Abstract
Background and purpose:
There is a discrepancy in the adverse effect of 3-hydroxy-3-methylglutaryl--CoA reductase inhibitors, statins between the clinical reports and the studies using skeletal muscle cell models. In the clinical reports, both hydrophilic and lipophilic statins induce myotoxicity, whereas in in vitro experiments using cell lines of myoblasts, lipophilic, but not hydrophilic, statins exert myotoxicity. We investigated the cause of this discrepancy.
Experimental approach:
Skeletal myofibres, fibroblasts and satellite cells were isolated from rat flexor digitorum brevis (FDB) muscles. Using these primary cultured cells as well as the L6 myoblast cell line, we compared the toxicity of hydrophilic pravastatin and lipophilic fluvastatin. The mRNA expression levels of possible drug transporters for statins were also examined in these cells using reverse transcriptase-PCR.
Key results:
In the skeletal myofibres, both pravastatin and fluvastatin induced vacuolation and cell death, whereas in the mononuclear cells only fluvastatin, but not pravastatin, was toxic. mRNA of the organic anion transporting polypeptides (Oatp) 1a4 and Oatp2b1 were expressed in the skeletal myofibres, but not in mononucleate cells. Estrone-3-sulphate, a substrate for Oatps, attenuated the effects of pravastatin and fluvastatin in skeletal myofibres; p-aminohippuric acid, a substrate for the organic anion transporters (Oats), but not Oatps, failed to do so.
Conclusions and implications:
The statin transporters Oatp1a4 and Oatp2b1 are expressed in rat skeletal myofibres, but not in satellite cells, fibroblasts or in L6 myoblasts. This is probably why hydrophilic pravastatin affects skeletal muscle, but not skeletal myoblasts.
Keywords: drug transporters, organic anion transporting polypeptide, rhabdomyolysis, skeletal muscle, statins
Introduction
Statins, or 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, attenuate cholesterol biosynthesis and are widely used for treating hyperlipidemia (Endo, 1992). The major adverse effect of statins is myotoxicity, including myalgia, myositis and rhabdomyolysis (Ucar et al., 2000). The cause of this adverse effect has not been elucidated. We recently found that statins induce vacuolation in primary cultured rat skeletal myofibres by inactivating Rab small GTPases as a result of the depletion of geranylgeranyl pyrophosphate (Sakamoto et al., 2007). As Rab GTPases are essential for intracellular vesicle transport, our finding will be an important step in clarifying the mechanism of statin-induced myotoxicity. During the course of our previous study, we noticed that not only fluvastatin but also pravastatin induced vacuolation in the skeletal myofibres, although the potency was slightly different. This suggested the lines of inquiry we followed in this study.
Statins are classified into hydrophilic (for example, pravastatin and rosuvastatin) and lipophilic (for example, atorvastatin, cerivastatin, fluvastatin, lovastatin, pitavastatin and simvastatin) types according to the difference in their aqueous solubility (Holdgate et al., 2003). Hydrophilic statins are membrane-impermeable and therefore require drug transporters to enter cells. In contrast, lipophilic statins can pass through the cell membrane by passive diffusion, and therefore can be widely distributed in a variety of cells and tissues (Koga et al., 1992).
As skeletal myofibres are long, multinucleated and difficult to culture, primary cultures of neonatal myoblasts or myoblast cell lines and rhabdomyosarcoma cells have been used as substitute systems for the study of myotoxicity. In these skeletal cell models, hydrophilic pravastatin was shown to be non-toxic even at a millimolar concentration range (Nakahara et al., 1994; Masters et al., 1995; Kaufmann et al., 2006). Thus, hydrophilic statins have been considered to be less toxic than lipophilic statins (Ichihara and Satoh, 2002). However, in clinical studies, the myotoxic risk of hydrophilic pravastatin was not different from that of lipophilic statins (Bruckert et al., 2005), and furthermore, the risk of hydrophilic rosuvastatin was even significantly higher (Alsheikh-Ali et al., 2005). Furthermore, the relationship between tissue selectivity and lipophilicity is unclear (Sirtori, 1993). The cause of this discrepancy in statin-induced toxicity between experimental results and clinical data has not been resolved.
Statins are ionized as anions and are thus taken up or extruded by the membrane transporters for organic anions, including organic anion transporting polypeptides (OATP in humans, Oatp in rodents) and organic anion transporters (OAT/Oat) (Shitara and Sugiyama, 2006). OATPs/oatps are mainly expressed in the liver (Hsiang et al., 1999; Tokui et al., 1999), and OATs/Oats mainly in the kidney (Hasegawa et al., 2002). Some are expressed in other tissues such as endothelial cells and the retina (Gao et al., 2002; Sugiyama et al., 2003).
The skeletal muscles account for nearly half of mammalian body weight and are important organs for drug distribution (Fichtl and Kurz, 1978). However, there have been few studies on the drug transporters in skeletal muscle and there has been no report on the involvement of drug transporters in the myotoxicity of the statins.
In this study, using primary cultured skeletal myofibres isolated from rat flexor digitorum brevis (FDB) muscles, we examined the cytotoxicity of pravastatin and compared it with that of fluvastatin. We also examined the effects of the statins on mononuclear cells isolated from rat FDB muscles, including satellite cells and fibroblasts, and also on commercially available L6 myoblasts. Furthermore, the mRNA expression levels of possible statin transporters were examined using the reverse transcriptase-PCR (RT-PCR). We then examined the functional aspect of the statin transporter candidates detected by RT-PCR by using a substrate saturation method in the FDB myofibres.
Methods
Isolation of cells from rat FDB muscles
All experiments were performed in accordance with the regulation of the Animal Research Committee of Fukushima Medical University. A total of 11 female rats (Wistar; 8–16 weeks old; 160–210 g) were used. The rats were anesthetized with ether by being placed in an airtight glass container (2 L in volume) that had been filled with evaporated ether (from about 5 mL liquid ether absorbed in cotton) and then exsanguinated.
Single skeletal myofibres were isolated as described previously (Sakamoto et al., 2007). In brief, the FDB muscles isolated from both soles were incubated in 3% collagenase (Wako, Tokyo, Japan) -containing Ringer's solution for 2.5–3 h at 37 °C. The drugs were applied within 6 h after the myofibres were isolated.
