Abstract
Objectives
To determine serum concentrations of proinflammatory (C reactive protein, complement C3 and C4) and anti‐inflammatory (α1 antitrypsin, C1 esterase inhibitor (C1‐INH)) acute phase proteins in elite cyclists before and during a three week cycle tour.
Methods
Seventeen professional cyclists participating in the Vuelta a Espańa volunteered for the study. Their mean (SD) physical characteristics were: age 28 (1) years; height 1.7 (0.06) m; weight 65 (7) kg; body fat 7.6 (0.8)%; Vo2max 75.3 (2.3) ml/kg/min. Venepuncture was performed on each subject 24 hours before the tour began (T0), on day 11 (the first rest day; T1) and day 21 (the second to last stage of the tour; T2). Samples at T1 and T2 were taken about 17 hours after the previous stage. Analysis of variance was used to determine changes over time. Where significance was found, a Tukey post hoc test was performed.
Results
C reactive protein concentrations were consistently within the normal range, although there was a 228%, non‐significant increase at T1. C3 concentrations fell within the normal range at all times assessed. C4 concentrations before the race were within the normal range and were significantly increased 10 days (T1) into the race. C1‐INH concentrations did not change significantly throughout the race. α1 Antitrypsin concentration before the race was at the lower end of the normal range and was only significantly raised at T2.
Conclusions
Although not as pronounced as those reported in marathon/ultramarathon runners, elite cyclists participating in a three week cycle tour experienced increases in selected proinflammatory and anti‐inflammatory acute phase proteins, indicating an acute phase/inflammatory response. It is tenable that the increase in α1 antitrypsin and C1‐INH (anti‐inflammatory mediators) at T2 served to attenuate the acute phase/inflammatory response. The lower than normal resting concentrations of the acute phase proteins supports the notion that chronic aerobic exercise induces an anti‐inflammatory state.
Keywords: acute phase response, inflammation, immunity, cycling, endurance exercise
Unaccustomed, long duration and/or intense exercise has been shown to elicit aspects of an acute phase response.1,2 This response, which ultimately serves to protect the body and restore homoeostasis, can be initiated by a wide variety of stimuli, including microbial invasion, chemical/physical trauma, and ischaemic necrosis.3,4 C reactive protein (CRP), α1 antitrypsin, and complement proteins are classified as acute phase proteins. Traditionally, an acute phase protein has been defined as a protein that either increases (positive acute phase protein) or decreases (negative acute phase protein) in concentration in response to homoeostatic disturbance in which cell damage and/or tissue death has occurred.5,6 This response is a generalised systemic reaction closely linked to inflammation and forms part of the innate immune response.
Most studies investigating the response of acute phase proteins to physical activity have examined prolonged bouts of running1,7 short term maximal and submaximal exercise,2,8 or the changes in acute phase proteins over extended periods (nine months).9 The focus of these investigations has been proinflammatory mediators, with anti‐inflammatory proteins receiving less attention. In addition, few studies have investigated this response in exercise that involves less mechanical loading—that is, less eccentric damage—such as cycling.
Thus the primary aim of this study was to determine the concentrations of proinflammatory (CRP, complementary proteins C3 and C4) and anti‐inflammatory (α1 antitrypsin, C1 esterase inhibitor (C1‐INH)) acute phase proteins in elite cyclists at two time points during a three week cycle tour. The secondary aim was to determine if the resting (pre‐race) concentrations of the acute phase proteins were within normal ranges.
Methods
Subject screening
The subjects were screened at the European University of Madrid one month before the start of the race. The 17 subjects provided written informed consent in accordance with the regulations of the university, completed a health history questionnaire, and had a physical examination (including blood pressure and electrocardiographic and echocardiographic evaluation). Height, weight, and percentage body fat were recorded for each participant. All subjects performed a graded exercise test for determination of maximal oxygen uptake (Vo2max).
