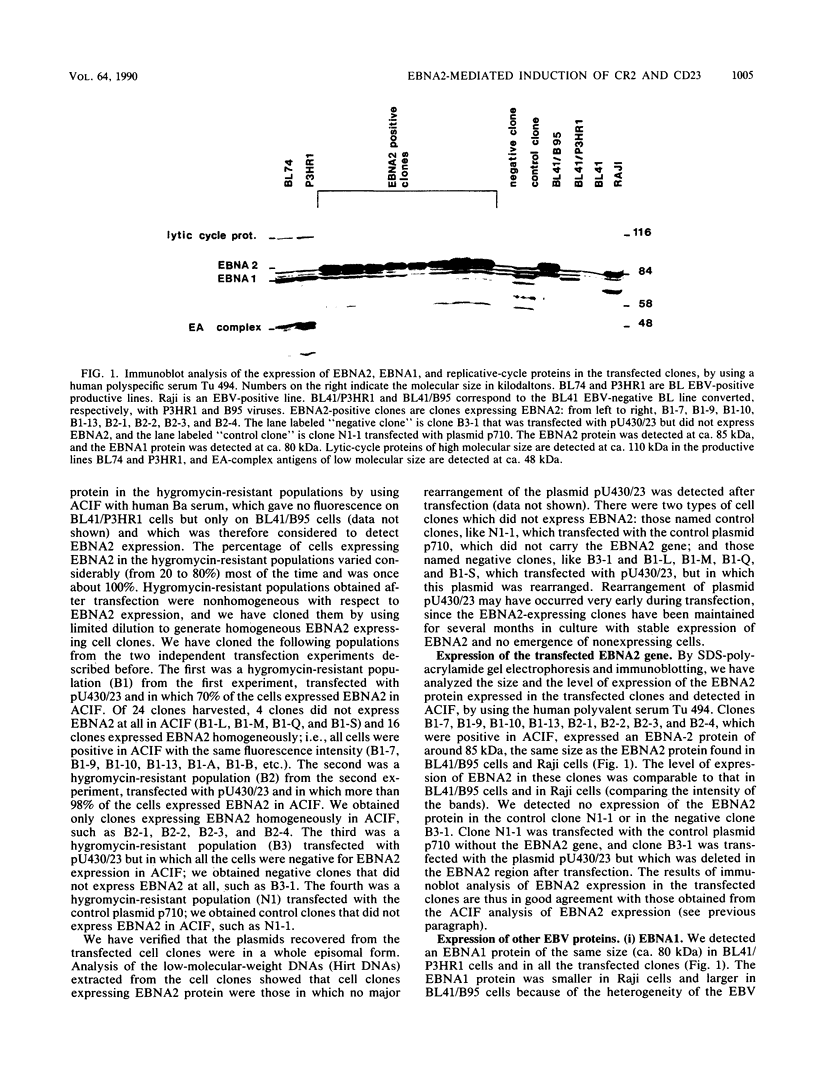
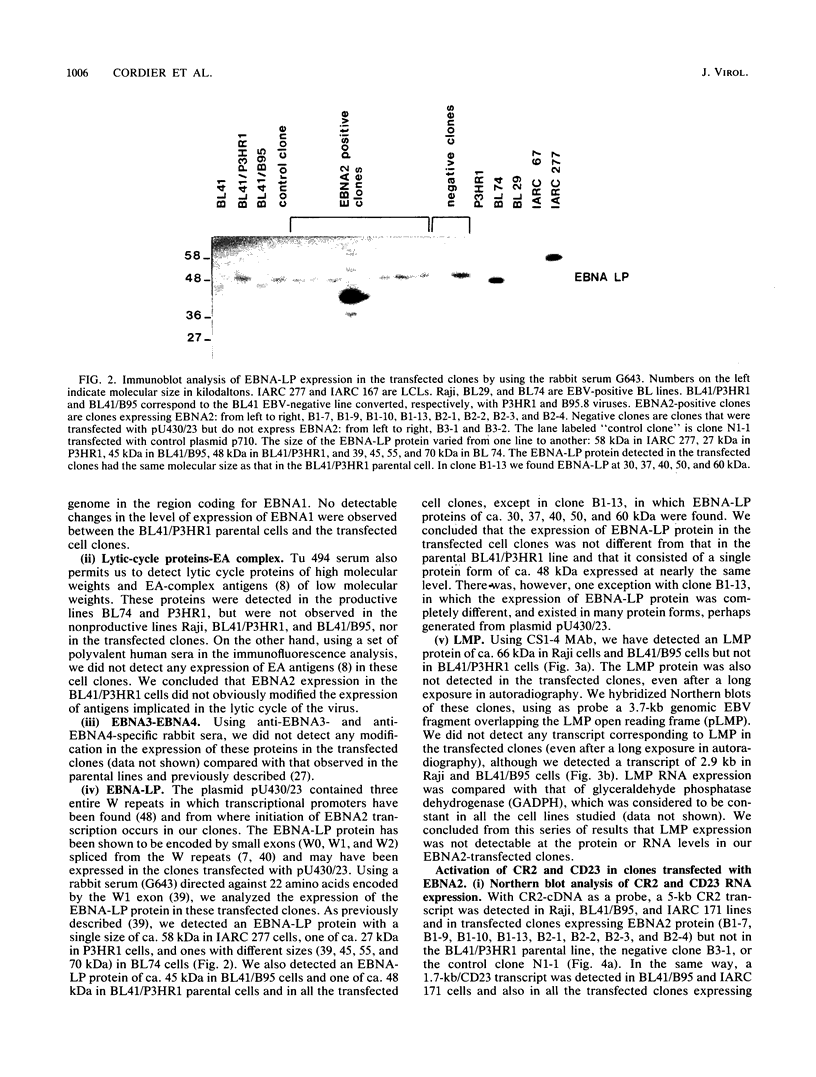
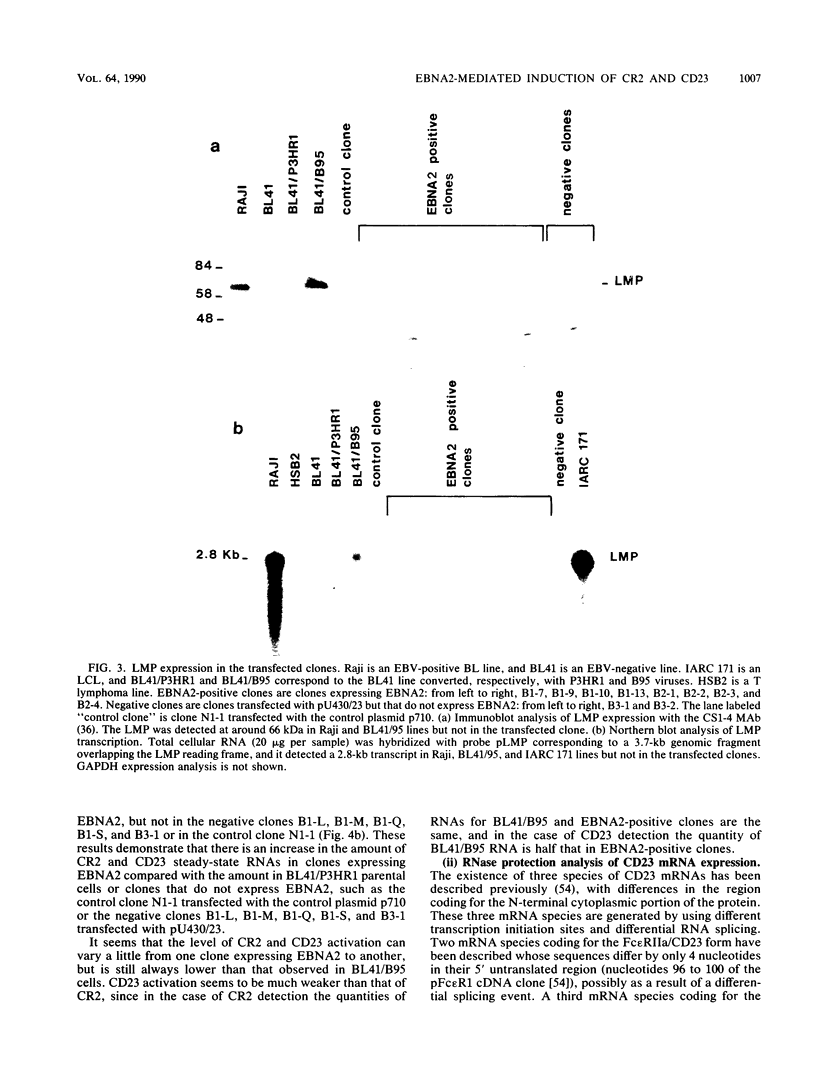
Abstract
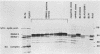
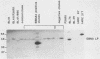
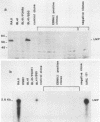
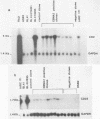
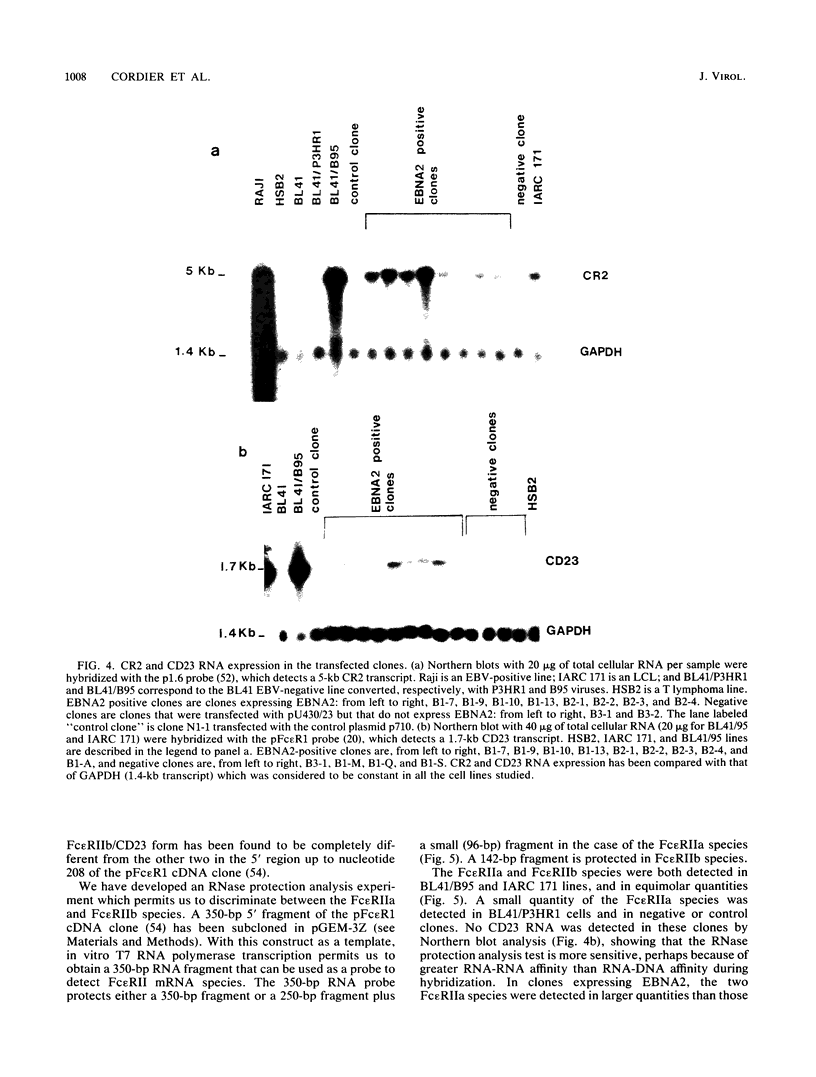
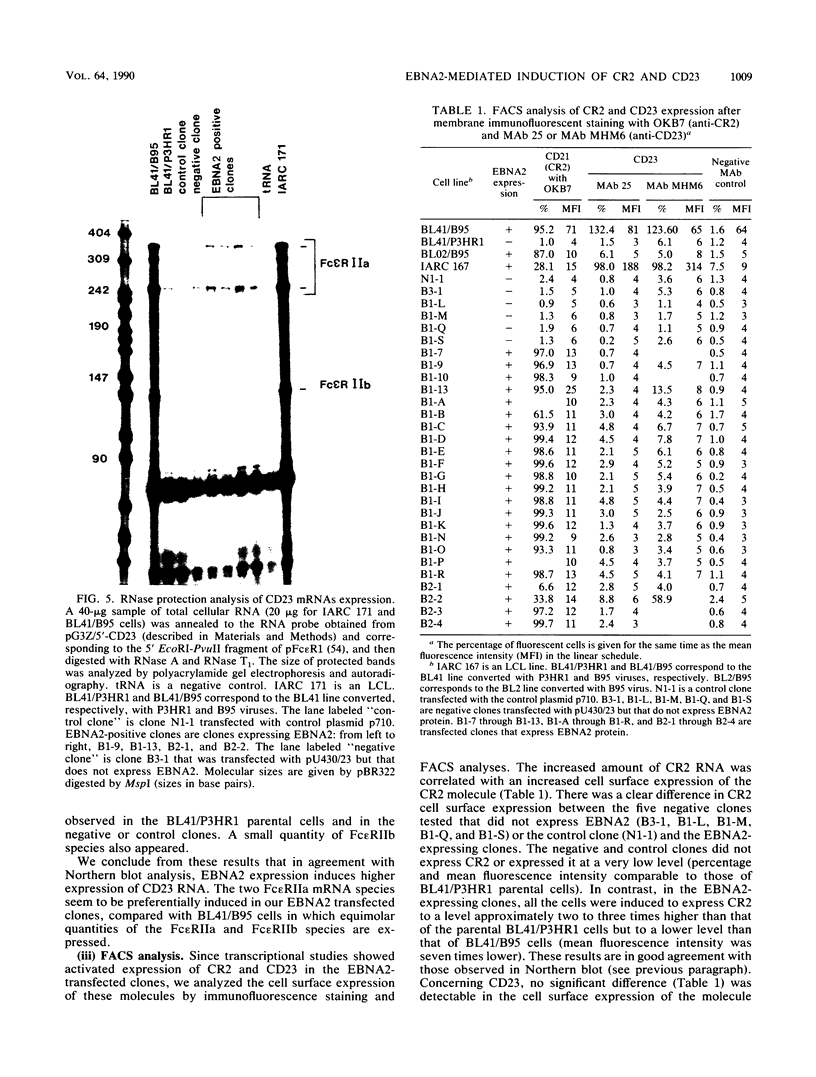
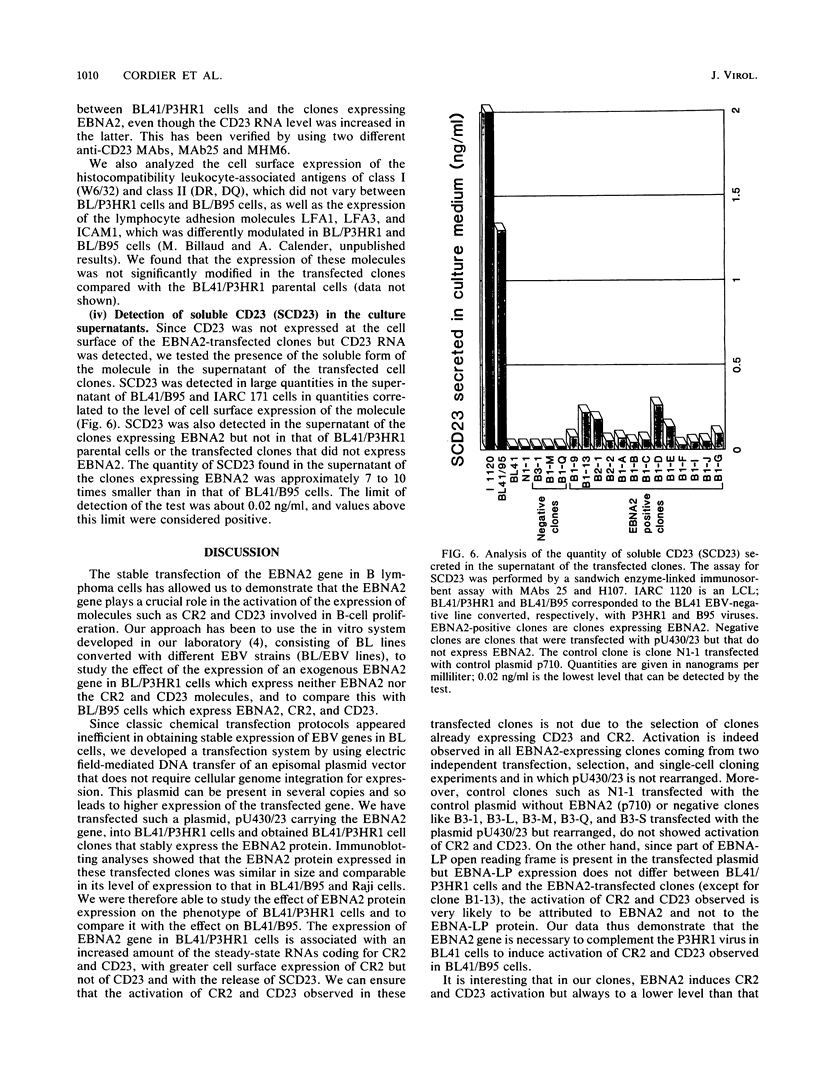
A set of B-cell activation molecules, including the Epstein-Barr virus (EBV) receptor CR2 (CD21) and the B-cell activation antigen CD23 (Blast2/Fc epsilon RII), is turned on by infecting EBV-negative B-lymphoma cell lines with immortalizing strains of the viruslike B95-8 (BL/B95 cells). This up regulation may represent one of the mechanisms involved in EBV-mediated B-cell immortalization. The P3HR1 nonimmortalizing strain of the virus, which is deleted for the entire Epstein-Barr nuclear antigen 2 (EBNA2) protein open reading frame, is incapable of inducing the expression of CR2 and CD23, suggesting a crucial role for EBNA2 in the activation of these molecules. In addition, lymphoma cells containing the P3HR1 genome (BL/P3HR1 cells) do not express the viral latent membrane protein (LMP), which is regularly expressed in cells infected with immortalizing viral strains. Using electroporation, we have transfected the EBNA2 gene cloned in an episomal vector into BL/P3HR1 cells and have obtained cell clones that stably express the EBNA2 protein. In these clones, EBNA2 expression was associated with an increased amount of CR2 and CD23 steady-state RNAs. Of the three species of CD23 mRNAs described, the Fc epsilon RIIa species was preferentially expressed in these EBNA2-expressing clones. An increased cell surface expression of CR2 but not of CD23 