Abstract
The zebrafish is a well-established model organism used in developmental biology. In the last decade, this technology has been extended to the generation of high-value knowledge on safety risks of novel drugs. Indeed, the larval zebrafish appear to combine advantages of whole organism phenotypic assays and those (rapid production of results with minimal resource engagement) of in vitro high-throughput screening techniques. Thus, if appropriately evaluated, it can offer undeniable advantages in drug discovery for identification of target and off-target effects. Here, we review some applications of zebrafish to identify potential safety liabilities, particularly before lead/candidate selection. For instance, zebrafish cardiovascular system can be used to reveal decreases in heart rate and atrial–ventricular dissociation, which may signal human ether-a-go-go-related gene (hERG) channel blockade. Another main area of interest is the CNS, where zebrafish behavioural assays have been and are further being developed into screening platforms for assessment of locomotor activity, convulsant and proconvulsant liability, cognitive impairment, drug dependence potential and impaired visual and auditory functions. Zebrafish also offer interesting possibilities for evaluating effects on bone density and gastrointestinal function. Furthermore, available knowledge of the renal system in larval zebrafish can allow identification of potential safety issues of drug candidates on this often neglected area in early development platforms. Although additional validation is certainly needed, the zebrafish is emerging as a versatile in vivo animal model to identify off-target effects that need investigation and further clarification early in the drug discovery process to reduce the current, high degree of attrition in development.
Keywords: zebrafish, safety pharmacology, liability, high throughput, model organism
Introduction
The initial interest on zebrafish as a model system goes back to the early 1970s when George Streisinger selected zebrafish larvae to develop the first vertebrate assay enabling forward genetic screening (Streisinger et al., 1981; Grunwald and Eisen, 2002). During the subsequent 20 years, zebrafish was almost exclusively used to study organ development. This resulted in the characterization of an exceptionally large number of genes involved in vertebrate pathways, which contributed to the establishment of zebrafish as a relevant model for human disease and pharmaceutical research (Driever et al., 1996; Alestrom et al., 2006).
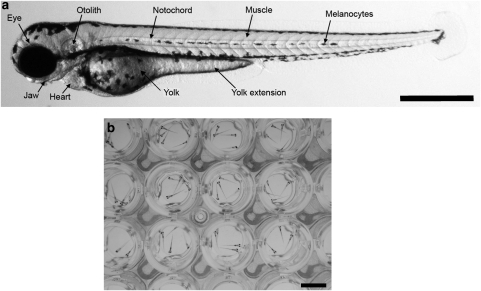
An attractive feature of zebrafish assays for pharmacology investigations is the potential to use them in medium-to-high-throughput screening mode, because the zebrafish is a small (5 cm for an adult and 5 mm for 7 days post-fertilization (d.p.f.) larvae) and robust freshwater tropical cyprinid that is easy to maintain in large stocks due to their high fecundity. Experimental manipulation and direct observation of organ function can be easily performed as embryos are transparent (Figure 1a) and develop rapidly ex utero with most organs becoming fully functional between 3 and 5 d.p.f. (Westerfield, 1995). The organization of the genome and the genetic pathways controlling signal transduction and development appear to be highly conserved between zebrafish and humans (Postlethwait et al., 2000). The zebrafish genome is sequenced and multiple genetic markers and gene chips are available commercially (http://www.sanger.ac.uk/Projects/D_rerio/). Both larval and adult zebrafish have been used to validate this model organism at various developmental ages but its unique advantage resides in the larval stage as it can be used in multi-well plate screening technologies. In contrast to rodents, the zebrafish larvae are not foetal but are closer to the juvenile state in that the nervous system is mature, vital organs are functioning and tissue architecture is fully developed at the time of the assays. Furthermore, only milligrams of compound are needed for screening in 96-well plates as the larvae can live in as little as 50 μL of fluid (Figure 1b). Finally, chemical screening is facilitated by the fact that zebrafish are reasonably tolerant to dimethylsulphoxide concentrations generally used in such technologies and small molecule compounds dissolved in the swimming medium can reach target tissues via passage through the skin of the larvae (Rombough, 2002). Given these advantages, it is not surprising that screening platforms using zebrafish are now emerging as they provide the high content of an in vivo assay that can be easily and inexpensively applied throughout the crucial hit to lead and lead optimization stages of the drug discovery process (Goldsmith, 2004; Parng, 2005; Zon and Peterson, 2005; Rubinstein, 2006).
Figure 1.
(a) Zebrafish larva at 3 d.p.f. with organs such as the heart clearly visible due to the optical clarity of the larva at this age. Bar, 0.5 mm. (b) Zebrafish larvae at 7 d.p.f in a 96-well plate. Bar 5 mm.
Zebrafish have been used historically for evaluating the toxicity of environmental and agrochemical agents (Bretaud et al., 2004) but more recently, their use for toxicity evaluation of pharmaceutical agents has been earnestly pursued (Hill et al., 2005). In zebrafish larvae, an in vivo toxicology assessment can be achieved in a week, a much shorter time frame than that required when performing comparable mammalian assays. Human disease models for efficacy screening have also been developed in zebrafish across a wide range of therapeutic areas (cardiovascular disease, infection, cancer, inflammation and metabolic diseases) (MacRae and Peterson, 2003; Zon and Peterson, 2005; Murphey and Zon, 2006; Rubinstein, 2006; Lieschke and Currie, 2007).
This review will outline the application of zebrafish for assessing safety liabilities of drug candidates before lead/candidate selection. The International Conference on Harmonisation (ICH) S7A guideline requires that any clinical candidate is evaluated before first exposure to man on basic vital (cardiac, central nervous and respiratory system) functions. These safety pharmacology investigations as well as toxicology studies often reveal effects that require further experimentation for clarification purposes that are expensive and time consuming and may result in the abandonment of the clinical candidate. Screening technologies exist and are being further developed in zebrafish, which should provide very early readouts of potential off-target effects on the cardiac and CNS as well as other functions (such as effects on the intestinal tract, proconvulsant potential, auditory and visual functions and bone formation) prior to lead or candidate selection. Additional advantages of the zebrafish technology are that it can also provide early knowledge on safety pharmacological areas (for example, dependency potential, cognitive impairment and renal function) not generally considered as a prerequisite to first-in-man evaluation. Thus, the zebrafish technology complemented by early non-good laboratory practice (GLP) studies should be seen as a useful pre-filter to aid selection of the safest lead candidates as early as possible in the drug discovery process.
In conclusion, the armamentarium of assays/models available in the safety pharmacology arena could benefit from novel in vivo screening tools as a gap exists between the high-throughput, time- and cost-effective in vitro screens used early in the discovery process and the slower, time-consuming mammalian in vivo assays that are generally considered to have greater predictive and translational power to the human situation (Bass et al., 2005; Suter, 2006). Once adequately validated, zebrafish could be of great help to the safety pharmacology objective, which is to identify as early as feasibly possible potential safety liabilities of drugs selected for human evaluation.
Cardiac function
Drugs delaying cardiac repolarization by inhibiting human ether-a-go-go-related (hERG) channel prolong ECG QT interval, an effect which, in patients with concurrent cardiac risk factors (for example, inherited disease, electrolyte abnormalities, metabolic inhibitors, etc), can evolve into life-threatening pro-arrhythmic episodes. This off-target property is currently a major source of attrition in drug development (Suter, 2006) and has been a predominant reason in drugs being recalled from the market in the last decade (EMEA, 1997; Shah, 2004). For this reason, a specific ICH guideline (FDA, 2005) has been developed requiring that any drug candidate prior to human evaluation is tested in vitro for its effect on hERG channel expressed in cell lines and in vivo for QT prolongation. However, a shortcoming of this guidance is that it does not directly deal with other possible mechanisms of cardiac toxicity, such as sodium channel blockade or calcium channel activation. Methods to unveil these properties are also available but they are generally costly and time consuming (Eckhardt et al., 1998; Yang et al., 2001). The availability of a zebrafish model with the ability to reveal QT interval-prolonging effects of compounds through multiple mechanisms could be very useful.
Because of the transparency of the larval state, observation of the heart rhythm as well as the vasculature and circulation in zebrafish is possible and does not require physical intervention. The heart of zebrafish embryo starts beating within 26 h of fertilization (Baker et al., 1997) and by 2 d.p.f. undergoes looping (Stainier et al., 1996). A fully functioning vascular tree is present by 3 d.p.f. (Sehnert and Stainier, 2002). At 4 d.p.f. cardiomyocyte proliferation thickens the ventricular wall (Antkiewicz et al., 2005) and by 5 d.p.f. the heart has developed valves (Forouhar et al., 2004). Zebrafish organs and tissues do not rely on the cardiac output for oxygen delivery as aerobic metabolism is largely dependent on oxygen diffusion through the skin up to 14 d.p.f. from the swimming medium (Jacob et al., 2002; Rombough, 2002).
