Abstract
Background
Some studies have suggested that the insertion allele of the ACE gene is associated with endurance performance, including the Ironman triathlon. It is possible that this association is due to genetic linkage between the ACE I/D locus and the T/A variant in intron 4 of the neighbouring GH1 gene. The A variant is associated with lower levels of growth hormone production. Growth hormone has multiple effects, especially on metabolism during exercise and recovery from exercise. Its production during exercise has also been shown to stimulate sweat rate and heat loss.
Objective
To determine whether the GH1 gene is associated with the performance and/or post‐race rectal temperatures of competitors in the South African Ironman triathlon.
Methods
A total of 169 of the fastest finishing white male triathletes who completed the 2000 and/or 2001 South African Ironman triathlon and 155 control subjects were genotyped for the T/A variant in the GH1 gene. Post‐race rectal temperature was also determined in 103 of these triathletes.
Results
There was no significant difference in the frequency of this polymorphism in the GH1 gene when the fastest finishing triathletes were compared with the control subjects. Post‐race rectal temperatures were, however, significantly higher in those triathletes with an AA genotype (mean (SD) 37.7 (0.8)°C) compared with those with a TT genotype (37.2 (0.8)°C) (p = 0.019).
Conclusions
The T/A polymorphism in intron 4 of the GH1 gene was not associated with performance of the fastest finishers of the South African Ironman triathlon. Post‐race rectal temperatures were, however, significantly higher in the fastest finishing athletes, who were homozygous for a GH1 genotype associated with lower growth hormone production.
Keywords: genetics, thermoregulation, endurance performance, growth hormone, triathlon
Physical performance is, at least in part, determined by genetic background.1 To date, polymorphisms in two genes have been shown to be associated with athletic ability, namely the insertion/deletion (I/D) polymorphism in the angiotensin converting enzyme (ACE) gene2,3,4,5 and a truncating mutation in the α actinin 3 (ACTN3)6 gene.
Some studies have shown that the I allele of the ACE gene2,3,4,5 is associated with elite endurance performance, whereas the D allele of this gene4,7 or the absence of the truncating mutation in the ACTN3 gene are associated with superior ability in sprinting‐type activities.6 Previous work from this laboratory has shown that the I allele of the ACE gene was associated with the performance of the fastest South African born, consenting white male finishers of South African Ironman triathlons.8 However, not all studies have shown an association between the ACE I/D polymorphism and athletic ability.9,10
Although investigators have suggested that ACE affects athletic performance through the local skeletal muscle renin‐angiotensin system11 or the kallikrein‐kinin system,12 the mechanisms of these effects remain unclear. It is therefore possible that other gene(s) closely linked to the ACE gene on chromosome 17q2313 encode a protein directly involved in athletic performance.
The human growth hormone 1 (GH1) gene, together with the other members of the growth hormone gene family, has been mapped to chromosome 17q24.2, 397 kb downstream from the ACE gene.14 The GH1 gene encodes a family of hormones produced by the anterior pituitary gland in response to a number of stimuli. A 22 kDa monomeric protein is the major growth hormone isoform found in the circulation. Other isoforms are produced by alternative splicing, post‐translational modification, proteolytic cleavage, and/or oligomerisation.15,16,17 Endurance and resistance exercise, as well as sleep, are potent physiological stimuli for growth hormone secretion.18 The secretion of growth hormone and insulin‐like growth factor‐I (IGF‐I), an intermediate substance through which growth hormone exerts much of its effect, is affected by the type, intensity, frequency, and duration of exercise, as well as the training status of the athlete.16,17,18,19,20
The most noticeable beneficial effects of growth hormone on muscle and bone mass, muscle strength, metabolism, and exercise performance are observed in growth hormone deficient patients receiving growth hormone treatment.18,19,21,22,23 Although some isolated studies have suggested that growth hormone is able to improve athletic performance,24 most investigators suggest that it has little or no beneficial effect on skeletal muscle mass or strength and performance in healthy people.18,19,21,22 In spite of the lack, or minimal response, of muscle to growth hormone in healthy people at doses used in clinical trials, it has nevertheless been suggested that growth hormone may still play an important role in muscle anabolism, as it stimulates IGF‐I production in muscle.21 Increases in growth hormone production during exercise are believed to cause responses in carbohydrate, fat, and protein metabolism,16,17,25,26 as well as enhancing tissue repair after exercise.26 Exercise induced growth hormone secretion has also been shown to stimulate sweat secretion and heat dissipation through sweat evaporation, thereby influencing heat loss from the body during exercise.27
The T to A variant at position 1663 in intron 4 of the GH1 gene is associated with lower levels of production of growth hormone and IGF‐I.28,29 The aim of this study was therefore to determine whether the GH1 gene T/A polymorphism is associated with performance and/or post‐race rectal temperature of the fastest consenting white male finishers of the 2000 and/or 2001 South African Ironman triathlon.