was observed, and the soluble form of CD23 molecule (SCD23) was released. We were, however, not able to detect any expression of LMP in these cell clones. These data demonstrate that EBNA2 gene is able to complement P3HR1 virus latent functions to induce the activation of CR2 and CD23 expression, and they emphasize the role of EBNA2 protein in the modulation of cellular gene implicated in B-cell proliferation and hence in EBV-mediated B-cell immortalization. Nevertheless, EBNA2 expression in BL/P3HR1 cells is not able to restore the level of CR2 and CD23 expression observed in BL/B95 cells, suggesting that other cellular or viral proteins may also have an important role in the activation of these molecules: the viral LMP seems to be a good candidate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billaud M., Busson P., Huang D., Mueller-Lantzch N., Rousselet G., Pavlish O., Wakasugi H., Seigneurin J. M., Tursz T., Lenoir G. M. Epstein-Barr virus (EBV)-containing nasopharyngeal carcinoma cells express the B-cell activation antigen blast2/CD23 and low levels of the EBV receptor CR2. J Virol. 1989 Oct;63(10):4121–4128. doi: 10.1128/jvi.63.10.4121-4128.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy J. Y., Aubry J. P., Peronne C., Wijdenes J., Banchereau J. Production and characterization of a monoclonal antibody specific for the human lymphocyte low affinity receptor for IgE: CD 23 is a low affinity receptor for IgE. J Immunol. 1987 May 1;138(9):2970–2978. [PubMed] [Google Scholar]