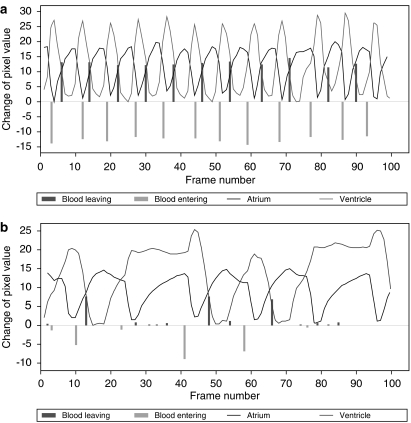
hERG and its zebrafish homologue (zERG) show high similarities, suggesting an evolutionary conserved role for this protein channel. zERG is expressed solely in the two chambers of the zebrafish heart with the drug-binding and pore domains showing a 99% conserved amino-acid sequence with the human orthologue (Langheinrich et al., 2003). The zERG gene has not, to date, been heterologously expressed and characterized for biochemical and kinetic similarities to hERG. Such data, when available, will facilitate the interpretation of zebrafish data. Knocking down the zERG gene results in a characteristic arrhythmia, with two atrial beats coupled to each ventricular beat. Known QT-prolonging drugs when tested in 3 d.p.f. embryos cause this specific arrhythmia in a concentration-dependent manner with lower concentrations inducing bradycardia and higher concentrations leading to 2:1 decoupling followed in some cases by a more pronounced decoupling (3:1 and 4:1), irregular arrhythmia, fibrillation or complete ventricular block (Langheinrich et al., 2003; Berghmans, 2006) (Figure 2). The assay was able to detect QT-prolonging drugs known to block hERG such as terfenadine, cisapride and pimozide, as well as compounds such as YS-035, an L-type calcium channel blocker, not affecting the hERG channel. The distinctive atrial–ventricular decoupling feature has also been observed in human neonates and newborns harbouring QT prolongation (Phillips et al., 2001). The atrial–ventricular decoupling effect produced by a test article in zebrafish may therefore be taken as a surrogate signalling QT prolongation.
Figure 2.
Data analysis of a high-resolution video of a 3 d.p.f zebrafish larvae heart where atrium beat (black line), ventricular beat (grey line) and outflow strength (black bars) are shown for: (a) normal heart rate; (b) 2:1 atrial/ventricular ratio arrhythmia in response to treatment with terfenadine (Berghmans, 2006).
Milan et al. (2003) reported that 18 out of 23 drugs known to cause QT prolongation and torsades de pointes in man caused bradycardia in 3 d.p.f. zebrafish when dissolved in the swimming medium, whereas the remaining 4 false-negative drugs were found to cause bradycardia when administered through microinjection into the yolk sac, suggesting poor absorption through the skin barrier. Furthermore, erythromycin (1 mg mL−1) potentiated the cardiac depressant effects of cisapride (3 μg mL−1) replicating the well-documented clinical interaction between these two drugs. Indeed, erythromycin by inhibiting cytochrome P450 3A4 impairs the metabolic detoxification of cisapride by the zebrafish larvae and thereby causing higher levels of cisapride in the larvae (Milan et al., 2003). A similar effect has been observed with cimetidine (31 μg mL−1) and terfenadine (10 μg mL−1) (Milan et al., 2003), as well as ketoconazole (30 μM) or amiodarone (3 μM) and terfenadine (3 μM) (S Berghmans, unpublished data). Thus, the measurement of atrial–ventricular rate in zebrafish larvae can provide an early, simple in vivo assessment of dangerous QT prolongation and arrhythmia, which is the outcome of drug–drug interaction.
A critical issue with the described assay is that the concentrations needed to cause dissociation between the atrium and ventricle in zebrafish are significantly higher than those required to block the hERG channel in a patch clamp assay and also higher than those present in the human plasma to cause this effect. Recently, Mittelstadt et al. (2008) found that the lowest concentration required to produce atrial–ventricular decoupling was higher than the reported in vitro hERG IC50 in six out of the seven QT-prolonging compounds tested. However, the results of the Mittelstadt et al. (2008) study indicate that the zebrafish assay is considerably less sensitive than the hERG assay by approximately 8- to 10-fold. This may limit the applicability of the zebrafish assay, particularly when testing compounds with limited solubility (Mittelstadt et al., 2008). Despite these problems, this study reported a statistically significant correlation between the hERG IC50 values determined with patch clamp assays and effective concentrations in zebrafish.
Burns et al. (2005) have produced embryos that express green fluorescent protein in the myocardium. They were able to use an automated microscope to scan a 96-well plate, with software detecting the fluorescent heart and variations in the pixel intensity to give a read out of the heart rate in 95% of the larvae. The authors were able to detect bradycardia with some known QT-prolonging drugs and this assay format may aid easier detection of cardiac rate in zebrafish in future assay development (Burns et al., 2005).
An assessment of the ECG in zebrafish can aid in the validation of the use of zebrafish in early cardiac assessment but would not be high throughput enough to provide a convenient early screening assay. By immobilizing anaesthetized 5 d.p.f. larval zebrafish in ultra low melt agarose gel, it is possible to take ECG readings that clearly show a repeating oscillating signal with obvious P (atrial) and R (ventricular) depolarization waves (Forouhar et al., 2004). Perfusion with known QT-prolonging drugs astemizole (50 μM), haloperidol (100 μM), pimozide (0.01 μM) and terfenadine (0.1 μM) resulted in a measurable increase in corrected QT interval (QTc; QT=QTc × RRα) interval in adult zebrafish. Further experiments with astemizole showed this increase in QTc to be concentration dependent (Milan et al., 2006).
Patch clamp data from 3 d.p.f. zebrafish showed that cardiomyocytes have a large repertoire of cardiac currents that are comparable to other species. This includes several potassium channels, including the IKr, sodium as well as, T-type and L-type calcium channels (Baker et al., 1997). Alterations to these other ion channels or ion channel protein trafficking could have the potential to cause repolarization abnormalities and predispose an individual to QT prolongation or torsades de pointes (Cordes et al., 2005; Eckhardt et al., 2005). As effects of compounds on the overall cardiac electrophysiology are often investigated in in vivo models, a significant advantage of the zebrafish with respect to single in vitro single channel assays is that it can signal cardiac effects due to a variety of mechanisms including the hERG channel.
As the methods of examining the cardiovascular system using the zebrafish continue to be refined and validated, the utility and applicability of this model for early identification of safety liabilities will increase. Zebrafish are able to detect the effects of known QT-prolonging drugs in a relatively high-throughput assay in larvae by using the surrogate readout of atrial–ventricular decoupling. Image capture systems also yield important information regarding the circulatory system and have the potential to provide information on the contractile activity of the heart.
In the larval zebrafish, the blood cells circulating around the body can be followed by using a microscope. Very early in development (∼26 h.p.f. (hours post-fertilization)), primitive erythrocytes start circulating. They are later replaced by a second wave of erythropoeisis at 5 d.p.f. (de Jong and Zon, 2005). Erythrocytes play an important role in generating shear forces that might affect the morphogenesis of the heart and vascular system (Hove et al., 2003; Isogai et al., 2003). Available image capture systems enable following the movements of a single erythrocyte within the vascular system to be traced by subtracting the odd and even fields of one video frame and generating the difference image (Schwerte and Pelster, 2000). This provides a wide variety of information such as erythrocyte velocity, blood vessel diameter and blood distribution. This system could be applied to develop a system for examining the effect of compounds on the contractility of the zebrafish heart.
CNS assessment
According to the ICH S7A guidelines, clinical candidates need to be investigated as a prerequisite to human testing for their effects on general behaviour, motor activity, behavioural changes, coordination, sensory/motor reflex responses and body temperature. Additional CNS follow-up studies are expected to be carried out prior to product approval to cover behavioural pharmacology, learning and memory, ligand-specific binding, neurochemistry, visual, auditory and/or electrophysiology examinations (FDA, 2001). Omitted from the guideline requirements, but traditionally tested, are convulsive liability, pain sensitivity and interaction with barbiturates (Porsolt et al., 2002).
Zebrafish have recently emerged as a model organism for CNS studies. The overall organization of the zebrafish brain is similar to other vertebrates, having matched defined areas such the hypothalamus and olfactory bulb, encompassing structures of the lateral pallium, which appear to be homologous to the mammalian hippocampus (Tropepe and Sive, 2003). In addition, the main neurotransmitter systems such as the cholinergic, 5-hydroxytryptaminergic, dopaminergic and noradrenergic pathways are also present and have been mapped throughout the brain (Rink and Wullimann, 2004; Wullimann and Mueller, 2004). Zebrafish larvae have recently become the focus of neurobehavioural studies as they display learning, sleep, drug addiction and neurobehavioural phenotypes that are quantifiable and related to those seen in man (Zhdanova et al., 2001; Cahill, 2002; Guo, 2004; Orger et al., 2004; Ninkovic et al., 2006). Zebrafish have a developmentally regulated blood–brain barrier that is functional at 10 d.p.f. (Goldsmith and Fleming, 2007). Additionally, P-glycoprotein transporters are present in the microvasculature of the endothelium of the CNS at 8 d.p.f., which coincides with the efflux of the P-glycoprotein substrates from the zebrafish brain. These data suggest that the zebrafish can be used to develop relevant models for the study of neurological activity of drugs and to assess whether or not compounds are excluded from the CNS.