Methods
Subjects
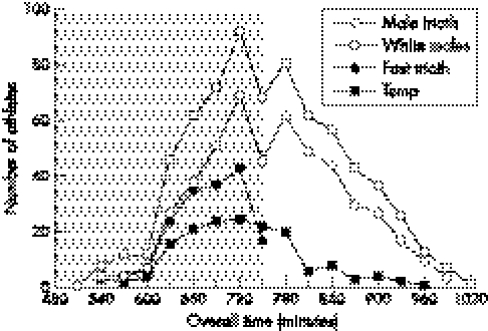
Participants were recruited from the 2000 (272 male finishers) and 2001 (544 male finishers) South African Ironman triathlons, which consisted of a 3.8 km swim, a 180 km cycle, and a 42.2 km run.30 A total of 701 male triathletes completed either one (n = 586) or both (n = 115) events (fig 1). The athletes completed an informed consent form and personal particulars questionnaire. DNA samples were obtained from 447 of the white male triathletes, of which 169 of the fastest finishers (Fast Triath) were included in this study. An additional 54 slower finishers, for whom post‐race rectal temperature was measured, were also included.
Figure 1 Comparison of the overall race time distribution of the whole field of male triathletes (Male Triath, n = 701) and the various subgroups of athletes who completed either the 2000 and/or the 2001 South African Ironman triathlon. A total of 115 male athletes completed both events, and the fastest times for these athletes were included in the analysis. The subgroups were the white male finishers (White Males, n = 488), the fastest finishers (Fast Triath, n = 169), and triathletes for whom post‐race rectal temperature data were available (Temp; n = 103 for the triathletes who completed the race in ⩽737 minutes and n = 54 for those who completed the race in >737 minutes). The Fast Triath athletes completed the events within the time period (737 minutes) indicated by the shaded area.
In addition, 155 apparently healthy control subjects (Con) who had not participated in or trained for an ultraendurance event were recruited from the greater Cape Town metropolitan area. Approval for this study was obtained from the Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town.
Rectal temperature
Post‐race rectal temperature was measured in 103 (70 from 2001 and 33 from 2000) of the triathletes in the Fast Triath group and in the 54 slower finishers. The remaining athletes did not consent to having their post‐race rectal temperatures measured. Rectal temperatures were obtained, using calibrated electronic clinical thermometers (Microlife, Berneck, Switzerland), within five minutes of completion of the race from the athletes while they lay supine on a treatment cot in the medical tent. The thermometer was inserted 5 cm into the rectum and left there until a stable reading was obtained (30 seconds).
Total DNA extraction and GH1 genotyping
A 5 ml sample of venous blood was drawn from the forearm antecubital vein of each subject and collected in an EDTA vacutainer tube. The blood samples were stored at 4°C until DNA extraction as described by Lahiri and Nurnberger.31 The subjects were genotyped for the GH1 gene 1663 T/A polymorphism using a nested polymerase chain reaction (PCR) assay as described by Le Marchand et al.29 The secondary PCR products were digested with the restriction enzyme AatII. The resulting fragments were resolved on 7.5% polyacrylamide gels and visualised by ethidium bromide staining. The A alleles produced a 180 bp fragment, and the T alleles produced 149 and 31 bp fragments. Nine of the samples (two AA, four TA, and three TT) were PCR amplified, and the PCR products digested with AatII two to six times to check the reliability of the digestion. Identical results were obtained for each analysis.
Environmental conditions
The South African Weather Service provided details of the environmental conditions on the two race days.