Satellite cells and fibroblasts were also isolated from the FDB muscles. Isolation of satellite cells was according to the explants method with minor modifications (Bischoff, 1986; Allen et al., 1997). Following the isolation of myofibres from FDB muscles, 5–10 fibres were plated on a laminin-coated dish. As the satellite cells attached to the skeletal myofibres migrated to the floor of the dish, it was impossible to estimate the density of satellite cells. Three days after isolation, we applied drugs to the medium. To culture fibroblasts, the supernatant of the medium used for the isolation of myofibres was collected and poured into a non-coated dish. After 1 h, the medium was agitated and changed to remove the satellite cells. Then the fibroblasts were plated onto 12-well plates at 53.3±5.6 cells per mm−2 (n=7). FDB muscles, satellite cells and fibroblasts were plated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and maintained at 37 °C in a humidified atmosphere of 5% CO2/95% air.
Culture of L6 myoblasts
The L6 myoblast cell line originally derived from rat skeletal muscle was obtained from Human Science Research Sources Bank (Osaka, Japan). L6 myoblasts were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and maintained at 37 °C in a humidified atmosphere of 5% CO2/95% air. The cells were plated onto 12-well plates at 21.1±2.5 cells per mm−2 (n=4).
Cytotoxicity assays
To evaluate the cytotoxicity of the drugs, cell viability was examined using 0.2% Trypan blue/phosphate-buffered saline (Invitrogen, Carlsbad, CA, USA). FDB fibres were treated with Trypan blue for 5 min. Then the cells were washed three times with Ringer's solution and the Trypan blue stained cells and non-stained cells were counted.
Total RNA isolation and RT-PCR
Total RNA was extracted from tissues and cells by the acid-guanidine thiocyanate/phenol/chloroform method (Chomczynski and Sacchi, 1987). First-strand cDNA primed by random hexamers was prepared from total RNA (1 μg) using Moloney murine leukaemia virus reverse transcriptase in a final reaction volume of 20 μL. The cDNA was diluted fivefold with water and was used as a template for PCR analysis. The sequences of the primers and the predicted lengths of the PCR products are summarized in the Table 1. The primer sequences for Oatp1a1 and Oatp1b2 were obtained from references (Ohtsuki et al., 2003; Serrano et al., 2003). PCR was carried out with 35 amplification cycles (Ohkubo et al., 2000) and annealing temperature at 59 °C. The PCR products were separated by 1.5–2% agarose gel electrophoresis and visualized by ethidium bromide staining.
Table 1.
Oligonucleotide primers used for PCR amplification of cDNAs
| Protein Name | Accession # | Primer sequences | Length (bp) | References |
|---|---|---|---|---|
| Oatp1a1 | NM_017111 | Fw: 5′-TGGGGAAGGTTGCTGGCCCAATTT-3′ (688–714) Rv: 5′-GGTGGTTAATCCAGCAACTGCTGC-3′ (1346–1323) | 659 | Ohtsuki et al., 2003 |
| Oatp1a4 | NM_131906 | Fw: 5′-ATGGCCTGGCATACATGTCA-3′ (1485–1504) Rv: 5′-GGGAACTGGAATGTCCTCGTA-3′ (2001–1981) | 517 | — |
| Oatp1b2 | NM_031650 | Fw: 5′- GCCCAACCTTCACGATCAAA-3′ (151–170) Rv: 5′-GCCAAGGATTGGTCCAATCAT-3′ (774–754) | 624 | Serrano et al., 2003 |
| Oatp2b1 | NM_080786 | Fw: 5′-CCGCTACGACCACAGCA-3′ (445–461) Rv: 5′-CCAAGACCTTCTGCCTGA-3′ (1002–985) | 558 | — |
| Oat3 | NM_031332 | Fw: 5′-GGAGCTGAAGTTCAACTTGCA-3′ (1008–1028) Rv: 5′-GTTCACCCGTGATTTTCACCA-3′ (1528–1508) | 521 | — |
| Mrp2 | NM_012833 | Fw: 5′-GTATCAGCCATGCTGGGAGA-3′ (2123–2142) Rv: 5′-TCTCAGCCATGTCTCCTCCA-3′ (2342–2323) | 219 | — |
| GAPDH | X02231 | Fw: 5′-TGCTGAGTATGTCGTGGAGT-3′ (338–357) Rv: 5′-CATACTTGGCAGGTTTCTCC -3′ (831–812) | 493 | — |
Data analysis
Each experiment was repeated three to eight times. The data were expressed as mean±s.e.m. The relationship between the concentration of statins (Statin) and the cell viability (CV) was fitted by the following equation:
where CVmax is the maximum value of cell viability (which equals cell viability under control conditions), CVmin is the minimum value of cell viability (which was calculated by curve fitting software: Origin V6.1, OriginLab, Northampton, MA, USA), LC50 is the median value of the concentration-viability curve, (Statin) is the concentration of statins in the medium, m is a Hill coefficient and C is a constant. Statistical significance between two groups or among multiple groups was evaluated using Student's t-test, Scheffé's and Dunnet's tests after the F-test or one-way ANOVA.
Drugs and materials
In this study, we used hydrophilic pravastatin and lipophilic fluvastatin (Figure 1), because these statins were readily available in our laboratory (Sakamoto et al., 2007). Most of the pharmacological agents used were purchased from Sigma-Aldrich (St Louis, MO, USA). Fluvastatin was a gift from Novartis (Basel, Switzerland). Pravastatin was dissolved in sterile phosphate-buffered saline. Other compounds were dissolved in dimethyl sulphoxide. The concentration of the vehicles was lower than 0.1% and had no significant effects.
