor in the prevalence of medical diagnoses after the race.
Clinical measures of fluid status, rectal temperature, blood pressure, absolute and percentage weight loss were also not different between groups.
Therefore predictions of the expected consequences of “large” Na+ losses during prolonged exercise are inaccurate either because athletes sweat less or have lower sweat [Na+] than are currently believed. Alternatively, during acute states of Na+ loss, additional Na+ may be released either from intracellular body stores—for example, bone, skin—or by contraction of the extracellular fluid volume, in order to buffer acute Na+ losses until these are replenished by Na+ ingestion during the next meal.
Acknowledgements
We acknowledge the contributions of David Hampson to this field study.
Footnotes
Competing interests: none declared
References
- 1.Twerenbold R, Knechtle B, Kakebeeke T H.et al Effects of different sodium concentrations in replacement fluids during prolonged exercise in women. Br J Sports Med 200337300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coyle E F. Fluid and fuel intake during exercise. J Sports Sci 20042239–55. [DOI] [PubMed] [Google Scholar]
- 3.Convertino V A, Armstrong L E, Coyle E F.et al American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 1996;28: i–vii, [DOI] [PubMed]
- 4.Montain S J, Sawka M N, Wenger C B. Hyponatremia associated with exercise: risk factors and pathogenesis. Exerc Sport Sci Rev 200129113–117. [DOI] [PubMed] [Google Scholar]
- 5.Murray B, Eichner E R. Hyponatremia of exercise. Curr Sports Med Rep 20043117–118. [DOI] [PubMed] [Google Scholar]
- 6.Smith H R, Dhatt G S, Melia W M.et al Cystic fibrosis presenting as hyponatraemic heat exhaustion. BMJ 1995310579–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine of the National Academies Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington DC: The National Academies Press, 2004
- 8.Heer M, Baisch F, Kropp J.et al High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol 2000278F585–F595. [DOI] [PubMed] [Google Scholar]
- 9.Titze J, Maillet A, Lang R.et al Long‐term sodium balance in humans in a terrestrial space station simulation study. Am J Kidney Dis 200240508–516. [DOI] [PubMed] [Google Scholar]
- 10.Titze J, Krause H, Hecht H.et al Reduced osmotically inactive Na storage capacity and hypertension in the Dahl model. Am J Physiol Renal Physiol 2002283F134–F141. [DOI] [PubMed] [Google Scholar]
- 11.Titze J, Lang R, Ilies C.et al Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol 2003285F1108–F1117. [DOI] [PubMed] [Google Scholar]
- 12.Noakes T D. Sodium ingestion and the prevention of hyponatraemia during exercise. Br J Sports Med 200438790–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speedy D B, Rogers I R, Noakes T D.et al Diagnosis and prevention of hyponatremia at an ultradistance triathlon. Clin J Sport Med 20001052–58. [DOI] [PubMed] [Google Scholar]
- 14.Noakes T D, Sharwood K, Collins M.et al The dipsomania of great distance: water intoxication in an Ironman triathlete. Br J Sports Med 200438E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharwood K, Collins M, Goedecke J.et al Weight changes, medical complications and performance during an Ironman triathlon. Br J Sports Med 200438718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders B, Noakes T D, Dennis S C. Sodium replacement and fluid shifts during prolonged exercise in humans. Eur J Appl Physiol 200184419–425. [DOI] [PubMed] [Google Scholar]
- 17.Sanders B, Noakes T D, Dennis S C. Water and electrolyte shifts with partial fluid replacement during exercise. Eur J Appl Physiol Occup Physiol 199980318–323. [DOI] [PubMed] [Google Scholar]