Statistical analysis
Data were analysed using the Statistica 7.0 (Stat‐soft Inc, Tulsa, Oklahoma, USA) and GraphPad InStat 2.05a (GraphPad Software, San Diego, California, USA) statistical programs. Where applicable, data are presented as mean (SD) with the number of subjects in a second set of parentheses. Pearson's χ2 analysis was used to detect differences in the genotype and allele frequencies between the groups. Lewontin's standardised disequilibrium coefficient (D′) and the χ2 test for Hardy‐Weinberg equilibrium were calculated using the Linkage Disequilibrium Analyzer 1.0 software.32 A one way analysis of variance was used to determine any significant differences between the characteristics of the triathlete and control groups, as well as any genotype effects on the physiological measurements. Where the overall F value was significant, a Tukey's honest significant difference post hoc test was used to identify where the differences were. Statistical significance was accepted when p<0.05. The required sample sizes were determined using Quanto 0.5 as previously described.8
Results
Subject characteristics
The Fast Triath (124 from 2001) group finished the Ironman triathlons within the top 49% (666 (48) minutes; range 521–737) of the whole field of 701 (515 from 2001) male triathletes (745 (96) minutes; range 508–998) (fig 1). There was no significant difference in the mean finishing times of the male athletes who completed the 2000 (756 (101) minutes; range 508–968) and 2001 (745 (96) minutes; range 535–998) events (p = 0.121). There was a strong correlation between the 2000 and 2001 finishing times of the 115 athletes in the whole field (r = 0.859) and the 48 athletes in the Fast Triath group (r = 0.837) who completed both events. The fastest times for these athletes were included in the analysis. These athletes ran their faster race 39.0 (37.4) minutes faster than their slower race.
As shown in table 1, the triathlete and control groups were matched for height. The triathlete group was, however, significantly older than the control group, as well as being significantly lighter and having a lower body mass index (BMI). In addition, the Fast Triath group had a significantly lower percentage of South African born members than the Con group.
Table 1 General physiological characteristics of the triathlete (Fast Triath) and control (Con) groups.
| Fast Triath (n = 169) | Con (n = 155) | p Value | |
|---|---|---|---|
| Age (years) | 33.2 (6.2) (169) | 29.0 (10.5) (150) | <0.001 |
| Height (cm) | 180.4 (6.3) (153) | 181.1 (8.0) (151) | 0.400 |
| Weight (kg) | 75.3 (7.3) (167) | 82.8 (11.2) (154) | <0.001 |
| BMI (kg/m2) | 23.1 (1.6) (152) | 25.3 (3.3) (151) | <0.001 |
| South African born (%) | 56.6 (166) | 85.6 (153) | <0.001 |
Except for the percentage South African born, values are expressed as mean (SD). The number of subjects is in a second set of parentheses.
BMI, Body mass index.
GH1 genotype and allele frequency
There was no significant difference between the genotype or allele frequency distributions of the GH1 gene 1663 T/A polymorphism when the Fast Triath and Con groups were compared (table 2). Although there were significantly fewer South African born members of the Fast Triath group, there were no effects of population stratification on the observed genotype (p = 0.857) and allele (p = 0.932) frequency distributions when only the South African born athletes were included in the analysis (data not shown). In addition, there were no GH1 genotype effects on the observed differences in age, weight, and BMI between the two groups (data not shown).
Table 2 GH1 A/T polymorphism genotype and allele frequencies in the South African Ironman triathlete (Fast Triath) and control (Con) groups.
| Fast Triath (n = 169) | Con (n = 155) | |
|---|---|---|
| AA genotype | 36 (21.3)* | 31 (20.0)* |
| TA genotype | 84 (49.7)* | 74 (47.7)* |
| TT genotype | 49 (29.0)* | 50 (32.3)* |
| A allele | 156 (46.2)† | 136 (43.9)† |
| T allele | 174 (53.8)† | 174 (56.1)† |
Values are expressed as the number of subjects or alleles, with the percentage in parentheses.
*Fast Triath v Con genotype distribution, Pearsons χ2 = 0.412 and p = 0.814.
†Fast Triath v Con allele distribution, χ2 = 0.254 and p = 0.614.
The GH1 allele distributions of all the groups included in this study were in Hardy‐Weinberg equilibrium. The GH1 gene 1663 T/A polymorphism and the previously published ACE gene I/D polymorphism of the same subjects8 were not in linkage disequilibrium (D′ = 0.146).