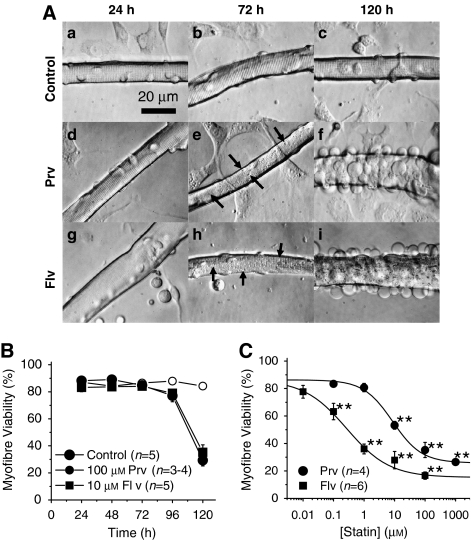
Figure 1.
Pravastatin and fluvastatin induced vacuolation and cell death in FDB fibres. (A) Phase contrast micrographs. (a–c) A control myofibre after 24 (a), 72 (b) and 120 h (c) in culture with fibroblasts in the background. (d–f) A myofibre cultured with 100 μM pravastatin (Prv) for 24 (d), 72 (e) and 120 h (f). Note that the background cells remained intact whereas damage was prominent in the skeletal myofibre. (g–i) A myofibre cultured with 10 μM fluvastatin (Flv) for 24 (g), 72 (h) and 120 h (i). Note that the background cells remained intact; whereas there was prominent damage in the skeletal myofibre. Unlike pravastatin treatment, the background cells disappeared after Flv treatment. Arrows indicate vacuoles induced by the statin treatment (e, h). (B) Time-dependent changes in the percentage of Trypan blue positive myofibres among control, pravastatin- and fluvastatin-treated cultures. (C) Comparison of cell death induced by Prv and Flv. Myofibres were incubated with various concentrations of Prv (closed circles) or Flv (closed squares) for 120 h. LC50 values for Prv and Flv are 8.6 and 0.3 μM, respectively (**P<0.01, compared with the control).
Results
Cytotoxicity of pravastatin and fluvastatin on FDB fibres
We first compared the toxicity of pravastatin with that of fluvastatin on FDB myofibres. In our previous study, both pravastatin and fluvastatin induced vacuolation in FDB myofibres after 72 h treatment (Sakamoto et al., 2007). The EC50 of pravastatin and fluvastatin for the vacuolation were 18.5 and 0.3 μM, respectively (calculated from Figure 1c of Sakamoto et al., 2007).
Figure 1Aa–c shows control myofibres cultured for 24, 72 and 120 h. Pravastatin at 100 μM (Figure 1Ad–f) and fluvastatin at 10 μM (Figure 1Ag–i) both induced numerous vacuoles in the myofibres after 72 h incubation (images with greater magnifications are shown in Supplementary Figure A). Figure 1B illustrates the time-dependent changes in the viability of myofibres treated with 100 μM pravastatin or 10 μM fluvastatin (see also Figure 1d of Sakamoto et al., 2007). At 120 h, both statins dramatically increased the number of Trypan blue staining myofibres. The concentration-viability relationships of the statins were obtained at 120 h for various statin concentrations (Figure 1c). The median lethal concentrations (LC50) of pravastatin and fluvastatin were 8.6 and 0.3 μM, respectively (Figure 1c). When cell death occurred, many blebs were observed on the surface of the sarcolemma of the statin-treated myofibres (Figure 1Af and i). This result clearly shows that not only lipophilic fluvastatin but also hydrophilic pravastatin induces toxicity in skeletal myofibres.
Cytotoxicity of statins in fibroblasts and satellite cells
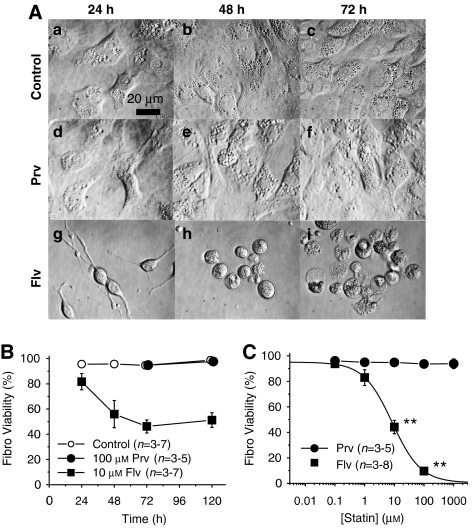
As shown in Figure 1A, we noticed that pravastatin affected myofibres, but not the background cells, whereas fluvastatin affected both myofibres and background cells: the background cells appeared intact in pravastatin-treated cultures, but had almost entirely disappeared after 72 and 120 h of incubation with fluvastatin (Figure 1Ad–i). To compare in detail the effect of the statins on the background cells, we separated fibroblasts from myofibres and selectively cultured fibroblasts (Figure 2Aa–c) that comprised the majority of the background cells. Pravastatin (100 μM) did not affect the viability of fibroblasts up to 120 h (Figure 2Ad–f and Figure 2B). However, fluvastatin (10 μM) started to induce morphological changes and cell death in fibroblasts within 24 h (Figures 2Ag–i and B). The concentration-viability relationship was obtained and is shown in Figure 2C. The LC50 value of fluvastatin against fibroblasts was 8.4 μM (Figure 2C). In contrast, pravastatin was without effect on fibroblasts even at 1 mM for 120 h (Figure 2C). Next, we analysed the toxicity of the statins on the satellite cells. The satellite cells were also resistant to pravastatin, but were susceptible to fluvastatin (Supplementary Figure).
Figure 2.
Fibroblasts isolated from flexor digitorum brevis (FDB) muscles were injured by incubation with fluvastatin, but not with pravastatin. (A) Phase contrast micrographs. (a–c) Fibroblasts after 24, 48 and 72 h in the control condition. (d–f) Fibroblasts cultured with 100 μM pravastatin (Prv) for 24–72 h. (g–i) Fibroblasts cultured with 10 μM fluvastatin (Flv). (B) Time-dependent changes in the percentage of Trypan blue positive fibroblasts in control, pravastatin- and fluvastatin-treated cultures. (C) Comparison of cell death induced by Prv and Flv. Fibroblasts (Fibro) were incubated with various concentrations of Prv (closed circles) or Flv (closed squares) for 72 h. LC50 value for Flv was 8.6 μM, whereas that for Prv was not measureable (**P<0.01, compared with the control).