Locomotor activity
From a safety pharmacology perspective, the basic information yielded from activity meter tests is whether a test substance increases or decreases the ability of the animal to move around (Porsolt et al., 2002). The need to quantify such a parameter in rodents has led to the development of automated systems that rely on interruption of photoelectric beams or video-image analysis allowing calculation of parameters, such as distance moved and the speed of movement. With the emergence of the zebrafish as a model organism in pharmacological studies, these technologies have been adapted to track zebrafish locomotor activity. Zebrafish larvae are capable of free swimming from 96 h.p.f. and can be tested for locomotor activity by immersion in the medium containing test compound (Drapeau et al., 2002).
The effect of a range of sedative compounds on zebrafish larvae locomotor activity has been documented (Table 1). Clozapine (12.5–50 μM), fluoxetine (4.6 μM), melatonin (10 nM–100 μM), diazepam (10 nM–100 μM) and pentobarbital (10 nM–100 μM) cause hypomotility in zebrafish larvae (Zhdanova et al., 2001; Airhart et al., 2007; Boehmler et al., 2007). Ethanol, on the other hand, causes hyperactivity at intermediate concentrations (1–2%), whereas treatment at higher concentrations (4%) depress spontaneous activity and causes toxicity (Lockwood et al., 2004; Parng et al., 2007).
Table 1.
The effects of drugs on larval zebrafish locomotor activity
| Drugs | Effects in man | Effects in zebrafish | Reference |
|---|---|---|---|
| Clozapine | Sedative | Sedative | Boehmler et al. (2007) |
| Fluoxetine | Sedative | Sedative | Airhart et al. (2007) |
| Melatonin | Sedative | Sedative | Zhdanova et al. (2001) |
| Diazepam | Sedative | Sedative | Zhdanova et al. (2001) |
| Pentobarbital | Sedative | Sedative | Zhdanova et al. (2001) |
| Ethanol | Stimulant/sedative | Stimulant/sedative | Lockwood et al. (2004) |
Thus, these results support the use of zebrafish as a model organism to test locomotor activity upon drug treatment that could be applied to the early identification of potential safety liabilities to aid in prioritization of hit series and in lead optimization for the selection of the most promising candidate before regulatory preclinical studies are performed. Moreover, there is a body of evidence emerging to suggest that the zebrafish model can also predict hyperactivity. Further validation of the system is needed to demonstrate its utility for hyperactivity and to fully characterize the correlation with drugs known to cause hypoactivity in other mammalian species. It would also be interesting to investigate the equivalent of an Irwin test, where a range of zebrafish behaviours are studied for a potential correlation with the known effects of drugs in rodent Irwin tests.
Convulsant and proconvulsant liability
A number of drugs can cause typical tonic/clonic convulsions (seizures) and/or a range of characteristic clinical effects spanning from simple tingling to mood changes either by acting directly on the CNS or indirectly by affecting blood–brain barrier permeability (Easter et al., 2007). Generally, convulsant or proconvulsant clinical candidates are discarded from pharmaceutical development pathway as they are potentially life threatening. Although convulsants can cause seizure-linked symptoms in their own right, a proconvulsant reduces the threshold to reach a seizure-like pre-existing condition or enhances seizure-inducing triggers. Thus, safety pharmacology approaches this liability by evaluating the capacity of test substances to cause convulsions or to lower the seizure threshold in animals subjected to an electroconvulsive shock (Rundfeldt et al., 1995); or treated with reference proconvulsant agents such as pentylenetetrazol (PTZ) or picrotoxin (Porsolt et al., 2002). Although in vitro models using cell or tissue have been developed (Easter et al., 2007), they do not replicate the complex phenomenon of seizures in integrated systems. Therefore, the availability of a pharmacologically validated high-throughput zebrafish assays would be highly beneficial to aid in prioritizing and selecting compounds for further study in rodent models.
Zebrafish larvae at 7 d.p.f. respond to PTZ with a distinct series of movements (Baraban et al., 2005) evolving from increased swimming activity to rapid ‘whirlpool-like' behaviour to generalized tonic clonic seizures. The latencies to the beginning of this three-stage phenomenon depend on the concentration of the convulsant agent. The zebrafish seizure model has been further validated by demonstrating the presence of ictal spike and sharp wave activity postictal depression and interictal slow wave activity, using tectal whole-field recordings (Baraban et al., 2005). In addition, these are accompanied by a rapid upregulation of c-fos gene in the CNS, which is known to underlie neuronal activation. Using a video-tracking system, evidence of reduction and suppression of PTZ-induced seizure was obtained with 13 known antiepileptic drugs (Berghmans et al., 2007). An additional blinded study using 17 compounds known to produce seizure liability and 8 negatives in a video-tracking system achieved a predictability of 72% (Winter et al., 2008). This screening approach will also allow investigation of potential proconvulsants. Compounds such as theophylline (100 μM), pilocarpine (1 mM) or caffeine (100 μM) are able to potentiate subthreshold concentrations of PTZ (1 mM) or picrotoxin (10 mM) (S Berghmans, unpublished data). The timing of seizure induction has been investigated by exposing zebrafish larvae to domoic acid, which was found to reduce latency time to first PTZ-induced seizure (Tiedeken and Ramsdell, 2007). Therefore, with further validation it is very much possible that zebrafish could be used in the detection of convulsant and proconvulsant liabilities of early stage compounds.
Cognitive impairment
Cognitive impairment (learning, memory and attention) is an adverse effect associated with several classes of drugs, including antiepileptics, antidepressants and antipsychotics. Commonly used assays are passive avoidance tests and the Morris water maze carried out in rodents.
There have been a number of models developed to study learning and memory in zebrafish and these could, with further modification and validation, be used as assays to identify potential safety liabilities in CNS active drugs in early drug discovery. Non-associative learning has been studied in larval zebrafish at 7 d.p.f. by measuring the reduction in a startle response to a series of acoustic stimuli (Best et al., 2008). Donepezil (3 μM) and memantine (30 μM), which are current therapies for improving cognitive ability in Alzheimer's disease patients, and the phosphodiesterase-4 inhibitor rolipram (3 μM), which has been shown to increase learning and memory in rodent studies, modulated habituation to the acoustic stimuli in this assay. Profiling compounds that impair memory, such as scopolamine as well as antiepileptic and antidepressant drugs, in this simple assay would be a straightforward way to assess whether or not this system could be applied to measuring cognitive impairment as well as improvement in cognition.
More sophisticated experimental paradigms have also been developed for testing associative learning in adult zebrafish in both tanks and mazes. Zebrafish have been shown to display rapid and reliable learning by swimming to certain locations within a tank to avoid adverse stimuli, such as electrical shocks (Xu et al., 2007) or a moving net (Arthur and Levin, 2001). Zebrafish have shown cognitive flexibility by demonstrating reversal when these contingencies are changed. A spatial alternation paradigm was used to show that adult zebrafish learn to respond to food reward by alternating between ends of the test tank and rapidly achieving high correct response levels (Williams et al., 2002). On retesting after 10 days, this behaviour is remembered. A number of researchers have studied learning and memory in a T-maze where food reward is linked to specific colours placed on the side of the tank (Peitsaro et al., 2003; Swain et al., 2004; Colwill et al., 2005). The adult zebrafish learned to associate one of a pair of colours with food; reversal and extinction were also demonstrated in this system. A three-compartment maze with a central chamber and a choice of left or right chambers has been used to demonstrate spatial and non-spatial visual discrimination learning (Arthur and Levin, 2001) and to demonstrate the improvement of learning in response to nicotine treatment (100–200 μg mL−1) (Levin et al., 2006). Treatment with ethanol (10 mM) or lead (10 μM) for the first 24 h.p.f. of development resulted in significant learning and memory deficits when the adult zebrafish were tested (Carvan et al., 2004). Although several of the studies above have measured improvements in cognition, the recent progress in learning and memory experimental paradigms in zebrafish suggests the possible utility of this model organism in studies to determine cognitive impairment. Whether a sufficiently predictive and well-validated assay can be developed in larval zebrafish for the early identification of potential cognitive impairment properties in a novel compound to be applied to screening in lead optimization remains to be seen.
Dependence liability
In 2006, the European Medicines Agency (EMEA) issued guidelines on the non-clinical investigation of the dependence potential of all new CNS-active medical products and for compounds with a novel mechanism of action. Furthermore, the initial investigations are to be conducted prior to clinical trials in man followed by additional assessments, if indicated, alongside clinical trials and before approval (EMEA, 2006).