- Cairns J., Flores-Romo L., Millsum M. J., Guy G. R., Gillis S., Ledbetter J. A., Gordon J. Soluble CD23 is released by B lymphocytes cycling in response to interleukin 4 and anti-Bp50 (CDw40). Eur J Immunol. 1988 Mar;18(3):349–353. doi: 10.1002/eji.1830180305. [DOI] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Kallin B., Alexander H., Ernberg I., Uno M., Ono Y., Klein G., Lerner R. A. An Epstein-Barr virus (EBV)-determined nuclear antigen (EBNA5) partly encoded by the transformation-associated Bam WYH region of EBV DNA: preferential expression in lymphoblastoid cell lines. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6641–6645. doi: 10.1073/pnas.83.17.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Barel M., Ehlin-Henriksson B., Klein G. gp140, the C3d receptor of human B lymphocytes, is also the Epstein-Barr virus receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1490–1493. doi: 10.1073/pnas.82.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade R., Crevon M. C., Barel M., Vazquez A., Krikorian L., Charriaut C., Galanaud P. Enhancement of human B cell proliferation by an antibody to the C3d receptor, the gp 140 molecule. Eur J Immunol. 1985 Jan;15(1):73–76. doi: 10.1002/eji.1830150114. [DOI] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., English L. S., Hughes-Jones N. C. Immortalized B lymphocytes produce B-cell growth factor. Nature. 1984 Jul 12;310(5973):145–147. doi: 10.1038/310145a0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Rowe M., Walker L., Guy G. Ligation of the CD23,p45 (BLAST-2,EBVCS) antigen triggers the cell-cycle progression of activated B lymphocytes. Eur J Immunol. 1986 Sep;16(9):1075–1080. doi: 10.1002/eji.1830160908. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Fennewald S., Hummel M., Cole T., Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7207–7211. doi: 10.1073/pnas.81.22.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Wang F., Bushman E. W., Kieff E. Definitive identification of a member of the Epstein-Barr virus nuclear protein 3 family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5693–5697. doi: 10.1073/pnas.83.