Cytotoxicity of pravastatin and fluvastatin on L6 myoblasts
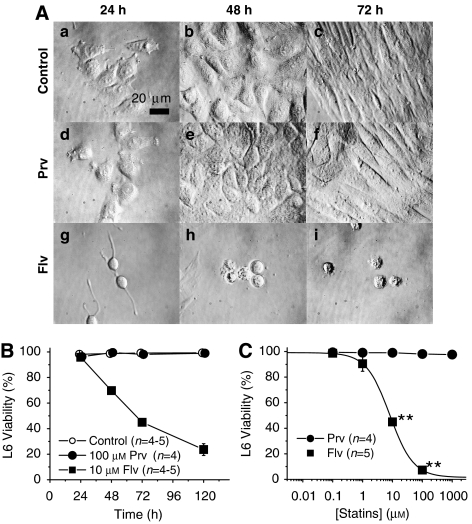
L6 myoblasts are an immortalized cell line established from neonatal rat skeletal myoblasts (Yaffe, 1968). We tested the effects of pravastatin or fluvastatin on L6 cells for up to 120 h (Figure 3A). Pravastatin did not induce any morphological changes or cell death in L6 cells even at 1 mM for 120 h (Figures 3Ad–f and B). However, fluvastatin prevented proliferation and induced cell death in L6 myoblasts within 48 h of treatment (Figures 3Ag–i and B). Figure 3C shows the concentration-viability relationships at 72 h of the statin treatment. The LC50 value for fluvastatin was 8.2 μM in L6 myoblasts at 72 h (Figure 3C).
Figure 3.
L6 rat skeletal myoblasts were injured by incubation with fluvastatin, but not with pravastatin. (A) Phase contrast micrographs. (a–c) L6 myoblasts after 24 (a), 72 (b) and 120 h (c) in the control condition. (d–f) L6 myoblasts cultured with 100 μM pravastatin (Prv) for 24 (d), 72 (e) and 120 h (f). (g–i) L6 myoblasts cultured with 10 μM fluvastatin (Flv) for 24 (g), 72 (h) and 120 h (i). (B) Time-dependent changes in the percentage of Trypan blue positive cells in control, pravastatin- and fluvastatin-treated cultures. (C) Comparison of cell death induced by Prv and Flv. L6 myoblasts were incubated with various concentrations of Prv (closed circles) or Flv (closed squares) for 72 h. LC50 value for Flv is 8.2 μM, whereas that for Prv was not measurable (**P<0.01, compared with the control).
Drug transporter expressions
In the above experiments, lipophilic fluvastatin affected all four types of cells we examined, but hydrophilic pravastatin affected only the primary cultured skeletal myofibres. To explain this difference, we hypothesized that drug transporters for pravastatin uptake might be expressed in the myofibres, but not in the other cell membranes. Alternatively, transporters for pravastatin extrusion might be expressed in all but the skeletal myofibres.
Four transporters have been so far reported to carry pravastatin in rats: Oatp1a1 (Hsiang et al., 1999), Oatp1a4 (Tokui et al., 1999), Oatp1b2 (Sasaki et al., 2004) and Oat3 (Hasegawa et al., 2002). In addition, Oatp2b1 is the rat orthologue of human OATP2B1, which mediates pravastatin uptake across human intestinal apical membranes (Kobayashi et al., 2003). We examined the mRNA expression levels of these transporters in several rat skeletal muscles and mononucleate cells of skeletal origin.
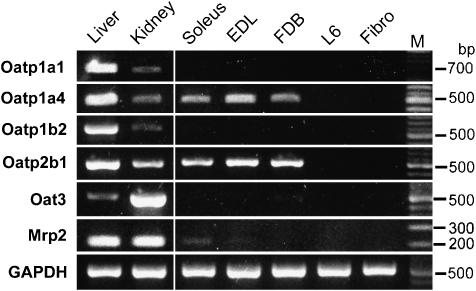
Skeletal muscles are composed of three different myofibres: type I myofibres (red, slow and glycolytic muscles), and types IIA and IIB (white, fast and aerobic muscles). The soleus is a type I muscle, whereas the extensor digitorum longus and the FDB are composed of predominantly type IIA and type IIB muscle fibres. We examined the transporter expression in the FDB, soleus and extensor digitorum longus. We also checked the liver and the kidney as positive controls for transporter expression. As shown in Figure 4, Oatp1a4 and Oatp2b1 mRNAs were expressed in all three skeletal muscles but not in fibroblasts and L6 myoblasts. Similar results were obtained in all four female rats and one male rat.
Figure 4.
Expression of the genes of drug transporters which carry pravastatin. Reverse transcriptase (RT)-PCR detection of transporters in rat liver, kidney, soleus muscle, extensor digitorum brevis (EDL) muscle, flexor digitorum brevis (FDB) muscle, L6 myoblasts (L6) and fibroblasts isolated from FDB muscle (Fibro). PCR products were generated through the use of gene-specific primers for Oatp1a1, Oatp1a4, Oatp1b2, Oatp2b1, Oat3 and Mrp2. A 100-bp molecular weight marker (M) was used to estimate the size of the amplicon.
We also examined the expression of Oat3 and the multidrug-resistance associate protein 2, because OAT3 is expressed in human skeletal muscles and is reported to be involved in pravastatin uptake (Takeda et al., 2004) and multidrug-resistance associate protein 2 excretes pravastatin from the cell by consuming ATP (Yamazaki et al., 1997). Only a faint band of multidrug-resistance associate protein 2 appeared in the soleus from one female rat (Figure 4), but was undetectable in the muscle preparations from four other rats. Other transporters such as Oatp1a1, Oatp1b2 and Oat3 mRNA were expressed in the liver and/or kidney, but not in the skeletal muscles, fibroblasts or in L6 myoblasts. This result suggests that pravastatin affected skeletal myofibres but not the mononuclear cells, because pravastatin transporters Oatp1a4 and Oatp2b1 were expressed in the skeletal muscle but not in fibroblasts and L6 myoblasts.