The tour (Vuelta a Espańa/Tour of Spain) included 21 consecutive daily stages with only two days of rest. During this three week period, a total of 2956 km was covered. The total time spent during the race by the overall winner was about 68 hours. The mean distance covered per daily stage was about 141 km (?55 km) at altitudes of 0–2500 m above sea level. Daily stages began at 1200 and finished between 1630 and 1730. Temperatures ranged from 18 to 30°C during the stages.
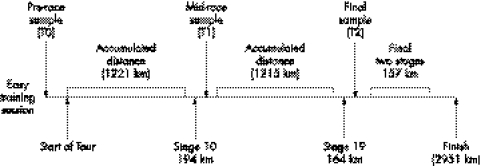
Three blood samples were obtained from each participant during the tour (fig 1): at T0 (pre‐competition control), T1 (first rest day and after stage 10), andT2 (after stage 19). The samples were taken between 0900 and 0930, after an overnight fast, under “basal conditions”. T1 and T2 samples were obtained 16–17 hours after the previous stage and, in the case of T0, the day after an easy training session and a whole “tapering week” (3–4 hours a day of easy to moderate cycling, most of which was performed at heart rates below 150 beats/min). Venepuncture was performed on each subject. The samples were allowed to clot in serum separator tubes at room temperature and then centrifuged at 2000 g for 20 minutes at 4°C. Separated serum was divided into aliquots and stored at −80°C until analysed.
Figure 1 Study design.
Measurement of acute phase proteins
CRP
Concentrations of CRP in serum were determined using the N Latex CRP kit (Behring Diagnostics, Frankfurt, Germany). Specimens were mixed with polystyrene particles coated with monoclonal antibodies, and the intensity of light scatter was measured in a Behring nephelometer against standards of known concentration (obtained from the supplier).
C1‐INH, C3 and C4
The concentration of complement proteins C1, C3, and C4 in serum was determined using specific antisera to C1, C3c, and C4 (Behring Diagnostics). The immune complexes formed were measured in a Behring nephelometer and the amount of C1, C3, and C4 was calculated by comparison with standards of known concentration.
α1 Antitrypsin
α1 Antitrypsin was determined using an enzyme immunoassay kit (Dade Behring, Newark, NJ, USA) on a BN II prospec nephelometer.
Data analysis
A repeated measures analysis of variance (1 × 3) was used to determine whether there were any overall significant differences (p⩽0.05) between serum samples taken before (T0), during (T1), and after (T2) competition. Where appropriate, Tukey post hoc tests were performed.
Results
Descriptive characteristics for the 17 professional cyclists were (mean (SD)): age 28 (1) years; height 1.7 (0.06) m; weight 65 (7) kg; body fat 7.6 (0.8)%; Vo2max 75.3 (2.3) ml/kg/min. Plasma volume did not differ significantly over the three sampling points and body mass remained close to pre‐race values (individual changes <10% and 5% respectively).
Proinflammatory acute phase proteins
Serum CRP concentration at all three time points (table 1) was within the normal range (table 2); it had increased (p = 0.06) at T1 (mid‐race). C3 concentration had decreased at T1 but rose again at T2. All values fell within the normal range and none of the changes were significant. C4 concentration was also within the normal range at all time points, but the increase at 10 days into the tour (T1) (table 1) was a significant change from the baseline. C4 returned to pre‐race concentration at T2.
Table 1 Concentrations of acute phase proteins during the Vuelta a Espańa.
| Protein | T0 | T1 | T2 |
|---|---|---|---|
| CRP (mg/l) | 0.51 (0.72) | 1.67 (0.56) | 0.56 (0.13) |
| C3 (g/l) | 0.85 (0.04) | 0.84 (0.04) | 0.86 (0.04) |
| C4 (g/l) | 0.16 (0.01) | 0.18 (0.01)* | 0.16 (0.03) |
| C1‐INH (mg/dl) | 24.97 (0.46) | 25.60 (0.67) | 25.31 (0.66) |
| α1 Antitrypsin (g/l) | 1.05 (0.03) | 1.11 (0.05) | 1.10 (0.04)* |
Values are mean (SD).