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Miesner D. R., Atkinson J. P., Holers V. M. Identification of an alternative polyadenylation site in the human C3b/C4b receptor (complement receptor type 1) transcriptional unit and prediction of a secreted form of complement receptor type 1. J Exp Med. 1988 Oct 1;168(4):1255–1270. doi: 10.1084/jem.168.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M. Synergistic activation of cells by Epstein-Barr virus and B-cell growth factor. J Virol. 1987 Mar;61(3):774–781. doi: 10.1128/jvi.61.3.774-781.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Laux G., Perricaudet M., Farrell P. J. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J. 1988 Mar;7(3):769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernhardt W., Raschke W. C., Melchers F. Alpha-type B cell growth factor and complement component C3: their possible structural relationship. Curr Top Microbiol Immunol. 1986;132:98–104. doi: 10.1007/978-3-642-71562-4_13. [DOI] [PubMed] [Google Scholar]
- Melchers F., Erdei A., Schulz T., Dierich M. P. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985 Sep 19;317(6034):264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Lenoir G. M., Sauter M., Takaki K., Béchet J. M., Kuklik-Roos C., Wunderlich D., Bornkamm G. W. Identification of the coding region for a second Epstein-Barr virus nuclear antigen (EBNA 2) by transfection of cloned DNA fragments. EMBO J. 1985 Jul;4(7):1805–1811. doi: 10.1002/j.1460-2075.1985.tb03854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. J., Wang D., Young L. S., Wang F., Rowe M., Kieff E., Rickinson A. B. Epstein-Barr virus-specific cytotoxic T-cell recognition of transfectants expressing the virus-coded latent membrane protein LMP. J Virol. 