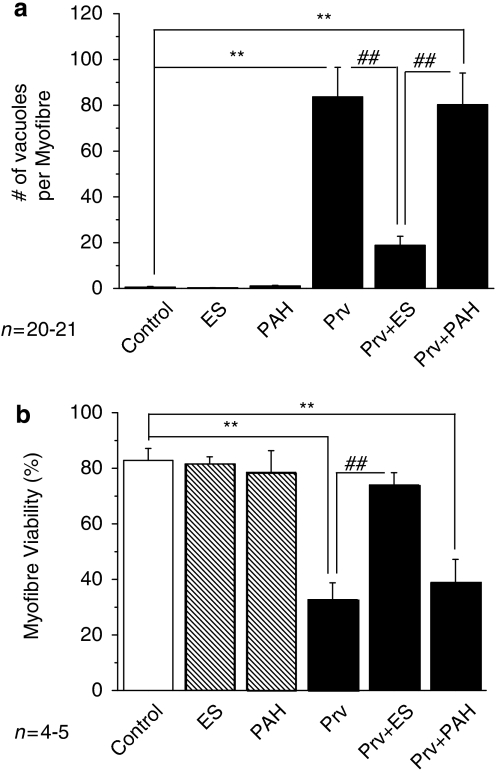
Effect of estrone-3-sulphate on pravastatin toxicity
We next tested whether Oatp1a4 and Oatp2b1 are functionally responsible for pravastatin toxicity in FDB myofibres. Estrone-3-sulphate (ES) is a typical substrate for OAT/Oat and OATP/Oatp (including Oatp1a4 and Oatp2b1), whereas p-aminohippuric acid (PAH) is a substrate for OAT/Oat, but not for OATP/Oatp (Anzai et al., 2006). If Oatp1a4 and Oatp2b1 were functionally responsible transporters for pravastatin in FDB myofibres, then the effect of pravastatin would be competitively inhibited by ES, but not by PAH.
We treated FDB myofibres with 300 μM ES or 300 μM PAH for 6 h before co-application of 100 μM pravastatin. These levels of ES or PAH alone had no effect on the morphology and viability of myofibres (Figures 5a and b). At 120 h, the number of vacuoles induced by pravastatin in the presence of PAH was similar to that with pravastatin alone. However, it was dramatically reduced in the presence of ES (P<0.01, n=20) (Figure 5a). At 120 h, the viability of FDB fibres was also significantly improved by ES, but not by PAH (n=5; P<0.01) (Figure 5b). As ES, but not PAH, inhibited the effect of pravastatin, we conclude that Oatp1a4 and Oatp2b1 are responsible for the uptake of pravastatin in FDB skeletal myofibres.
Figure 5.
Effect of substrates of drug transporters on pravastatin-induced vacuolation and injury in flexor digitorum brevis (FDB) fibres. (a) Numbers of vacuoles averaged for a myofibre cultured for 72 h under control, with 300 μM estrone-3-sulphate (ES) alone, with 300 μM p-aminohippuric acid (PAH) alone, with 100 μM pravastatin alone (Prv), with 100 μM Prv and 300 μM ES (Prv+ES) and with 100 μM Prv and 300 μM PAH (Prv+PAH). **P<0.01, compared with control. ##P<0.01, compared with Prv. (b) Viability of FDB fibres cultured for 120 h under the same treatments as in (a).
Effect of ES on fluvastatin toxicity
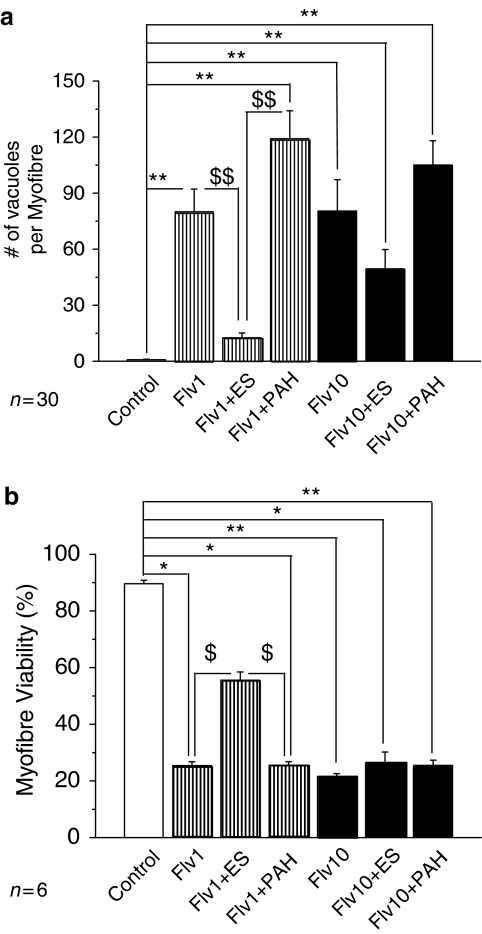
The LC50 value of fluvastatin was 0.3 μMin myofibres (Figure 1), but was 8.2–8.4 μM in fibroblasts (Figure 2) and L6 myoblasts (Figure 3 and Supplementary Figure for satellite cells). This difference in the potency of fluvastatin between myofibres and mononucleate cells suggests that not only hydrophilic pravastatin but also lipophilic fluvastatin is carried by the transporters in myofibres. Therefore, we repeated the above experiment for fluvastatin.
Two different concentrations (1 or 10 μM) of fluvastatin were tested. At 120 h with 1 μM fluvastatin, the number of vacuoles was significantly reduced by ES (P<0.01, Figure 6a), but not by PAH (P>0.05). The viability of myofibres at 120 h with 1 μM fluvastatin was partially but significantly improved by ES (P<0.05), but not by PAH (P>0.05). At a higher concentration of 10 μM fluvastatin, ES did not significantly improve vacuolation or cell viability (Figures 6a and b). These results demonstrate that Oatps are involved in fluvastatin transport in rat skeletal muscles.
Figure 6.