*p<0.05 compared with T0 (analysis of variance).
T0, Before the race; T1, first rest day (after stage 10); T2, after stage 19 (2nd last stage); CRP, C reactive protein; C1‐INH, C1 esterase inhibitor.
Table 2 Reference ranges of acute phase proteins.
| Protein | Normal range |
|---|---|
| CRP | 0–5 mg/l |
| C3 | 0.5–1.53 g/l |
| C4 | 0.1–1.0 g/l |
| C1‐INH | 31–43 mg/dl |
| α1 Antitrypsin | 0.9–2.0 g/l |
CRP, C reactive protein; C1‐INH, C1 esterase inhibitor.
Anti‐inflammatory acute phase proteins
Serum C1‐INH concentration was below the normal range (table 2) at T0, and, although it was raised at T1 and T2 (table 1), these increases were not significant. Serum α1 antitrypsin concentration was significantly (p<0.05) increased at T2 compared with T0.
Discussion
The aim of this study was to determine the concentrations of proinflammatory and anti‐inflammatory acute phase proteins in elite cyclists before and during a three week cycle tour. Most studies investigating the acute phase/inflammatory response to physical activity have used running to elicit this response. Running is associated with greater mechanical stress than cycling, predominantly as a result of the eccentric component, a key factor that results in greater muscle damage with this type of activity. Therefore it would not be surprising if the acute phase response in cyclists is less pronounced than in runners. Overall, there were only minor changes in the concentrations of the acute phase proteins despite the fact that events of this nature are associated with pronounced hormonal disturbance.10 Reports from competitors suggest that it is common for cyclists to experience progressive muscular soreness as a tour progresses. We therefore expected a “cumulative” inflammatory response towards the end of the race (T2). Contrary to this, the acute phase proteins (except α1 antitrypsin) exhibited no significant increase and had returned to baseline concentrations toward the end of the tour.
Although non‐significant and within the normal range, the concentration of CRP had increased at T1 to 1.67 (0.56) mg/l . The day before this sample was taken, the cyclists had completed a stage of 194 km averaging 46.40 km/h with an average heart rate of 124 beats/min. CRP concentrations had returned to 0.56 (0.13) mg/l at T2. The day before the T2 sample was taken, the cyclists had completed a stage of 164 km averaging 42.54 km/h with an average heart rate of 131 beats/min. This was only a slight increase compared with T0 (resting concentration). With the cyclists racing on consecutive days and accumulating almost 2800 km up to T2, it was surprising not see a cumulative elevated inflammatory response in the concentrations of the proinflammatory acute phase proteins, CRP, C3 and C4. CRP concentrations of less than 5 mg/l are considered normal in our laboratory, with the average person having a concentration of about 2 mg/l.5 Even in response to the long strenuous stages, the cyclists maintained a CRP concentration below these norms. Although moderate increases in CRP are noted, these were not significant. Moreover, the concentrations at T2 had returned to baseline, suggesting possible CRP use in the circulation.
C4 (proinflammatory) was significantly increased at T1, a trend mirroring that of CRP. The increase in CRP and C4 would support the notion that the classical pathway of complement was activated after this particular stage of the race. At T2, C4 was equal to pre‐race concentrations, indicating that either the 19th stage was not intense enough to elicit changes in this acute phase protein, or C1‐INH (anti‐inflammatory), which was slightly raised at this point, was preventing the activation of C4.
The proinflammatory protein C3 is the most abundant complement protein and serves to promote activation of all three pathways of complement.11 In contrast with CRP and C4, C3 concentration had declined at T1. Although minimal, the unexpected decrease may be attributed to the fact that C3 is continually and rapidly being cleaved to form C3b and C3a for the inflammatory/complement cascade to progress. Smith et al12 found no significant increases in C3 after short term aerobic exercise, but a significant increase in C3a was observed.