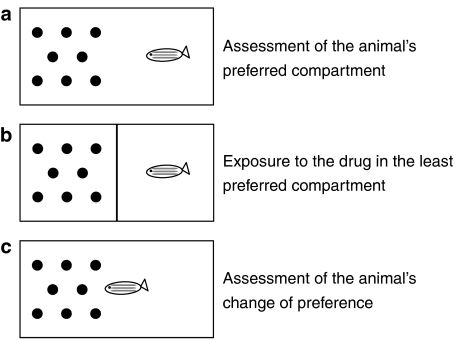
Conditioned place preference has been the preferred approach to investigate the reinforcing properties of drugs in rodents as well as in fish. It follows the same principle whereby the animal is allowed to explore the test box and the preferred compartment of the box is determined, followed by exposure to the drug in the least preferred compartment and then the animal is assessed for change in preference (Guo, 2004) (Figure 3). Using this approach, the reinforcing properties of cocaine (5–15 mg L−1) and D-amphetamine (40 μg per g of body weight) have been demonstrated in adult zebrafish (Darland and Dowling, 2001; Ninkovic et al., 2006). Another paradigm, the choice chamber paradigm, was used to show the reinforcing properties of morphine (0.8 μM) in 14 d.p.f. larvae. In this assay, larval zebrafish have a choice of spending their time in either a water- or morphine-containing compartment. Larvae that have previously experienced morphine spend significantly more time in the compartment containing morphine. This behaviour can be attenuated by pretreatment with antagonists of the opioid receptor, naloxone (0.5 μM) or the dopamine receptor D1 antagonist SCH 23390 (9 μM) (Bretaud et al., 2007). Thus, both adult and larval zebrafish have the potential to be used in behavioural studies to assess rewarding properties of drugs, although further validation studies are required to ensure that this complex neurological behaviour can be adequately modelled.
Figure 3.
The experimental paradigm for the investigation of conditioned place preference in zebrafish. The fish preferred compartment is assessed (a), the fish is then exposed to the drug in the least preferred compartment (b). Finally the fish is tested for change in preference (c).
Whereas, according to the guidelines, drug discrimination studies alone do not comprise strong evidence of (absence of) dependence potential, withdrawal syndrome assessment is a requirement. However, withdrawal symptoms are subjective and self-reported, making them difficult to assess in animal models. Furthermore, withdrawal from different classes of compounds can cause different symptoms (Warner et al., 2006). Despite the difficulties in assessing withdrawal symptoms, a recent report suggests that adult zebrafish could be used as a model to assess the effects of drug withdrawal. Zebrafish treated with non-anaesthetic cocaine doses (1.5 μM) showed an increase in motility upon drug withdrawal. This hyperactive behaviour is counteracted by reinstatement of the cocaine treatment or treatment with diazepam (5 μM), suggesting that cocaine withdrawal produces behavioural effects in zebrafish, which are consistent with an anxiety-like state (Lopez-Patino et al., 2008). Therefore, zebrafish could potentially be an early model for assessing possible effects of drug withdrawal, using hyperactivity as a measurement.
Visual function
Numerous systemic drugs cause adverse ocular events by affecting the function of the retina or visual pathways, or by causing overt retinal toxicity, including bisphosphonates, antiepileptic drugs, erectile dysfunction agents and tuberculosis treatments (Fraunfelder and Fraunfelder, 2004). The rich blood supply and small volume of the eye make it particularly susceptible to drug-induced adverse effects, many of which occur with chronic dosing and in older patients. Of the candidate drugs that were stopped in development between 1993 and 2006, 6.8% were due to retinal toxicity (Car, 2006). Although this is a small but measurable incidence, ocular toxicity does not lend itself to ‘risk management', so the impact on a development project is potentially serious (Verdugo-Gazdik et al., 2006). Ocular safety is currently assessed at a relatively late stage in the development of a compound and conventional studies, such as the measurement of the electroretinogram in dogs, are technically difficult and labour intensive.
The zebrafish visual system shows utility for the assessment of visual function as the zebrafish retina is very similar to humans (Goldsmith and Harris, 2003). Zebrafish have a cone dense retina and thus, like humans, have rich colour vision, providing an advantage over testing in nocturnal rodents. Visual system development is very rapid in zebrafish embryos to enable feeding and predator avoidance, and therefore the larvae exhibit visually mediated behaviours that can be used in the assessment of vision (Fleisch and Neuhauss, 2006). The earliest quantifiable visual behaviour is the visual startle response, manifest at 68 h.p.f., whereby larvae respond to a sudden decrease in light intensity with a rapid body movement (Kimmel et al., 1974). This behavioural response starts at the time when outer segments of photoreceptors and synaptic ribbons have formed in the retina (Neuhauss, 2003). By 5 d.p.f. the visual system is well developed according to electrophysiological, morphological and behavioural criteria (Bilotta and Saszik, 2001).
To assess visual function in zebrafish, assays such as the optokinetic response and optomotor response (OMR) have previously been developed and take advantage of inherent visual reflexes (Neuhauss et al., 1999). The optokinetic response assay is carried out with larvae immobilized in methylcellulose inside a striped drum, the rotation of which elicits a series of smooth ocular pursuits followed by a rapid saccade as the eyes flick onto the next stripe. Fish with defective visual function show a reduced number of saccades compared with normal fish. The OMR is the locomotor behaviour of an animal induced in response to a repetitive pattern. This response can be elicited in zebrafish by moving horizontal stripes below long transparent channels in which the larvae swim. The larvae perceive that they are being swept downstream and swim to keep a constant position with a stripe and therefore visually normal fish accumulate at one end of the channel. The number of larvae in a ‘pass area' designated at the end of the channel can then be counted and the proportion of fish in the pass area will be reduced in groups with defective visual function. The OMR assay has the capacity to evaluate the effect of compounds on zebrafish vision at a higher throughput; however, compounds that affect the locomotor ability of the zebrafish will appear positive in OMR as hypomotility can decrease the number of larvae scored in the pass area of the chamber. Therefore, the OMR assay may be used as a primary screen, with positive compounds being further evaluated for defects in visual function by secondary screening in optokinetic response.
In a preliminary validation study of the zebrafish OMR assay, six compounds (chloroquine (100 μM), chlorpromazine (10 μM), diazepam (10 μM), nicotine (6.2 μM), ouabain (50 μM) and phenytoin (100 μM)) out of eight were correctly predicted to have effects on visual function, with aspirin correctly identified as a negative control (Richards et al., 2006). A more detailed recent study of the effects of 27 compounds in the OMR assay revealed a good correlation between the effects of compounds in larval zebrafish at 8 d.p.f. with the data available from other in vivo and in vitro models or the clinic: 13 out of 19 positive compounds produced the expected effect, whereas 6 of the 8 negative compounds were correctly predicted. This gave an overall predictability of 70% for adverse effects on visual function (Alderton et al., 2007). These studies suggest that the OMR assay in zebrafish can be useful in predicting the adverse effects of drugs on visual function in man and would support its use as a screen for ‘frontloading' safety pharmacology assessment of this end point in vivo.
Auditory function
Assessment of adverse drug reactions (ADRs) in the auditory system is not a required test prior to first-in-man evaluation. Widely used drugs, such as aminoglycoside antibiotics or platin drugs for cancer treatment often cause permanent hearing loss in man (Rybak and Ramkumar, 2007). However, routine behavioural assessment of the auditory system in animal models can fail to be predictive for ADRs because rodents and other species can lose most of their high-frequency hearing and still be able to respond to predominant ambient noises (Mattsson, 2000).
Despite the clear differences in the general anatomy of the ear, the structure and function of the hair cells are highly conserved between fish and mammals. Zebrafish do not have outer or middle ears, but have a typical vertebrate inner ear with biomineralized structures, the otoliths, that deflect the sensory hair bundles situated beneath them (Fekete, 2003). Hair cells within the otoliths play a crucial role in hearing, and are also found in a related sensory system, the lateral line in zebrafish (Whitfield, 2002). However, unlike humans the hair cells in zebrafish larvae will regenerate after damage and this important difference needs to be taken into account.
A simple approach to screen for auditory defects in zebrafish is to look for abnormal swimming patterns. Adult wild-type fish maintain a normal upright position with respect to their dorsoventral axis and their lateral stripes are rarely seen from above. A hallmark of auditory system mutations is swimming in circles or loops and resting on their side or upside down. The sputnik mutant fish exhibit spontaneous head-forward ventral and lateral looping patterns of swimming (Nicolson et al., 1998). On the other hand, the cosmonaut mutant fish spin in a pinwheel manner around an axis centred near the head (Nicolson et al., 1998). An alternative approach to assess hearing in zebrafish makes use of the acoustically triggered startle response. The startle response is a fast contraction of body muscles caused by a sudden acoustic, tactile or visual stimulus mediated by simple neuronal circuitry (Koch, 1999). Zebrafish larvae from 5 d.p.f. display a characteristic startle response to auditory and visual stimuli that is maintained through to adulthood (Easter and Nicola, 1996; Zeddies and Fay, 2005). Bang et al. (2002) designed an automated, high-throughput behavioural screen to assess hearing in adult zebrafish that relies on the observation of the acoustically triggered startle response. Non-responders were further assessed with radiological analysis for morphological defects of the auditory system (Bang et al., 2002). Moreover, this approach has been applied in larval zebrafish. Groups of 7 d.p.f. larvae were exposed to an acoustic stimulus that gave rise to an acute and quantifiable increase in locomotor activity (Best et al., 2008).