Effect of substrates of drug transporters on fluvastatin-induced vacuolation and injury in flexor digitorum brevis (FDB) fibres. (a) Number of vacuoles averaged for a myofibre cultured for 72 h under control, with 1 μM fluvastatin alone (Flv1), with 1 μM fluvastatin and 300 μM estrone-3-sulphate (Flv1+ES), with 1 μM fluvastatin and 300 μM p-aminohippuric acid (Flv1+PAH), with 10 μM fluvastatin alone (Flv10), with 10 μM fluvastatin and 300 μM ES (Flv10+ES) and with 10 μM fluvastatin and 300 μM PAH (Flv10+PAH). *P<0.05, compared with control; **P<0.01, compared with control. $$P<0.01, compare with Flv1+ES. (b) Viability of FDB fibres cultured for 120 h under the same treatments as in (a). $P<0.05, compared with Flv1+ES.
Discussion
In this study, we found that pravastatin induced vacuolation and cell death in FDB fibres, but not in mononucleate cells isolated from the skeletal muscle, including fibroblasts, satellite cells and L6 myoblasts. This effect of hydrophilic pravastatin is different from that of lipophilic fluvastatin, because fluvastatin affected both the skeletal myofibres and the mononucleate cells that we examined. RT-PCR analysis revealed that the known transporters for pravastatin, namely Oatp1a4 and Oatp2b1, were expressed in the skeletal muscles, but not in either fibroblasts or L6 myoblasts. The vacuolation and cell death induced by pravastatin were prevented by the presence of a high concentration of ES, which is a substrate for Oatps. PAH, which is a substrate for Oats but not for Oatps, did not prevent the toxic effects of pravastatin. These results suggest that the different sensitivities to pravastatin between the skeletal myofibres and the mononucleate cells, including fibroblasts, satellite cells and L6 myoblasts, are due to the different expression of the statin transporters, that is, Oatp1a4 and Oatp2b1, in the different types of cells.
Contribution of drug transporters to statin myotoxicity in skeletal muscles
We noticed that the cytotoxic potency of fluvastatin was higher in skeletal myofibres than in mononucleate cells—the LC50 value for fluvastatin was 0.3 μMin the myofibres and about 8 μM in the latter cells. As the vacuolation and cell death induced by 1 μM fluvastatin were inhibited by ES but not by PAH, fluvastatin must also be carried by Oatps expressed in skeletal fibres. Therefore, Oatp1a4 and Oatp2b1 are statin transporters, and can carry both hydrophilic and lipophilic statins. In agreement with our results, human OATP2B1 recognized not only pravastatin but also fluvastatin and atorvastatin as substrates: in recombinant studies, the Km values of OATP2B1 for fluvastatin and atorvastatin are 0.7–0.8 and 0.2 μM, respectively (Grube et al., 2006; Noe et al., 2007). These values are similar to the LC50 value for fluvastatin (0.3 μM). These results suggested that the influx of fluvastatin into myofibres was not only due to passive diffusion but also occurred through Oatps expressed in skeletal muscles. ES, however, failed to decrease the myotoxicity induced by 10 μM fluvastatin, indicating that at higher concentration of fluvastatin, the passive diffusion overwhelms the transporter function. This further indicates that at therapeutically low concentrations, lipophilic statins must be mainly carried into cells by membrane transporters such as OATP/Oatp, and the contribution of passive diffusion must be small, if there is any such contribution at all.
Comparison with clinical reports
Pravastatin and fluvastatin directly inhibit rat 3-hydroxy-3-methylglutaryl-CoA reductase with similar IC50 values of 6.9 and 3.8 nM, respectively (McTaggart et al., 2001). Furthermore, the therapeutic serum concentration, or the maximum serum drug concentration value, of pravastatin is 65 ng mL−1, which is also not very different from that of fluvastatin, which is 190 ng mL−1 at a 40 mg day−1 oral dose (Kivisto et al., 1998; Kyrklund et al., 2004). Furthermore, the myotoxic risks in the clinical reports are not different between pravastatin and other statins, including lipophilic statins (Alsheikh-Ali et al., 2005; Bruckert et al., 2005). Yet, in our study using rat FDB fibres, the LC50 value of 0.3 μM for pravastatin was about 30-fold less than that of fluvastatin (LC50=8 μM). One possible reason for this discrepancy is the species difference in the expression of drug transporters. It has been reported that not only OATP2B1 (Nishimura and Naito, 2005) but also OAT3 is expressed in human skeletal muscle (Cha et al., 2001; Takeda et al., 2004), but not in the rat (Kusuhara et al., 1999). Our results with rats agreed with this finding. The affinity of pravastatin for OAT3 (Km=13.4 μM) is higher than that to Oatp1a4 (Km=37.5 μM) (Tokui et al., 1999; Hasegawa et al., 2002) and that to OATP2B1 (Km=2.3 mM) (Kobayashi et al., 2003). Therefore, the uptake of pravastatin by human skeletal muscle may be higher than that in rodent skeletal muscle. Another possibility is due to the drug–drug interactions; the serum concentration of pravastatin is sensitive to interactions with other drugs and this sensitivity is greater for pravastatin than for other statins. For example, co-administration of cyclosporin A could alter the pharmacokinetics of statins, because cyclosporin A inhibits OATP1B1, which is involved in the uptake of statins in the liver (Shitara et al., 2003, 2004). In humans, cyclosporin A has been reported to increase the area under the curve and maximum serum drug concentration of pravastatin by 5–7.9 times and 22.8 times, respectively (Regazzi et al., 1994). In contrast, the area under the curve and maximum serum drug concentration of fluvastatin was only slightly increased by 1.9–3.5 times and 1.3 times, respectively (Spence et al., 1995; Goldberg and Roth, 1996). Thus, the pharmacokinetics of pravastatin seems to be markedly modified by interaction with cyclosporin A.
Potential physiological roles of OATP/Oatp in skeletal muscles
The physiological roles of OATP/Oatp have not been identified yet, although they have been implicated in the uptake of thyroid hormone, bile acid and prostanoids (Abe et al., 1998; Mikkaichi et al., 2004). If this is so, the OATP/Oatp expression in the skeletal muscles is not surprising, because the metabolism and development of skeletal muscles are highly regulated by thyroid hormones and bile acids accelerate the conversion from tetra-iodothyronine to tri-iodothyronine (Watanabe et al., 2006). Thus, Oatps may be important for the growth, maturation and metabolism of muscles.