GH1 gene genotype effects on post‐race rectal temperature
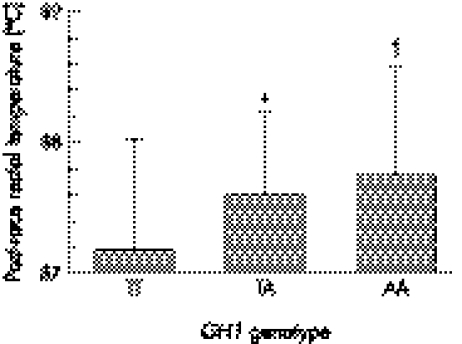
As shown in table 3, there were no significant differences in age, height, weight, BMI, and relative decrease in body weight during the event, as well as the overall and split times when the 103 Fast Triath subjects with post‐race rectal temperature data were divided into three groups on the basis of their GH1 genotype. There was, however, a significant difference in post‐race rectal temperatures between the genotype groups of these triathletes (p = 0.019) (fig 2). The post‐race rectal temperatures in the triathletes with a TT genotype (37.2 (0.8)°C, n = 29) were significantly lower than those with an AT (37.6 (0.6)°C, n = 50, p = 0.044) or AA (37.7 (0.8)°C, n = 24, p = 0.028) genotype. There was no significant difference between athletes with a TA or AA genotype (p = 0.819). Even when corrected for BMI (p = 0.014), weight (p = 0.009), or percentage weight loss (p = 0.012), there was still a genotype effect on post‐race rectal temperature in these athletes.
Table 3 General physiological characteristics and performance data of the three Fast Triath triathlete GH1 genotype subgroups (n = 103) who completed the 2000 and/or 2001 South African Ironman triathlon.
| TT genotype (n = 29) | TA genotype (n = 50) | AA genotype (n = 24) | p Value | |
|---|---|---|---|---|
| Age (years) | 34.3 (7.0) (29) | 32.9 (6.3) (50) | 34.0 (5.7) (24) | 0.584 |
| Height (cm) | 180.4 (7.6) (27) | 180.1 (4.8) (46) | 180.1 (7.8) (23) | 0.985 |
| Weight (kg) | 75.9 (9.5) (29) | 74.6 (5.9) (50) | 73.6 (8.4) (23) | 0.551 |
| BMI (kg/m2) | 23.2 (1.6) (27) | 22.9 (1.7) (46) | 22.8 (1.8) (22) | 0.677 |
| Decrease in body weight (%) | −4.1 (1.4) (24) | −4.3 (1.7) (45) | −4.7 (1.7) (23) | 0.526 |
| Overall time (min) | 658 (61) (29) | 671 (42) (50) | 662 (39) (24) | 0.483 |
| Swim time (min) | 63 (11) (27) | 62 (8) (48) | 62 (8) (22) | 0.976 |
| Cycle time (min) | 351 (31) (27) | 357 (24) (46) | 353 (23) (21) | 0.582 |
| Run time (min) | 237 (28) (28) | 243 (20) (49) | 243 (25) (24) | 0.553 |
Values are expressed as mean (SD), with the number of subjects in the second set of parentheses. The decrease in body weight was calculated as the difference between the initial and final weight divided by the initial weight and expressed as a percentage.
BMI, Body mass index.
Figure 2 Post‐race rectal temperatures of the three triathlete GH1 genotype subgroups who completed either the 2000 and/or 2001 South African Ironman triathlon within 737 minutes. *Significantly different from TT, p = 0.044. †Significantly different from TT, p = 0.028.