Hair cell integrity is indispensable for hearing and can be assessed through staining with vital dyes. Live hair cells can easily be visualized in vivo in the optically clear embryo by staining with DASPEI (2-(4-(dimethylamino)styryl)-N-ethylpyridiniumiodide) (Harris et al., 2003). Zebrafish larvae have been used to demonstrate lateral line hair cell loss upon treatment with a number of agents, including gentamicin (5 μM), neomycin (10 μM) and cisplatin (50 μM), and otoprotection was reported with glutathione (100 μM) and other antioxidants in cisplatin-treated fish (Harris et al., 2003; Ton and Parng, 2005; Santos et al., 2006).
Thus, zebrafish may show potential as a model to assess auditory function as behavioural assays and hair cell integrity assays have shown that zebrafish are affected by drugs that cause hearing impairment in man.
Gastrointestinal function
Gastrointestinal (GI) ADRs are frequent and include changes in gastric emptying and intestinal transit, changes in acid secretion, irritation of the gastric and intestinal mucosae, and nausea and vomiting. More than half (52%) of ADR-related hospital admissions that result in death are caused by GI bleeding (Pirmohamed et al., 2004). Although assessing the safety of drug development candidates on the GI system is not a regulatory requirement prior to conducting phase I trials, it is suggested as a follow-up study if necessary (FDA, 2001).
Despite the clear differences between the zebrafish and human GI systems, the former species has the potential to be used to assess some basic ADRs in the GI tract. As for other teleosts, the zebrafish does not possess a stomach and the intestine is continuous with the pharynx through a short oesophagus (Wallace and Pack, 2003) and no sphincters are present. However, like mammals, zebrafish have most of the cell types observed in the small intestine—absorptive, endocrine, goblet and interstitial cells of Cajal, although Paneth cells are absent (Wallace et al., 2005; Rich et al., 2007). The lumen is lined with crypt-like structures and peristalsis is achieved through contraction of the inner circular and outer longitudinal layers of smooth muscle and it is regulated by enteric nerves (Pack et al., 1996; Holmberg et al., 2003). However, zebrafish lack a muscularis mucosa and a thin layer of connective tissue separates the epithelium from the inner circular layer of smooth muscle (Wallace et al., 2005).
At 36 h.p.f. the zebrafish gut starts developing, with full patency of the anterior and posterior digestive tract being achieved by 72 and 96 h.p.f., respectively (Pack et al., 1996). The zebrafish digestive tract is colonized by bacteria as early as 4 d.p.f. (Bates et al., 2006) and it occurs within just a few hours after anus patency and the lumen first being open (Rawls et al., 2007). In parallel with the intestine, the liver and pancreas also develop within the first 4 d.p.f. (Pack et al., 1996). Thus, between 4 and 5 d.p.f. the zebrafish digestive tract is morphologically ready for the onset of exogenous feeding, which happens at 5 d.p.f.
Functionally, intestinal contractions start early in development with erratic and spontaneous contraction waves being observed as early as 3 d.p.f., before exogenous feeding starts (Pack et al., 1996; Holmberg et al., 2003). Between 4 and 7 d.p.f. more distinct contraction patterns can be observed. There are both anterograde (projecting towards the anus) and retrograde (projecting towards the mouth) peristaltic contractions along the intestine. In addition, there are local rectal contractions. Because of the transparency of the larvae, contractions can be observed under the microscope and can be quantified in vivo (Holmberg et al., 2003). The presence of functional cholinergic, tachykininergic and pituitary adenylate cyclase-activating polypeptide receptors has been shown, suggesting that intestinal motility in zebrafish is under the control of the enteric nervous system (Holmberg et al., 2004). Furthermore, a direct effect of Nω-nitro-L-arginine methyl ester (L-NAME) on zebrafish intestinal peristalsis indicates that an endogenous nitrergic tone is present in the larval gut (Holmberg et al., 2006). In a study of the effects of 10 compounds on gut motility in zebrafish larvae at 7 d.p.f., 8 of the 10 compounds tested showed the expected decrease in the number of contractions. Furthermore, two smooth muscle relaxants, isoprenaline and chlorpromazine, caused the intestinal lumen to expand (Berghmans et al., 2008).
These data suggest a positive correlation between the effects of a limited number of agents in zebrafish and the data available from other in vivo and in vitro models or man (Table 2), although more validation with a much wider range of pharmacological agents is required before zebrafish could be considered a useful and predictive model for assessing the effects of compounds on intestinal motility.
Table 2.
The effects of agents on larval zebrafish gut contraction frequency
| Agent | Concentration | Effects on zebrafish gut contraction frequency | Reference |
|---|---|---|---|
| Atropine | 1 μM | Decrease | Holmberg et al. (2004) |
| Acetylcholine | 10 μM | Increase | |
| PACAP-27 | 1 μM | Decrease | |
| Neurokinin A | 1 μM | Increase | |
| L-NAME | 1 mM | Increase | Holmberg et al. (2006) |
| Sodium nitroprusside | 100 μM | Decrease | |
| Chlorpromazine | 100 μM | Decrease | Berghmans et al. (2008) |
| Isoprenaline | 1 mM | Decrease | |
| Verapamil | 1 mM | Decrease | |
Abbreviations: L-NAME, Nω-nitro-L-arginine methyl ester; PACAP, pituitary adenylate cyclase-activating polypeptide.
The ease of observing and quantifying gut contractions in zebrafish means that they could be applied to assess emetic liability, one of the most commonly reported clinical ADR. From an evolutionary point of view, emesis is a body's defensive response to toxins accidentally ingested with food (Andrews and Horn, 2006). Many mammalian species, including rat, mouse, hamster, guinea-pig and rabbit, do not have a vomiting reflex in contrast to the ferret and dog, which are the most commonly used models for detecting the emetic potential of compounds (Andrews and Horn, 2006). Thus, the paucity of animal models highlights the need to find alternative models to assess emetic liability. Because of the absence of stomach, it would not be possible to study vomiting in zebrafish larvae per se; however, treatment with emetine (300 μM) causes pronounced increase in the frequency of retrograde movements in the gut (R Barrett, unpublished data and personal communication). These forceful contractions towards the mouth as opposed to the anterograde gut contraction, which characterize normal peristalsis, may be a useful surrogate for modelling emesis if further validation is successful. It would be necessary to investigate a substantial number of known emetic agents of varying pharmacological classes to indicate whether or not this experimental system has any utility.
Renal function
The kidney is the most irrigated organ in the human body, hence it is highly exposed to circulating drugs. Additionally, its function regulates water and salt homoeostasis, concentrating urine, so the drug concentration inside the kidney may be much higher than in the plasma. Damage to the kidneys can result in loss of function, acute renal failure and consequent retention of waste products. Glomerular filtration rate (GFR) is the best parameter to estimate renal function, and inulin clearance is the gold standard for its measurement, although creatine clearance can also be used. Clearance of a solute such as inulin, which is freely filterable at the glomerulus and is neither secreted nor reabsorbed in the tubules, must equate to GFR (Gad, 2004). Renal function impairment can be caused by several different classes of drugs, such as antibiotics, immunosuppressants and chemotherapeutic agents (Evenepoel, 2004; Taber and Mueller, 2006; Martinez-Salgado et al., 2007; Yao et al., 2007). Out of all ADR-related hospital admissions, 18% result in death due to renal failure (Pirmohamed et al., 2004). Moreover, acute renal failure in patients admitted to intensive care units is associated with high mortality (Evenepoel, 2004; Taber and Mueller, 2006). Thus, it is relevant to evaluate the effect of pharmacological agents on renal function and this is suggested as a follow-up study (FDA, 2001).
The teleost pronephros is a simple organ composed of a pair of nephrons with two glomeruli fused at the midline, pronephric tubules connecting directly to the glomeruli through a neck segment and paired bilateral pronephric ducts that direct the blood filtrate outside the animal. By 40 h.p.f the pronephros begins blood filtration (Drummond et al., 1998). The glomerulus, although simple in form, shares many of the cell types with higher vertebrate kidneys, including fenestrated capillary endothelial cells, podocytes and polarized tubular epithelial cells (Drummond et al., 1998).