C1‐INH is one of many controlling or regulatory molecules that serves to inhibit the formation of complement and thus plays an inherently anti‐inflammatory role.13 In keeping with the trend of CRP and C4, C1‐INH had increased at T1 and returned to just above baseline value at T2. The increase in C1‐INH at T1 is not surprising given the fact that CRP and C4 were shown to have increased at the same time point. Whether the increase in the concentrations of C1‐INH follows as a consequence of rises in C4 or vice versa is not clear. This was not an objective of this study, but the results do suggest a direct link between the concentrations of the two proteins in peripheral circulation.
α1 Antitrypsin, the most abundant serum proteinase inhibitor, assists in the resolution of inflammation.14,15 Specifically, it inhibits the proteases (elastase) produced by polymorphonuclear cells (neutrophils) during an inflammatory response.15,16 Numerous studies have shown that exercise can induce leucocytosis (for a review see McCarthy and Dale17). The associated production of neutrophil elastase is capable of activating complement.18 Thus it is plausible that the increase in α1 antitrypsin at T1 serves to suppress the negative effects of elastase production and indirectly inhibit complement activation—that is, an anti‐inflammatory role. The increase in α1 antitrypsin due to neutrophil elastase activity during and/or after exercise has also been supported by Dufaux and Order,19 who showed a significant increase in α1 antitrypsin 60 minutes into a 2.5 hour run.
Of additional interest were the resting concentrations of these proinflammatory and anti‐inflammatory mediators. The resting concentrations of acute phase proteins could provide insight into how the body adapts to physical activity: do proinflammatory or anti‐inflammatory mechanisms dominate? This has important implications for both serious athletes and those suffering from various chronic diseases that have been defined as inflammatory in nature. CRP at T0 was substantially below the average 2 mg/l concentration found in the general population as assessed in our laboratory and by others. This would support the notion that chronic physical activity is associated with lower resting concentrations of CRP, a concept that has been highlighted by numerous investigators.9 Similarly, C3 and C4 at T0 were found to be below and towards the lower end of the normal reference ranges respectively. Again this would imply an exercise induced adaptive process that downregulates proinflammatory proteins. Smith et al12 and Nieman et al20 both found similar trends in runners compared with non‐exercising controls. With resting concentrations of proinflammatory acute phase proteins seemingly suppressed in athletes, and exercise more commonly being defined as anti‐inflammatory in nature,21 it would seem unnecessary (although speculative) for anti‐inflammatory proteins to be upregulated. Our study shows that α1 antitrypsin and C1‐INH were towards the lower end and substantially below the reference ranges respectively.
What is already known on this topic
Prolonged/unaccustomed exercise that usually incorporates eccentric muscle action may initiate an acute phase inflammatory response
Exercise induced muscle damage is thought to be responsible for the observed increases in inflammatory markers after exercise and that chronic exercise may “suppress” resting concentrations of certain inflammatory markers
What this study adds
Prolonged cycling (minimal eccentric action) may elicit systemic changes in both proinflammatory and anti‐inflammatory markers
Resting concentrations of certain acute phase proteins in elite cyclists are lower than in sedentary subjects
In summary, increases in two out of the three proinflammatory (CRP, C4) and both of the anti‐inflammatory (C1‐INH, α1 antitrypsin) acute phase proteins, indicative of an acute phase/inflammatory response, occurred at mid‐race of a three week cycle tour. This finding is consistent with other studies of acute phase proteins. A clearer understanding of the balance between proinflammatory and anti‐inflammatory mechanisms associated with chronic acute phase/inflammatory sequelae will provide insight into how the body adapts to physical activity. The acute phase/inflammatory response is classified as an integral component of non‐specific/innate immunity. Further studies are needed to shed light on how this response affects adaptive immunity as evidence is mounting to support a close link between the two systems.