As mentioned above the slowest athlete in the Fast Triath group completed the race in 737 minutes. This was therefore defined as the cut‐off point between the fastest and slowest athletes analysed in this study. When the 54 triathletes who finished the event at a slower race pace (>737 minutes) were analysed, there was no longer a genotype effect on post‐race rectal temperature (AA genotype: 37.5 (0.9)°C, n = 7; AT genotype: 37.6 (0.5)°C, n = 30; TT genotype: 37.9 (0.6)°C, n = 17; p = 0.200). These athletes ran the marathon split of the triathlon in a mean time of 309 (39) minutes and an estimated oxygen uptake (V̇o2) of 25.3 ml/kg/min, which was significantly slower and lower respectively than the fastest athletes who ran on average for 241 (24) min (p<0.001) at an estimated V̇o2 of 34.3 ml/kg/min (p<0.001). Similar results were obtained when only those athletes who completed the race during 2001 were analysed separately in a similar way (data not shown). There was in fact a high degree of correlation (r = 0.515) between the post‐race rectal temperatures for the 26 triathletes who completed both events. The relative decreases in body weight during the triathlons between fastest (4.4 (1.6)%) and slowest (4.2 (1.7)%) finishers were similar (p = 0.392).
The average temperature while the fastest athletes were competing in the marathon split was 20.2 (1.9)°C, which was similar to the temperature of 20.3 (2.5)°C when the slowest athletes were competing in the marathon split (p = 0.697). On the other hand, the mean wind speed while the fastest athletes ran the marathon split (8.9 (0.9) m/s) was significantly higher than when the slowest athletes ran (8.0 (0.9) m/s) (p<0.001).
Discussion
The insertion or I allele of the ACE gene has been shown to be associated with endurance‐type athletic performance in high altitude mountaineers, Olympic rowers, distance runners, cyclists, and handball players.2,3,4,5 We have recently shown that the I allele of the ACE gene was also associated with the fastest 100 South African born consenting finishers of the South African Ironman triathlons.8 The D allele of this gene has, on the other hand, been shown to be associated with elite short distance swimmers and sprinters.4,7 Other studies have, however, not reported an association of this polymorphism with endurance activity.9,10 Because of the inconsistency in the literature over the possible association of the I allele in the ACE gene with endurance performance, and owing to the nature of genetic association studies, it is possible that another gene, such as the GH1 gene, which is in close proximity to the ACE gene on the long arm of chromosome 17, may encode a protein that is directly involved in the endurance phenotype.
The main finding of this study is that the 1663 T/A polymorphism in the GH1 gene was not associated with the performance of the fastest finishers of the 2000 and/or 2001 South African Ironman triathlon. This polymorphism was not in linkage disequilibrium with the ACE I/D polymorphism. The allele and genotype distributions of the GH1 gene 1663 T/A polymorphism agree with previously reported values for healthy white and Japanese populations.28,29
About 171 genes, together with the GH1 gene, have been mapped to chromosome 17q23–q24, a region encompassing 17.8 Mb, in close proximity to the ACE gene (www.ncbi.nlm.nih.gov). As some of these genes could theoretically encode a protein directly involved in endurance performance, the finding of this study does not exclude the possibility that another gene closely linked to the ACE and GH1 genes is responsible for determining athletic phenotype. Other possible associations between athletic ability and polymorphisms in candidate genes on this region of chromosome 17 need to be investigated.
This evidence does, however, suggest that the ACE gene, which encodes a key component of both the renin‐angiotensin and the kallikrein‐kinin systems, is associated with athletic ability. The potential mechanism by which the renin‐angiotensin system in particular could be involved in skeletal muscle function has been extensively studied and reviewed.33 At the genetic level, however, other genes encoding components of this system, namely the angiotensinogen (ANG) and angiotensin (AT1 and AT2) genes, have not been shown to be associated with the ability of endurance athletes.3,5 More recently, Williams et al12 have shown that the −9/+9 polymorphism in the bradykinin β2 receptor gene, which encodes a component of the kallikrein‐kinin system, is associated with physical performance, suggesting that this system may contribute to the performance phenotype. In addition, we have shown that the −9 allele of the bradykinin β2 receptor gene was associated with the actual performance of the fastest finishers of the Ironman triathlons (C Saunders, unpublished work).