Despite the simple renal system, the functional phenotype caused in the mammalian kidney by gentamicin is also observed in the zebrafish pronephros. Gentamicin is an aminoglycoside antibiotic that is nephrotoxic in mammals. Injection of gentamicin (10 mg mL−1) at 50–54 h.p.f. into the cardiac venous sinus causes several morphological changes to the fish: pericardial and endocranial oedema, accumulation of debris in the tubular lumen, tubular and glomerular distension, and renal tubular casts are extruded by the cloacae. Furthermore, there is peritubular accumulation of leukocytes with occasional infiltration into the glomerulus (Hentschel et al., 2005). This inflammatory response is an important component of acute renal failure in mammals (Kelly et al., 1996). The changes in glomerular and tubular morphology suggest impaired filtration. To evaluate this, the authors injected dextran and inulin into the fish and quantified clearance through changes in fluorescence over the heart area (Drummond et al., 1998; Hentschel et al., 2005). Gentamicin treatment produced a reduction in clearance of 75 and 67% for dextran and inulin, respectively. Treatment with cisplatin (1.5 mg mL−1), another nephrotoxic agent, also showed changes in GFR through reduction in clearance. Thus, despite the differences between simple pronephros of the zebrafish and the complex human kidney, the response to renal injury is conserved. Determination of clearance was further refined and the measurement region was shifted from the heart to the retina. The zebrafish pupil offers a better reflection of the amount of fluorescent marker delivered into the vascular system as it is far enough from the injection site that the readings are not confounded by the marker remaining there. Furthermore, the increase in GFR seen in the puromycin-induced human minimal change disease rat model could be replicated in zebrafish (Hentschel et al., 2007).
These literature reports show that the parameters required to evaluate the effects of drugs on zebrafish renal function have been identified: (i) the morphological changes, (ii) the inflammatory response and (iii) measurement of renal clearance. These combined with the simplicity of the fish pronephros and the ease to assess the renal system may qualify zebrafish as an early in vivo model to evaluate the renal liability potential of lead compounds.
Bone density
A study carried out in Switzerland in 2000 suggested musculoskeletal ADRs are the sixth major cause of ADR-related hospital admissions. Fractures due to steroid-induced osteoporosis were one of the main causes (Fattinger et al., 2000). Osteoporosis is developed by 30–50% of patients treated chronically with glucocorticoids (Gulko and Mulloy, 1996) and aromatase inhibitor therapy for hormone receptor-positive breast cancer can also cause reduction in bone mineral density (Gnant, 2006).
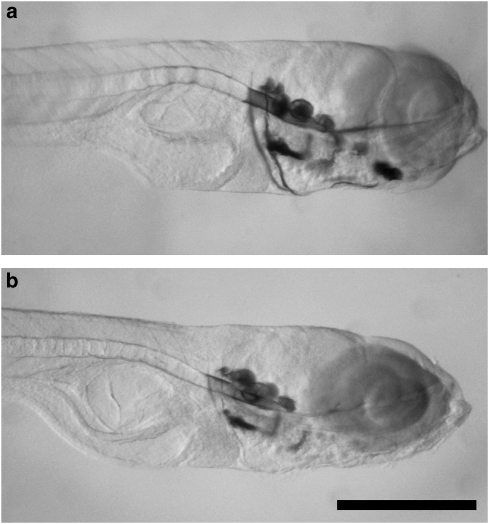
Zebrafish and mammalian bones are similar, with both intramembranous and endochondral ossification present in the craniofacial skeleton (Cubbage and Mabee, 1996; Fleming et al., 2004). The bones are vascularized, innervated and contain cavities filled with adipose tissue (Witten et al., 2001). Both osteoblasts and osteoclasts have been identified in zebrafish, with the former possessing active matrix formation and mineralization properties (Inohaya et al., 2007) and the latter being responsible for bone resorption (Witten et al., 2001). A high-throughput in vivo screening assay for compounds that cause changes to bone density has been developed (Fleming et al., 2005), taking advantage of the zebrafish larvae's small size and fast development. The larvae were treated from 5 to 10 d.p.f. in a 96-well plate followed by Alizarin red stains for mineralized bone that can then be quantified (Figure 4). Treatment with prednisolone (25 μM) in the media in which the larvae swim was shown to reduce mineralized area by 50% (Barrett et al., 2006). Such an assay could be used to screen any class of compounds that might be suspected to have an effect on bone demineralization.
Figure 4.
(a) Zebrafish larva at 10 d.p.f. has many bones of the head skeleton mineralized and these are stained with Alizarin red. B. Larva exposed to 25 μM prednisolone from 5 to 10 d.p.f. shows a marked reduction in the stained mineralized tissue. Quantification of the staining showed a 50% reduction in the mineralized area with steroid treatment (Barrett et al. (2006). Copyright Wiley-VCH Verlag GmbH & Co. KGaA (reproduced with permission)). Bar, 0.4 mm.
Concluding remarks
Early identification of unacceptable safety liabilities would be a major advancement to accelerate the drug discovery and development process and curb its incessantly increasing costs. The adoption of zebrafish models for screening compounds for selected off-target effects is supported by the organism's high biological relevance to mammals and from continuously emerging pharmacological data obtained for validation purposes. With adequate validation, high-throughput zebrafish assays could be used to rapidly test tens to hundreds of research compounds and should be able to provide early in vivo analysis in the hit to lead and lead optimization phases, thus enabling better decision-making in these critical stages of the drug discovery process. At the present time, experience with zebrafish safety pharmacology models is too limited to warrant the use of data generated for regulatory purposes. However, if the use of zebrafish permits the selection of compounds with an improved safety profile early on the drug discovery phase, then they will have a useful place in the screening cascade to maximize the detection of serious safety risks.
For obvious reasons, zebrafish cannot be used to assess the respiratory system, but this review suggests that the model can be applied for the evaluation of the effect of compounds on both cardiac and central nervous functions. Additionally, studies could also be performed in zebrafish with assays available for investigating off-target effects on GI function, auditory and visual functions, as well as convulsant or proconvulsant potential, and finally bone mineralization. The potential role of zebrafish in the early identification of possible off-target effects is not limited to these assays as a wider range of liability screens could be developed, examples of which are the renal function, drug dependency and abuse potential, and cognitive impairment.
The main issue with the use of zebrafish in assessing potential safety liabilities is the lack of extensive validation with diverse and comprehensive pharmacological compound sets to adequately understand its advantages and limitations in relation to its translation and predictability to humans. To gain acceptability for a particular biological system in zebrafish, it is essential that pharmacological responses are sufficiently conserved between man and zebrafish. To date four studies have been published in validation of the cardiac, visual function and convulsant assays (summarized in Table 3) and show predictability of 70% or greater compared with known effects in humans/rodent studies. However, more detailed studies for these and the other systems are needed.
Table 3.
Validation studies published to date on larval zebrafish assays for assessing potential safety liabilities
| Assay | End point | No. of compounds | Predictability (%) | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|---|
| Cardiac function | 2:1 atrial/ventricular ratio | 100 | 77 | 78 | 81 | Milan et al. (2003) |
| Cardiac function | 2:1 atrial/ventricular ratio | 18 | 90 | 80 | 100 | Mittelstadt et al. (2008) |
| Visual function | Optomotor response | 27 | 70 | 68 | 75 | Alderton et al. (2007) |
| Convulsant activity | Locomotor activity | 25 | 72 | 63 | 77 | Winter et al. (2008) |
A drawback of zebrafish assays is that uptake of compounds into the zebrafish larvae can vary and they should be measured for accurate interpretation of results, thereby avoiding false negatives and to enable ranking of compounds within a chemical series. However, bioanalysis is only possible on whole larval zebrafish and therefore specific tissue concentrations cannot be compared with mammalian tissue concentrations. In an liquid chromatography tandem mass spectrometry (LC-MS/MS) study of the uptake by zebrafish of nine compounds, there was a correlation between CLogP and the amount of compound penetrating the zebrafish, with less polar compounds with CLogP values under 3.8 showing lowest penetration (Berghmans et al., 2008). However, this correlation with logP is not clear cut and there are exceptions (S Berghmans, unpublished data), possibly due to active uptake mechanisms. Limited uptake of compounds in the zebrafish may result in a lack of sensitivity of the models and consequently, problems could be encountered when screening compounds with modest solubility. This issue of varying uptake into the zebrafish should be taken into account, particularly when validating a particular biological system with a definitive set of pharmacological reference compounds to ascertain the predictability and sensitivity of each assay.
Another limitation of the zebrafish system is that the blood–brain barrier of the larval zebrafish is not fully formed until 10 d.p.f. Therefore, the assessment of CNS-mediated effects in larvae may erroneously identify compounds that are excluded from the brain in older fish and in mammals.
Finally, the larval zebrafish is a rapidly developing system, which may not be appropriate for modelling the effects of compounds on adults, and this difference needs to be taken into account particularly during the validation phases of assay development.
Zebrafish offer interesting possibilities in emerging areas of potential safety liability assessment that cannot be easily addressed with current rodent models. For example, drug–drug interactions arising from the ever-increasing use of drug combinations in clinical use could be tackled in vivo relatively easily in zebrafish. The rapidly growing safety pharmacogenomics challenge could also be investigated using zebrafish as its genome has been sequenced and will be fully annotated in the near future. Transgenic zebrafish could be prepared to represent genetic variants/polymorphisms found in humans with a view to identifying rare ADRs.