Acknowledgements
This study was supported by Tshwane University of Technology, the National Research Foundation, and the National Health Laboratory Services, South Africa.
Footnotes
Competing interests: none declared
References
- 1.Weight L M, Alexander D, Jacobs P. Strenuous exercise: analogous to the acute‐phase response? Clin Sci 199181677–683. [DOI] [PubMed] [Google Scholar]
- 2.Meyer T M, Gabriel H, Ratz M.et al Anaerobic exercise induces moderate acute phase response. Med Sci Sports Exerc 200133549–555. [DOI] [PubMed] [Google Scholar]
- 3.Pepys M B, Baltz M L. Acute phase proteins with special reference to C‐reactive protein and related proteins (pentaxins) and serum amyloid A‐protein. Adv Immunol 198334141–211. [DOI] [PubMed] [Google Scholar]
- 4.Gerwuz H. Biology of C‐reactive protein and the acute phase response. Hosp Pract 198267–81. [DOI] [PubMed]
- 5.Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. N Engl J Med 1999340448–454. [DOI] [PubMed] [Google Scholar]
- 6.Kushner I, Rzewnicki D. The acute phase response: general aspects. Baillière's Clin Rheumatol 19948513–530. [DOI] [PubMed] [Google Scholar]
- 7.Semple S J, Smith L L, McKune A J.et al Alterations in acute‐phase reactants (CRP, rheumatoid factor, complement, Factor B, and immune complexes) following an ultramarathon. S Afr J Sports Med 20041617–21. [Google Scholar]
- 8.Dufaux B, Order U, Liesen H. Effect of a short maximal physical exercise on coagulation, fibrinolysis, and complement system. Int J Sports Med 199112S38–S42. [DOI] [PubMed] [Google Scholar]
- 9.Mattusch F, Dufaux B, Heine O.et al Reduction of the plasma concentration of C‐reactive protein following nine months of endurance training. Int J Sports Med 19992121–24. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez‐Garcia B, Lucia A, Hoyos J.et al The response of sexual and stress hormones of male pro‐cyclists during continuous intense competition. Int J Sports Med 200223555–560. [DOI] [PubMed] [Google Scholar]
- 11.Sahu A, Lambris J D. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev 200118035–48. [DOI] [PubMed] [Google Scholar]
- 12.Smith J K, Chi D S, Krish G.et al Effect of exercise on complement activity. Ann Allergy 199065304–310. [PubMed] [Google Scholar]
- 13.Caliezi C, Wuillemin W A, Zeerleder S.et al C1‐esterase inhibitor: an anti‐inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev 20005291–112. [PubMed] [Google Scholar]
- 14.Hercz A. The inhibition of proteinases by human alpha‐antitrypsin. Eur J Biochem 197449287–292. [DOI] [PubMed] [Google Scholar]
- 15.Brantly M. alpha1‐Antitrypsin: Not just an antiprotease. Am J Res Cell Mol Biol 200227652–654. [DOI] [PubMed] [Google Scholar]
- 16.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 200351317–1327. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy D A, Dale M M. The leucocytosis of exercise. A review and model. Sports Med 19886333–363. [DOI] [PubMed] [Google Scholar]
- 18.Roitt I, Brostoff J, Male D.Immunology. 6th ed. Edinburgh: Elsevier Science, 2001
- 19.Dufaux B, Order U. Plasma elastase alpha‐1‐antitrypsin, neopetrin, tumour necrosis factor, and soluble interleukin‐2 receptor after prolonged exercise. Int J Sports Med 198910434–438. [DOI] [PubMed] [Google Scholar]
- 20.Nieman D C, Tan S A, Lee J W.et al Complement and immunoglobulin levels in athletes and sedentary controls. Int J Sports Med 198910124–128. [DOI] [PubMed] [Google Scholar]
- 21.Peterson A, Pederson B K. The anti‐inflammatory effect of exercise. J Appl Physiol 2005981154–1162. [DOI] [PubMed] [Google Scholar]