1988 Oct;62(10):3747–3755. doi: 10.1128/jvi.62.10.3747-3755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. J., Young L. S., Calender A., Gregory C. D., Rowe M., Lenoir G. M., Rickinson A. B. Different patterns of Epstein-Barr virus gene expression and of cytotoxic T-cell recognition in B-cell lines infected with transforming (B95.8) or nontransforming (P3HR1) virus strains. J Virol. 1988 Mar;62(3):894–901. doi: 10.1128/jvi.62.3.894-901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., McNaughton M. E., Cooper N. R. Binding of monoclonal antibody to the Epstein Barr virus (EBV)/CR2 receptor induces activation and differentiation of human B lymphocytes. J Immunol. 1985 Nov;135(5):3068–3073. [PubMed] [Google Scholar]
- Norment A. M., Littman D. R. A second subunit of CD8 is expressed in human T cells. EMBO J. 1988 Nov;7(11):3433–3439. doi: 10.1002/j.1460-2075.1988.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro N., Yoshioka A., Adachi M., Yasuda K., Masuda T., Yodoi J. Monoclonal antibody (H107) inhibiting IgE binding to Fc epsilon R(+) human lymphocytes. J Immunol. 1986 Aug 15;137(4):1258–1263. [PubMed] [Google Scholar]
- Polack A., Hartl G., Zimber U., Freese U. K., Laux G., Takaki K., Hohn B., Gissmann L., Bornkamm G. W. A complete set of overlapping cosmid clones of M-ABA virus derived from nasopharyngeal carcinoma and its similarity to other Epstein-Barr virus isolates. Gene. 1984 Mar;27(3):279–288. doi: 10.1016/0378-1119(84)90072-6. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricksten A., Kallin B., Alexander H., Dillner J., Fåhraeus R., Klein G., Lerner R., Rymo L. BamHI E region of the Epstein-Barr virus genome encodes three transformation-associated nuclear proteins. Proc Natl Acad Sci U S A. 1988 Feb;85(4):995–999. doi: 10.1073/pnas.85.