Bearing in mind the caveats outlined above, the wealth of information that can be garnered from the larval zebrafish to evaluate the safety liabilities of compounds in early lead optimization makes this an exciting new tool to aid and advance drug discovery. This should permit earlier informed decisions on prioritization of lead series saving both time and money and consequently reducing attrition rate.
Acknowledgments
We extend our thanks to Dr Icilio Cavero for his independent, constructive advice and criticism in the preparation of this paper. Thanks also to Euan Harris for his help with artwork.
Abbreviations
- ADR
adverse drug reaction
- d.p.f.
days post-fertilization
- EMEA, European Medicines Agency; GFR
glomerular filtration rate
- GI
gastrointestinal
- h.p.f.
hours post-fertilization
- hERG
human ether-a-go-go-related gene
- ICH, International Conference on Harmonisation; OMR
optomotor response
- PTZ
pentylenetetrazol
Conflict of interest
The authors are employees of Summit plc., and are share and/or options holders in Summit plc.
References
- Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–664. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Kimber GM, Richards FR, Strang I, Redfern WS, Winter MJ, et al. Validation of an optomotor method for assessment of visual function in zebrafish larvae (poster) Safety Pharmacology Society Annual Meeting 2007Edinburgh, UK; 20–21.September 2007 [Google Scholar]
- Alestrom P, Holter JL, Nourizadeh-Lillabadi R. Zebrafish in functional genomics and aquatic biomedicine. Trends Biotechnol. 2006;24:15–21. doi: 10.1016/j.tibtech.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Horn CC. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Arthur D, Levin ED. Spatial and non-spatial visual discrimination learning in zebrafish (Danio rerio) Anim Cogn. 2001;4:125–131. [Google Scholar]
- Baker K, Warren KS, Yellen G, Fishman MC. Defective ‘pacemaker' current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci USA. 1997;94:4554–4559. doi: 10.1073/pnas.94.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang PI, Yelick PC, Malicki JJ, Sewell WF. High-throughput behavioral screening method for detecting auditory response defects in zebrafish. J Neurosci Methods. 2002;118:177–187. doi: 10.1016/s0165-0270(02)00118-8. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–768. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Barrett R, Chappell C, Quick M, Fleming A. A rapid, high content, in vivo model of glucocorticoid-induced osteoporosis. Biotechnol J. 2006;1:651–655. doi: 10.1002/biot.200600043. [DOI] [PubMed] [Google Scholar]
- Bass AS, Tomaselli G, Bullingham R, III, Kinter LB. Drugs effects on ventricular repolarization: a critical evaluation of the strengths and weaknesses of current methodologies and regulatory practices. J Pharmacol Toxicol Methods. 2005;52:12–21. doi: 10.1016/j.vascn.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 2006;297:374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Berghmans S. IIR's 4th Annual QT Prolongation and Safety Pharmacology Conference: Predicative Safety Assessment in Non-Clinical and Clinical Studies. Amsterdam, The Netherlands; 2006. Zebrafish: validation of a pro-arrhythmia in vivo model (abstract for oral communication) [Google Scholar]
- Berghmans S, Butler B, Goldsmith P, Waldron G, Gardner I, Golder Z, et al. Early drug safety assessment for the cardiac, visual and gut functions using the zebrafish J Pharmacol Toxicol Methods 2008(in press) [DOI] [PubMed]
- Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 2007;75:18–28. doi: 10.1016/j.eplepsyres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Best JD, Berghmans S, Hunt JJ, Clarke SC, Fleming A, Goldsmith P, et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Saszik S. The zebrafish as a model visual system. Int J Dev Neurosci. 2001;19:621–629. doi: 10.1016/s0736-5748(01)00050-8. [DOI] [PubMed] [Google Scholar]
- Boehmler W, Carr T, Thisse C, Thisse B, Canfield VA, Levenson R. D4 dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behaviour. Genes Brain Behav. 2007;6:155–166. doi: 10.1111/j.1601-183X.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Bretaud S, Lee S, Guo S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson's disease. Neurotoxicol Teratol. 2004;26:857–864. doi: 10.1016/j.ntt.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Bretaud S, Li Q, Lockwood BL, Kobayashi K, Lin E, Guo S. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- Cahill GM. Clock mechanisms in zebrafish. Cell Tissue Res. 2002;309:27–34. doi: 10.1007/s00441-002-0570-7. [DOI] [PubMed] [Google Scholar]
- Car B. Enabling technologies in reducing attrition due to safety failures. Am Drug Discov. 2006;1:31–34. [Google Scholar]
- Carvan MJ, III, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicol Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Raymond MP, Ferreira L, Escudero H. Visual discrimination learning in zebrafish (Danio rerio) Behav Processes. 2005;70:19–31. doi: 10.1016/j.beproc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Cordes JS, Sun Z, Lloyd DB, Bradley JA, Opsahl AC, Tengowski MW, et al. Pentamidine reduces hERG expression to prolong the QT interval. Br J Pharmacol. 2005;145:15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubbage CC, Mabee PM. Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, cyprinidae) J Morphol. 1996;229:121–160. doi: 10.1002/(SICI)1097-4687(199608)229:2<121::AID-JMOR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci USA. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JL, Zon LI. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet. 2005;39:481–501. doi: 10.1146/annurev.genet.39.073003.095931. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Brustein E. Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- Easter A, Sharp TH, Valentin JP, Pollard CE. Pharmacological validation of a semi-automated in vitro hippocampal brain slice assay for assessment of seizure liability. J Pharmacol Toxicol Methods. 2007;56:223–233. doi: 10.1016/j.vascn.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Nicola GN. The development of vision in the zebrafish (Danio rerio) Dev Biol. 1996;180:646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- Eckhardt LL, Haverkamp W, Borggrefe M. Experimental models of torsades de pointes. Cardiovasc Res. 1998;39:178–193. doi: 10.1016/s0008-6363(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Eckhardt LL, Rajamani S, January CT. Protein trafficking abnormalities: a new mechanism in drug-induced long QT syndrome. Br J Pharmacol. 2005;145:3–4. doi: 10.1038/sj.bjp.0706143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMEA Points to consider: the assessment for the potential for QT interval prolongation by non-cardiovascular medicial products (CPMP/986/96) 1997.
- EMEA Guideline on the non-clinical investigation of the dependence potential of medical products. 2006.
- Evenepoel P. Acute toxic renal failure. Best Pract Res Clin Anaesthesiol. 2004;18:37–52. doi: 10.1016/j.bpa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Fattinger K, Roos M, Vergeres P, Holenstein C, Kind B, Masche U, et al. Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol. 2000;49:158–167. doi: 10.1046/j.1365-2125.2000.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA ICH Guidance for Industry: S7A Safety Pharmacology Studies for Human Pharmaceutical: U.S. Department of Health and Human Services. 2001.
- FDA ICH Guidance for Industry: S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals: U.S. Department of Health and Human Services. 2005. [PubMed]
- Fekete DM. Developmental biology. Rocks that roll zebrafish. Science. 2003;302:241–242. doi: 10.1126/science.1091171. [DOI] [PubMed] [Google Scholar]
- Fleisch VC, Neuhauss SC. Visual behavior in zebrafish. Zebrafish. 2006;3:191–201. doi: 10.1089/zeb.2006.3.191. [DOI] [PubMed] [Google Scholar]
- Fleming A, Keynes R, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131:873–880. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- Fleming A, Sato M, Goldsmith P. High-throughput in vivo screening for bone anabolic compounds with zebrafish. J Biomol Screen. 2005;10:823–831. doi: 10.1177/1087057105279952. [DOI] [PubMed] [Google Scholar]
- Forouhar AS, Hove JR, Calvert C, Flores J, Jadvar H, Gharib M. Electrocardiographic characterization of embryonic zebrafish. Conf Proc IEEE Eng Med Biol Soc. 2004;5:3615–3617. doi: 10.1109/IEMBS.2004.1404016. [DOI] [PubMed] [Google Scholar]
- Fraunfelder FW, Fraunfelder FT. Adverse ocular drug reactions recently identified by the National Registry of Drug-Induced Ocular Side Effects. Ophthalmology. 2004;111:1275–1279. doi: 10.1016/j.ophtha.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Gad SC. Safety Pharmacology in Pharmaceutical Development and Approval. CRC Press LLC: Boca Raton; 2004. pp. 95–107. [Google Scholar]
- Gnant M. Management of bone loss induced by aromatase inhibitors. Cancer Invest. 2006;24:328–330. doi: 10.1080/07357900600633759. [DOI] [PubMed] [Google Scholar]
- Goldsmith P. Zebrafish as a pharmacological tool: the how, why and when. Curr Opin Pharmacol. 2004;4:504–512. doi: 10.1016/j.coph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Goldsmith P, Fleming A. Screening methods employing zebrafish and the blood brain barrier. Granted patent EP1644733. 2007.