Even if the protein products of the ACE gene itself or another gene in the vicinity of the ACE gene is eventually unequivocally shown to be directly involved in the athletic phenotype, it remains highly unlikely that a single gene and its protein product can explain this phenotype. It is more probable that many genes encoding proteins in multiple biochemical and physiological systems contribute to athletic performance, as many physiological and biochemical systems have been shown to be associated with this phenotype.34
Although there was no significant GH1 genotype association with performance, there was a significant GH1 genotype effect on post‐race rectal temperature of the fastest finishers of the triathlons. The post‐race rectal temperatures were significantly lower in the fastest triathlete finishers with the TT genotype than those homozygous for the A allele. Several studies have shown that polymorphisms in the GH1 gene are associated with growth hormone production. Hasegawa et al28 have shown that the 1663 T/A polymorphism in the GH1 gene is associated with secretion of growth hormone and IGF‐I in prepubertal short Japanese children and plasma IGF‐I concentrations in adults. Although they did not measure growth hormone secretion, Le Marchand et al29 reported that the AA genotype of this polymorphism is associated with lower plasma concentrations of IGF‐I, higher plasma concentrations of IGF binding protein 1, and a lower ratio of plasma IGF‐I to IGF binding protein 3. All these effects are consistent with lower growth hormone secretion. In addition, Dennison et al35 have recently shown that a polymorphism in the promoter region of the GH1 gene, which is in strong linkage disequilibrium with the 1663 T/A polymorphism, is also associated with circulating plasma growth hormone concentrations.
As this polymorphism is associated with growth hormone production,28,29 we postulate that triathletes homozygous for the T allele may produce more growth hormone than those with the AA genotype, resulting in a higher sweat rate. An increase in sweat rate is associated with a greater heat loss from evaporation and a lower core temperature. Investigators have shown that growth hormone stimulates sweat production and heat loss from evaporation during exposure to heat in either the absence or presence of exercise.27 Growth hormone deficiency, on the other hand, is associated with reduced sweat secretion and an increase in heat storage.27,36 Recently, Heled et al37 showed that the I allele of the ACE gene is associated with superior heat tolerance during exposure to two hours of exercise. Although it has been suggested that the renin‐angiotensin system plays a role in thermoregulation,37 there was no ACE I/D genotype effect on post‐race rectal temperature in the triathletes who participated in this study (p = 0.945) (data not shown). The reason for this is not clear and needs to be investigated further.
What is already known on this topic
Although some studies have suggested that the insertion allele of the ACE gene is associated with endurance performance, the exact mechanism whereby ACE affects athletic ability is unclear
It is possible that another gene that is in close proximity to the ACE gene on chromosome 17 is involved in endurance performance
Growth hormone production is not the only factor able to modulate heat loss during exercise. The ability to maintain a normal body temperature can be affected by: the intensity of the exercise; the prevailing environmental conditions, such as wind and temperature; clothing; heat acclimatisation; dehydration; other factors.38 Interestingly, the GH1 genotype effect on post‐race rectal temperature was lost when the slowest athletes were analysed. The effects of environmental conditions on thermoregulation between the fastest and slowest athletes in this study were probably minimal because both groups of athletes competed in the marathon leg under similar environmental conditions. In addition, any theoretical effects of dehydration on thermoregulation between these two groups of athletes was also minimal, as all three genotype groups lost the same amount of weight during exercise.
The fact that the triathletes with a TT genotype did not lose more weight during the race than those with an A allele is not surprising, as it is highly unlikely that the reported weight loss during the triathlon was solely due to increased sweat rates. Changes in body weight during athletic events are also affected by fluid and food intake and fuel utilisation. Neither of these variables was measured during the triathlons. Further studies would therefore have to be conducted to determine the proposed relation between GH1 genotype, growth hormone production, sweat production, weight changes, and thermoregulation during endurance exercise.
Although there was no association between the GH1 gene polymorphism investigated in this study and endurance performance during the South African Ironman triathlons, which were raced under mild environmental conditions, it is possible that this polymorphism may be associated with endurance performance under hot environmental conditions such as the marathon of the 2004 Olympics in Athens, in which prevailing temperatures were 35°C (women's marathon) and 31°C (men's marathon). The influence of this polymorphism on endurance performance under these conditions remains to be investigated.
In conclusion, the functional T/A polymorphism in intron 4 of the GH1 gene was not associated with the endurance performance of the fastest finishers of the South African Ironman triathlons. Post‐race rectal temperature was, however, significantly lower in the fastest finishing athletes with a TT genotype. This genotype effect on post‐race rectal temperature was lost when the slower finishers were analysed.