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Evans H. S., Young L. S., Hennessy K., Kieff E., Rickinson A. B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987 Jun;68(Pt 6):1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- Rowe M., Hildreth J. E., Rickinson A. B., Epstein M. A. Monoclonal antibodies to Epstein-Barr virus-induced, transformation-associated cell surface antigens: binding patterns and effect upon virus-specific T-cell cytotoxicity. Int J Cancer. 1982 Apr 15;29(4):373–381. doi: 10.1002/ijc.2910290403. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M., Boos H., Hirsch F., Mueller-Lantzsch N. Characterization of a latent protein encoded by the large internal repeats and the BamHI Y fragment of the Epstein-Barr virus (EBV) genome. Virology. 1988 Oct;166(2):586–590. doi: 10.1016/0042-6822(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Speck S. H., Pfitzner A., Strominger J. L. An Epstein-Barr virus transcript from a latently infected, growth-transformed B-cell line encodes a highly repetitive polypeptide. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9298–9302. doi: 10.1073/pnas.83.24.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Grogan E. A., Shedd D., Robert M., Liu C. R., Miller G. Stable expression in mouse cells of nuclear neoantigen after transfer of a 3.4-megadalton cloned fragment of Epstein-Barr virus DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5688–5692. doi: 10.1073/pnas.79.18.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Israelsohn E. S. Generation of specific cytotoxic T cells with a fragment of the Epstein-Barr virus-encoded p63/latent membrane protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5384–5388. doi: 10.1073/pnas.84.