Clinical significance of lipophilic statin-induced toxicity in fibroblasts
There have been clinical reports that statins induced tendinopathy (Chazerain et al., 2001). This adverse effect was caused not only by lipophilic but also by hydrophilic statins (Marie et al., 2008). Tendons consist of fibroblasts and collagen excreted from fibroblasts. In our study, fluvastatin, but not pravastatin, affected fibroblasts isolated from FDB muscles. We propose two possible reasons that may account for the discrepancy between the clinical reports and our result. One is a culture condition for the fibroblasts. Unlike skeletal myofibres, fibroblasts proliferate massively and these were cultured on the flat bottom of the dishes in our experiment. However, in physiological conditions, fibroblasts are quiescent and grow in extracellular cellular matrix. Thus, there must be some difference in gene expression and the character of fibroblasts between in vivo and culture conditions. Another is a possible involvement of skeletal myofibres in tendinopathy. In our study, as the statin-induced damage progress, myofibres are separated from the bottom of the laminin-coated dish and float in the medium. This may suggest that statins weaken interaction between skeletal myofibres and tendons. Currently, the mechanism of statin-induced tendinopathy is unclear. Further studies are required for elucidation of this problem.
Limitation of myoblasts as skeletal muscle models
Myogenic cell lines such as L6 myoblasts, C2C12 myoblasts and primary cultures of neonatal myoblasts have been used so far for the study of myotoxicity. Our results clearly demonstrated that myogenic cell lines are different from skeletal muscle myofibres in their susceptibility to statin-induced myotoxicity. L6 myoblasts were initially used for the study of myogenesis (Yaffe, 1968). More recently, they were also used for in vitro studies on statin myotoxicity (Nakahara et al., 1994; Kaufmann et al., 2006). As in fibroblasts isolated from FDB, we could not detect the expression of the statin transporters Oatp1a4 and Oatp2b1 in L6 myoblasts.
Conclusions
We compared the cytotoxicity of statins on rat FDB fibres, fibroblasts and L6 skeletal myoblasts, and found that pravastatin shows selective cytotoxicity in FDB fibres. RT-PCR revealed that Oatp-type drug transporters are expressed in rat skeletal muscle, but not in L6 myoblasts and fibroblasts. Our present study is the first report of a functional expression of Oatps or statin transporters in rat skeletal muscle. This will provide new insights for the contribution of skeletal muscles to drug distribution.
External data objects
Acknowledgments
We thank Ms Sanae Sato and Dr Tomoyuki Ono for their technical help and Dr Yayoi Shikama for her stimulating discussion. This study was supported by grants-in-aid from the Japan Foundation for Promotion of Science to KS (No.20790210), a project grant from Fukushima Medical University to KS, and in part from the Smoking Research Foundation (KI18003) to JK.
Abbreviations
- ES
estrone-3-sulphate
- FDB
flexor digitorum brevis
- LC50
median lethal concentration
- OAT/Oat
organic anion transporter in human or rodents
- OATP/Oatp
organic anion transporting polypeptide in human or rodents
- PAH
p-aminohippuric acid
- RT-PCR
reverse transcriptase-PCR
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, et al. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem. 1998;273:22395–22401. doi: 10.1074/jbc.273.35.22395. [DOI] [PubMed] [Google Scholar]
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Method Cell Biol. 1997;52:155–176. doi: 10.1016/s0091-679x(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111:3051–3057. doi: 10.1161/CIRCULATIONAHA.105.555482. [DOI] [PubMed] [Google Scholar]
- Anzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. J Pharmacol Sci. 2006;100:411–426. doi: 10.1254/jphs.crj06006x. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- Chazerain P, Hayem G, Hamza S, Best C, Ziza JM. Four cases of tendinopathy in patients on statin therapy. Joint Bone Spine. 2001;68:430–433. doi: 10.1016/s1297-319x(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–1582. [PubMed] [Google Scholar]
- Fichtl B, Kurz H. Binding of drugs to human muscle. Eur J Clin Pharmacol. 1978;14:335–340. doi: 10.1007/BF00611903. [DOI] [PubMed] [Google Scholar]
- Gao B, Wenzel A, Grimm C, Vavricka SR, Benke D, Meier PJ, et al. Localization of organic anion transport protein 2 in the apical region of rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2002;43:510–514. [PubMed] [Google Scholar]
- Goldberg R, Roth D. Evaluation of fluvastatin in the treatment of hypercholesterolemia in renal transplant recipients taking cyclosporine. Transplantation. 1996;62:1559–1564. doi: 10.1097/00007890-199612150-00005. [DOI] [PubMed] [Google Scholar]
- Grube M, Kock K, Oswald S, Draber K, Meissner K, Eckel L, et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006;80:607–620. doi: 10.1016/j.clpt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kusuhara H, Sugiyama D, Ito K, Ueda S, Endou H, et al. Functional involvement of rat organic anion transporter 3 (rOat3; Slc22a8) in the renal uptake of organic anions. J Pharmacol Exp Ther. 2002;300:746–753. doi: 10.1124/jpet.300.3.746. [DOI] [PubMed] [Google Scholar]
- Holdgate GA, Ward WH, McTaggart F. Molecular mechanism for inhibition of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase by rosuvastatin. Biochem Soc Trans. 2003;31:528–531. doi: 10.1042/bst0310528. [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Ichihara K, Satoh K. Disparity between angiographic regression and clinical event rates with hydrophobic statins. Lancet. 2002;359:2195–2198. doi: 10.1016/S0140-6736(02)09098-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Torok M, Zahno A, Waldhauser KM, Brecht K, Krahenbuhl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci. 2006;63:2415–2425. doi: 10.1007/s00018-006-6235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisto KT, Kantola T, Neuvonen PJ. Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br J Clin Pharmacol. 1998;46:49–53. doi: 10.1046/j.1365-2125.1998.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–708. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- Koga T, Fukuda K, Shimada Y, Fukami M, Koike H, Tsujita Y. Tissue selectivity of pravastatin sodium, lovastatin and simvastatin. The relationship between inhibition of de novo sterol synthesis and active drug concentrations in the liver, spleen and testis in rat. Eur J Biochem. 1992;209:315–319. doi: 10.1111/j.1432-1033.1992.tb17291.x. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, et al. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem. 1999;274:13675–13680. doi: 10.1074/jbc.274.19.13675. [DOI] [PubMed] [Google Scholar]
- Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2004;57:181–187. doi: 10.1046/j.1365-2125.2003.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie I, Delafenêtre H, Massy N, Thuillez C, Noblet C, Network of the French Pharmacovigilance Centers Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990–2005 and review of the literature. Arthritis Rheum. 2008;59:367–372. doi: 10.1002/art.23309. [DOI] [PubMed] [Google Scholar]
- Masters BA, Palmoski MJ, Flint OP, Gregg RE, Wang-Iverson D, Durham SK. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, pravastatin, lovastatin, and simvastatin, using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol. 1995;131:163–174. doi: 10.1006/taap.1995.1058. [DOI] [PubMed] [Google Scholar]
- McTaggart F, Buckett L, Davidson R, Holdgate G, McCormick A, Schneck D, et al. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol. 2001;87:28B–32B. doi: 10.1016/s0002-9149(01)01454-0. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Tanemoto M, Ito S, Abe T. The organic anion transporter (OATP) family. Drug Metab Pharmacokinet. 2004;19:171–179. doi: 10.2133/dmpk.19.171. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Yada T, Kuriyama M, Osame M. Cytosolic Ca2+ increase and cell damage in L6 rat myoblasts by HMG-CoA reductase inhibitors. Biochem Biophys Res Commun. 1994;202:1579–1585. doi: 10.1006/bbrc.1994.2112. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20:452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- Noe J, Portmann R, Brun ME, Funk C. Substrate dependent drug-drug interactions between gemfibrozil, fluvastatin and other OATP substrates on OATP1B1, OATP2B1 and OATP1B3. Drug Metab Dispos. 2007;35:1308–1314. doi: 10.1124/dmd.106.012930. [DOI] [PubMed] [Google Scholar]
- Ohkubo S, Kimura J, Matsuoka I. Ecto-alkaline phosphatase in NG108–15 cells: a key enzyme mediating P1 antagonist-sensitive ATP response. Br J Pharmacol. 2000;131:1667–1672. doi: 10.1038/sj.bjp.0703750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Takizawa T, Takanaga H, Terasaki N, Kitazawa T, Sasaki M, et al. In vitro study of the functional expression of organic anion transporting polypeptide 3 at rat choroid plexus epithelial cells and its involvement in the cerebrospinal fluid-to-blood transport of estrone-3-sulfate. Mol Pharmacol. 2003;63:532–537. doi: 10.1124/mol.63.3.532. [DOI] [PubMed] [Google Scholar]
- Regazzi MB, Iacona I, Campana C, Gavazzi A, Vigano M, Perani G. Altered disposition of pravastatin following comcominant drug therapy with cyclosporin A in transplant recipients. Transplant Proc. 1994;26:2644–2645. [PubMed] [Google Scholar]
- Sakamoto K, Honda T, Yokoya S, Waguri S, Kimura J. Rab-small GTPases are involved in fluvastatin and pravastatin-induced vacuolation in rat skeletal myofibers. FASEB J. 2007;21:4087–4094. doi: 10.1096/fj.07-8713com. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Suzuki H, Aoki J, Ito K, Meier PJ, Sugiyama Y. Prediction of in vivo biliary clearance from the in vitro transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II monolayer expressing both rat organic anion transporting polypeptide 4 and multidrug resistance associated protein 2. Mol Pharmacol. 2004;66:450–459. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Serrano MA, Macias RI, Vallejo M, Briz O, Bravo A, Pascual MJ, et al. Effect of ursodeoxycholic acid on the impairment induced by maternal cholestasis in the rat placenta-maternal liver tandem excretory pathway. J Pharmacol Exp Ther. 2003;305:515–524. doi: 10.1124/jpet.102.047977. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Hirano M, Adachi Y, Itoh T, Sato H, Sugiyama Y. In vitro and in vivo correlation of the inhibitory effect of cyclosporin A on the transporter-mediated hepatic uptake of cerivastatin in rats. Drug Metab Dispos. 2004;32:1468–1475. doi: 10.1124/dmd.32.12.. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Itoh T, Sato H, Li AP, Sugiyama Y. Inhibition of transporter-mediated hepatic uptake as a mechanism for drug-drug interaction between cerivastatin and cyclosporin A. J Pharmacol Exp Ther. 2003;304:610–616. doi: 10.1124/jpet.102.041921. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug–drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Sirtori CR. Tissue selectivity of hydroxymethylglutaryl coenzyme A (HMG CoA) reductase inhibitors. Pharmacol Ther. 1993;60:431–459. doi: 10.1016/0163-7258(93)90031-8. [DOI] [PubMed] [Google Scholar]
- Spence JD, Munoz CE, Hendricks L, Latchinian L, Khouri HE. Pharmacokinetics of the combination of fluvastatin and gemfibrozil. Am J Cardiol. 1995;76:80A–83A. doi: 10.1016/s0002-9149(05)80024-4. [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, et al. The blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem. 2003;278:43489–43495. doi: 10.1074/jbc.M306933200. [DOI] [PubMed] [Google Scholar]
- Takeda M, Noshiro R, Onozato ML, Tojo A, Hasannejad H, Huang XL, et al. Evidence for a role of human organic anion transporters in the muscular side effects of HMG-CoA reductase inhibitors. Eur J Pharmacol. 2004;483:133–138. doi: 10.1016/j.ejphar.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, oatp2. Pharm Res. 1999;16:904–908. doi: 10.1023/a:1018838405987. [DOI] [PubMed] [Google Scholar]
- Ucar M, Mjorndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf. 2000;22:441–457. doi: 10.2165/00002018-200022060-00003. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci USA. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Akiyama S, Ni'inuma K, Nishigaki R, Sugiyama Y. Biliary excretion of pravastatin in rats: contribution of the excretion pathway mediated by canalicular multispecific organic anion transporter. Drug Metab Dispos. 1997;25:1123–1129. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.