What this study adds
The growth hormone 1 (GH1) gene, which is closely linked to the ACE gene, was not associated with endurance performance during an Ironman triathlon
Post‐race rectal temperatures were higher in the fastest finishing athletes who had a variant of the GH1 gene that is associated with lower growth hormone production
Acknowledgements
Research at the 2000 and 2001 South African Ironman triathlons was funded by dedicated grants from the race organisers, with support from the University of Cape Town, the South African Medical Research Council, and Discovery Health. Special thanks go to the staff and student from the UCT/MRC Research Unit for Exercise Science and Sports Medicine as well as staff from Body iQ Corporate Wellness, Pathnet Laboratories, and the Shosholoza Outreach and Development Programme of the Sports Science Institute of South Africa who assisted in collection of the data and samples for this project. We also thank Dale Rae, Gaonyadiwe Mokone, Colleen Saunders, and Dr Andrew Bosch for helpful comments and assistance with this project and manuscript.
Abbreviations
ACE - angiotensin converting enzyme
BMI - body mass index
GH1 - growth hormone 1
IGF‐I - insulin‐like growth factor‐I
PCR - polymerase chain reaction
Footnotes
Competing interests: none declared
References
- 1.Rankinen T, Perusse L, Rauramaa R.et al The human gene map for performance and health‐related fitness phenotypes: the 2003 update. Med Sci Sports Exerc 2004361451–1469. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery H E, Marshall R, Hemingway H.et al Human gene for physical performance. Nature 1998393221–222. [DOI] [PubMed] [Google Scholar]
- 3.Gayagay G, Yu B, Hambly B.et al Elite endurance athletes and the ACE I allele: the role of genes in athletic performance. Hum Genet 199810348–50. [DOI] [PubMed] [Google Scholar]
- 4.Myerson S, Hemingway H, Budget R.et al Human angiotensin I‐converting enzyme gene and endurance performance. J Appl Physiol 1999871313–1316. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez R, Terrados N, Ortolano R.et al Genetic variation in the renin‐angiotensin system and athletic performance. Eur J Appl Physiol 200082117–120. [DOI] [PubMed] [Google Scholar]
- 6.Yang N, MacArthur D G, Gulbin J P.et al ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet 200373627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods D, Hickman M, Jamshidi Y.et al Elite swimmers and the D allele of the ACE I/D polymorphism. Hum Genet 2001108230–232. [DOI] [PubMed] [Google Scholar]
- 8.Collins M, Xenophontos S L, Cariolou M A.et al The ACE gene and endurance performance during the South African Ironman triathlons. Med Sci Sports Exerc 2004361314–1320. [DOI] [PubMed] [Google Scholar]
- 9.Taylor R R, Mamotte C D S, Fallon K.et al Elite athletes and the gene for angiotensin‐converting enzyme. J Appl Physiol 1999871035–1037. [DOI] [PubMed] [Google Scholar]
- 10.Rankinen T, Wolfarth B, Simoneau J A.et al No association between the angiotensin‐converting enzyme ID polymorphism and elite endurance athlete status. J Appl Physiol 2000881571–1575. [DOI] [PubMed] [Google Scholar]
- 11.Jones A, Woods D R. Skeletal muscle RAS and exercise performance. Int J Biochem Cell Biol 200335855–866. [DOI] [PubMed] [Google Scholar]
- 12.Williams A G, Dhamrait S S, Wootton P T.et al Bradykinin receptor gene variant and human physical performance. J Appl Physiol 200496938–942. [DOI] [PubMed] [Google Scholar]
- 13.Mattei M ‐ G, Hubert C, Alhenc‐Gelas F.et al Angiotensin‐I converting enzyme gene is on chromosome 17. Cytogenet Cell Genet 1989511041 [Google Scholar]
- 14.Bennani‐Baiti I M, Jones B K, Liebhaber S A.et al Physical linkage of the human growth hormone gene cluster and the skeletal muscle sodium channel alpha‐subunit gene (SCN4A) on chromosome 17. Genomics 199529647–652. [DOI] [PubMed] [Google Scholar]