- 18.Speedy D B, Thompson J M, Rodgers I.et al Oral salt supplementation during ultradistance exercise. Clin J Sport Med 200212279–284. [DOI] [PubMed] [Google Scholar]
- 19.Noakes T D. Fluid replacement during exercise. Exerc Sport Sci Rev 199321297–330. [PubMed] [Google Scholar]
- 20.Riley W J, Pyke F S, Roberts A D.et al The effect of long‐distance running on some biochemical variables. Clin Chim Acta 19756583–89. [DOI] [PubMed] [Google Scholar]
- 21.Noakes T D, Carter J W. Biochemical parameters in athletes before and after having run 160 kilometres. S Afr Med J 1976501562–1566. [PubMed] [Google Scholar]
- 22.Muir A L, Percy‐Robb I W, Davidson I A.et al Physiological aspects of the Edinburgh commonwealth games. Lancet 197021125–1128. [DOI] [PubMed] [Google Scholar]
- 23.Noakes T D, Norman R J, Buck R H.et al The incidence of hyponatremia during prolonged ultraendurance exercise. Med Sci Sports Exerc 199022165–170. [PubMed] [Google Scholar]
- 24.Vrijens D M, Rehrer N J. Sodium‐free fluid ingestion decreases plasma sodium during exercise in the heat. J Appl Physiol 1999861847–1851. [DOI] [PubMed] [Google Scholar]
- 25.Pastene J, Germain M, Allevard A M.et al Water balance during and after marathon running. Eur J Appl Physiol Occup Physiol 19967349–55. [DOI] [PubMed] [Google Scholar]
- 26.Kavanagh T, Shephard R J. On the choice of fluid for the hydration of middle‐aged marathon runners. Br J Sports Med 19771126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers G, Goodman C, Rosen C. Water budget during ultra‐endurance exercise. Med Sci Sports Exerc 1997291477–1481. [DOI] [PubMed] [Google Scholar]
- 28.Weschler L B. Exercise‐associated hyponatremia: a mathematical review. Sports Med 200535899–922. [DOI] [PubMed] [Google Scholar]
- 29.Wolfson A B. Acute hyponatremia in ultra‐endurance athletes. Am J Emerg Med 199513116–117. [DOI] [PubMed] [Google Scholar]
- 30.Noakes T D. Overconsumption of fluids by athletes. BMJ 2003327113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noakes T. Fluid replacement during marathon running. Clin J Sport Med 200313309–318. [DOI] [PubMed] [Google Scholar]
- 32.Noakes T D, Sharwood K A, Speedy D B.et al Dehydration prevents the development of hyponatremia during exercise: evidence from 1423 weighed competitive athletic performances. Proc Natl Acad Sci USA 200510218550–18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orenstein D M, Henke K G, Costill D L.et al Exercise and heat stress in cystic fibrosis patients. Pediatr Res 198317267–269. [DOI] [PubMed] [Google Scholar]
- 34.Bar‐Or O, Blimkie C J, Hay J A.et al Voluntary dehydration and heat intolerance in cystic fibrosis. Lancet 1992339696–699. [DOI] [PubMed] [Google Scholar]
- 35.Stanghelle J K, Maehlum S, Skyberg D.et al Biochemical changes and endocrine responses in cystic fibrosis in relation to a marathon race. Int J Sports Med 19889(suppl.)45–50. [DOI] [PubMed] [Google Scholar]
- 36.Kriemler S, Wilk B, Schurer W.et al Preventing dehydration in children with cystic fibrosis who exercise in the heat. Med Sci Sports Exerc 199931774–779. [DOI] [PubMed] [Google Scholar]
- 37.Denton D A, Eichberg J W, Shade R.et al Sodium appetite in response to sodium deficiency in baboons. Am J Physiol 1993264R539–R543. [DOI] [PubMed] [Google Scholar]
- 38.Epstein A N. The dependence of the salt appetite of the rat on the hormonal consequences of sodium deficiency. J Physiol (Paris) 198479496–498. [PubMed] [Google Scholar]
- 39.Stellar E. Salt appetite: its neuroendocrine basis. Acta Neurobiol Exp (Wars) 199353475–484. [PubMed] [Google Scholar]