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Nadler L. M., Bhan A. K., Schooley R. T. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. J Immunol. 1985 May;134(5):3007–3012. [PubMed] [Google Scholar]
- Toneguzzo F., Hayday A. C., Keating A. Electric field-mediated DNA transfer: transient and stable gene expression in human and mouse lymphoid cells. Mol Cell Biol. 1986 Feb;6(2):703–706. doi: 10.1128/mcb.6.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. J., Fearon D. T., Klickstein L. B., Wong W. W., Richards S. A., de Bruyn Kops A., Smith J. A., Weis J. H. Identification of a partial cDNA clone for the C3d/Epstein-Barr virus receptor of human B lymphocytes: homology with the receptor for fragments C3b and C4b of the third and fourth components of complement. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5639–5643. doi: 10.1073/pnas.83.15.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Yokota A., Kikutani H., Tanaka T., Sato R., Barsumian E. L., Suemura M., Kishimoto T. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988 Nov 18;55(4):611–618. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Yukawa K., Kikutani H., Owaki H., Yamasaki K., Yokota A., Nakamura H., Barsumian E. L., Hardy R. R., Suemura M., Kishimoto T. A B cell-specific differentiation antigen, CD23, is a receptor for IgE (Fc epsilon R) on lymphocytes. J Immunol. 1987 Apr 15;138(8):2576–2580. [PubMed] [Google Scholar]
- van Santen V., Cheung A., Hummel M., Kieff E. RNA encoded by the IR1-U2 region of Epstein-Barr virus DNA in latently infected, growth-transformed cells. J Virol. 1983 May;46(2):424–433. doi: 10.1128/jvi.46.2.424-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]