- 15.Nindl B C, Kraemer W J, Marx J O.et al Growth hormone molecular heterogeneity and exercise. Exerc Sport Sci Rev 200331161–166. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey R J, Madgwick Z, Whyte G P. The exercise‐induced growth hormone response in athletes. Sports Med 200333599–613. [DOI] [PubMed] [Google Scholar]
- 17.De Palo E F, Gatti R, Lancerin F.et al Correlations of growth hormone (GH) and insulin‐like growth factor I (IGF‐I): effects of exercise and abuse by athletes. Clin Chim Acta 20013051–17. [DOI] [PubMed] [Google Scholar]
- 18.Rennie M J. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes? Br J Sports Med 200337100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins P J. Growth hormone and exercise: physiology, use and abuse. Growth Horm IGF Res 200111(suppl A)S71–S77. [DOI] [PubMed] [Google Scholar]
- 20.Stokes K. Growth hormone responses to sub‐maximal and sprint exercise. Growth Horm IGF Res 200313225–238. [DOI] [PubMed] [Google Scholar]
- 21.Sheffield‐Moore M, Urban R J. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab 200415110–115. [DOI] [PubMed] [Google Scholar]
- 22.Weber M M. Effects of growth hormone on skeletal muscle. Horm Res 200258(suppl 3)43–48. [DOI] [PubMed] [Google Scholar]
- 23.Abdul Shakoor S K, Shalet S M. Effects of GH replacement on metabolism and physical performance in GH deficient adults. J Endocrinol Invest 200326911–918. [DOI] [PubMed] [Google Scholar]
- 24.Stacy J J, Terrell T R, Armsey T D. Ergogenic AIDS: human growth hormone. Curr Sports Med Rep 20043229–233. [DOI] [PubMed] [Google Scholar]
- 25.Juhn M. Popular sports supplements and ergogenic aids. Sports Med 200333921–939. [DOI] [PubMed] [Google Scholar]
- 26.Roemmich J N, Rogol A D. Exercise and growth hormone: does one affect the other? J Pediatr 1997131S75–S80. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen J O, Krag M, Kanaley J.et al Exercise, hormones, and body temperature. regulation and action of GH during exercise. J Endocrinol Invest 200326838–842. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa Y, Fujii K, Yamada M.et al Identification of novel human GH‐1 gene polymorphisms that are associated with growth hormone secretion and height. J Clin Endocrinol Metab 2000851290–1295. [DOI] [PubMed] [Google Scholar]
- 29.Le Marchand L, Donlon T, Seifried A.et al Association of a common polymorphism in the human GH1 gene with colorectal neoplasia. J Natl Cancer Inst 200294454–460. [DOI] [PubMed] [Google Scholar]
- 30.Sharwood K, Collins M, Goedecke J.et al Weight changes, sodium levels, and performance in the South african ironman triathlon. Clin J Sport Med 200212391–399. [DOI] [PubMed] [Google Scholar]
- 31.Lahiri K, Nurnberger J I. A rapid non‐enzymaticmethod for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res 1991195444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding K, Zhou K, He F.et al LDA‐‐a java‐based linkage disequilibrium analyzer. Bioinformatics 2003192147–2148. [DOI] [PubMed] [Google Scholar]
- 33.Woods D R, Humphries S E, Montgomery H E. The ACE I/D polymorphism and human physical performance. Trends Endocrinol Metab 200011416–420. [DOI] [PubMed] [Google Scholar]
- 34.Myburgh K H. What makes an endurance athlete world‐class? Not simply a physiological conundrum. Comp Biochem Physiol A Mol Integr Physiol 2003136171–190. [DOI] [PubMed] [Google Scholar]
- 35.Dennison E M, Syddall H E, Rodriguez S.et al Polymorphism in the growth hormone gene, weight in infancy, and adult bone mass. J Clin Endocrinol Metab 2004894898–4903. [DOI] [PubMed] [Google Scholar]
- 36.Juul A, Hjortskov N, Jepsen L T.et al Growth hormone deficiency and hyperthermia during exercise: a controlled study of sixteen GH‐deficient patients. J Clin Endocrinol Metab 1995803335–3340. [DOI] [PubMed] [Google Scholar]
- 37.Heled Y, Moran D S, Mendel L.et al Human ACE I/D polymorphism is associated with individual differences in exercise heat tolerance. J Appl Physiol 20049772–76. [DOI] [PubMed] [Google Scholar]
- 38.Noakes T D. Temperature regulation. In: Horn JM, ed. Lore of running. Cape Town: Oxford University Press Southern Africa, 2004222–327.