- Goldsmith P, Harris WA. The zebrafish as a tool for understanding the biology of visual disorders. Semin Cell Dev Biol. 2003;14:11–18. doi: 10.1016/s1084-9521(02)00167-2. [DOI] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Gulko PS, Mulloy AL. Glucocorticoid-induced osteoporosis: pathogenesis, prevention and treatment. Clin Exp Rheumatol. 1996;14:199–206. [PubMed] [Google Scholar]
- Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish. Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, et al. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol. 2007;293:F1746–F1750. doi: 10.1152/ajprenal.00009.2007. [DOI] [PubMed] [Google Scholar]
- Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923–F929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Holmberg A, Olsson C, Holmgren S. The effects of endogenous and exogenous nitric oxide on gut motility in zebrafish Danio rerio embryos and larvae. J Exp Biol. 2006;209:2472–2479. doi: 10.1242/jeb.02272. [DOI] [PubMed] [Google Scholar]
- Holmberg A, Schwerte T, Fritsche R, Pelster B, Holmgren S. Ontogeny of intestinal motility in correlation to neuronal development in zebrafish embryos and larvae. J Fish Biol. 2003;63:318–331. [Google Scholar]
- Holmberg A, Schwerte T, Pelster B, Holmgren S. Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J Exp Biol. 2004;207:4085–4094. doi: 10.1242/jeb.01260. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Inohaya K, Takano Y, Kudo A. The teleost intervertebral region acts as a growth center of the centrum: in vivo visualization of osteoblasts and their progenitors in transgenic fish. Dev Dyn. 2007;236:3031–3046. doi: 10.1002/dvdy.21329. [DOI] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- Jacob E, Drexel M, Schwerte T, Pelster B. Influence of hypoxia and of hypoxemia on the development of cardiac activity in zebrafish larvae. Am J Physiol Regul Integr Comp Physiol. 2002;283:R911–R917. doi: 10.1152/ajpregu.00673.2001. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Patterson J, Kimmel RO. The development and behavioral characteristics of the startle response in the zebra fish. Dev Psychobiol. 1974;7:47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Langheinrich U, Vacun G, Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol. 2003;193:370–382. doi: 10.1016/j.taap.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology (Berl) 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacol Biochem Behav. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Patino MA, Yu L, Cabral H, Zhdanova IV. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol Behav. 2008;93:160–171. doi: 10.1016/j.physbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem Biol. 2003;10:901–908. doi: 10.1016/j.chembiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Mattsson JL. Ototoxicity: an argument for evaluation of the cochlea in safety testing in animals. Toxicol Pathol. 2000;28:137–141. doi: 10.1177/019262330002800117. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol. 2006;291:H269–H273. doi: 10.1152/ajpheart.00960.2005. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- Mittelstadt SW, Hemenway CL, Craig MP, Hove JR. Evaluation of zebrafish embryos as a model for assessing inhibition of hERG. J Pharmacol Toxicol Methods. 2008;57:100–105. doi: 10.1016/j.vascn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Murphey RD, Zon LI. Small molecule screening in the zebrafish. Methods. 2006;39:255–261. doi: 10.1016/j.ymeth.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Neuhauss SC. Behavioral genetic approaches to visual system development and function in zebrafish. J Neurobiol. 2003;54:148–160. doi: 10.1002/neu.10165. [DOI] [PubMed] [Google Scholar]
- Neuhauss SC, Biehlmaier O, Seeliger MW, Das T, Kohler K, Harris WA, et al. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J Neurosci. 1999;19:8603–8615. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson T, Rusch A, Friedrich RW, Granato M, Ruppersberg JP, Nusslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Folchert A, Makhankov YV, Neuhauss SC, Sillaber I, Straehle U, et al. Genetic identification of AChE as a positive modulator of addiction to the psychostimulant D-amphetamine in zebrafish. J Neurobiol. 2006;66:463–475. doi: 10.1002/neu.20231. [DOI] [PubMed] [Google Scholar]
- Orger MB, Gahtan E, Muto A, Page-McCaw P, Smear MC, Baier H. Behavioral screening assays in zebrafish. Methods Cell Biol. 2004;77:53–68. doi: 10.1016/s0091-679x(04)77003-x. [DOI] [PubMed] [Google Scholar]
- Pack M, Solnica-Krezel L, Malicki J, Neuhauss SC, Schier AF, Stemple DL, et al. Mutations affecting development of zebrafish digestive organs. Development. 1996;123:321–328. doi: 10.1242/dev.123.1.321. [DOI] [PubMed] [Google Scholar]
- Parng C. In vivo zebrafish assays for toxicity testing. Curr Opin Drug Discov Devel. 2005;8:100–106. [PubMed] [Google Scholar]
- Parng C, Roy NM, Ton C, Lin Y, McGrath P. Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods. 2007;55:103–112. doi: 10.1016/j.vascn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Peitsaro N, Kaslin J, Anichtchik OV, Panula P. Modulation of the histaminergic system and behaviour by alpha-fluoromethylhistidine in zebrafish. J Neurochem. 2003;86:432–441. doi: 10.1046/j.1471-4159.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Case CL, Gillette PC. Atrioventricular block in a newborn with acquired long QT syndrome. Cardiol Young. 2001;11:680–682. doi: 10.1017/s1047951101001111. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Lemaire M, Durmuller N, Roux S. New perspectives in CNS safety pharmacology. Fundam Clin Pharmacol. 2002;16:197–207. doi: 10.1046/j.1472-8206.2002.00061.x. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, et al. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 2000;10:1890–1902. doi: 10.1101/gr.164800. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Mahowald MA, Goodman AL, Trent CM, Gordon JI. In vivo imaging and genetic analysis link bacterial motility and symbiosis in the zebrafish gut. Proc Natl Acad Sci USA. 2007;104:7622–7627. doi: 10.1073/pnas.0702386104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A, Leddon SA, Hess SL, Gibbons SJ, Miller S, Xu X, et al. Kit-like immunoreactivity in the zebrafish gastrointestinal tract reveals putative ICC. Dev Dyn. 2007;236:903–911. doi: 10.1002/dvdy.21086. [DOI] [PubMed] [Google Scholar]
- Richards FM, Kimber G, Butler P, Waldron G, Knight T, Goldsmith P, et al. Prediction of ophthalmic effects in man by screening drugs for the optokinetic and optomotor responses in zebrafish Invest Ophthalmol Vis Sci 200647ARVO E-Abstract 2622, page 123 [Google Scholar]
- Rink E, Wullimann MF. Connections of the ventral telencephalon (subpallium) in the zebrafish (Danio rerio) Brain Res. 2004;1011:206–220. doi: 10.1016/j.brainres.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Rombough P. Gills are needed for ionoregulation before they are needed for O(2) uptake in developing zebrafish, Danio rerio. J Exp Biol. 2002;205:1787–1794. doi: 10.1242/jeb.205.12.1787. [DOI] [PubMed] [Google Scholar]
- Rubinstein AL. Zebrafish assays for drug toxicity screening. Expert Opin Drug Metab Toxicol. 2006;2:231–240. doi: 10.1517/17425255.2.2.231. [DOI] [PubMed] [Google Scholar]
- Rundfeldt C, Wlaz P, Honack D, Loscher W. Anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. Comparison of diazepam, bretazenil and abecarnil. J Pharmacol Exp Ther. 1995;275:693–702. [PubMed] [Google Scholar]
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Schwerte T, Pelster B. Digital motion analysis as a tool for analysing the shape and performance of the circulatory system in transparent animals. J Exp Biol. 2000;203:1659–1669. doi: 10.1242/jeb.203.11.1659. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Stainier DY. A window to the heart: can zebrafish mutants help us understand heart disease in humans. Trends Genet. 2002;18:491–494. doi: 10.1016/s0168-9525(02)02766-x. [DOI] [PubMed] [Google Scholar]
- Shah RR. Pharmacogenetic aspects of drug-induced torsade de pointes: potential tool for improving clinical drug development and prescribing. Drug Saf. 2004;27:145–172. doi: 10.2165/00002018-200427030-00001. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Suter W. Predictive value of in vitro safety studies. Curr Opin Chem Biol. 2006;10:362–366. doi: 10.1016/j.cbpa.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26:725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Taber SS, Mueller BA.Drug-associated renal dysfunction Crit Care Clin 200622357–374.viii [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS. Embryonic exposure to domoic acid increases the susceptibility of zebrafish larvae to the chemical convulsant pentylenetetrazole. Environ Health Perspect. 2007;115:1547–1552. doi: 10.1289/ehp.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005;208:79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism. Genes Brain Behav. 2003;2:268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Verdugo-Gazdik ME, Simic D, Opsahl AC, Tengowski MW. Investigating cytoskeletal alterations as a potential marker of retinal and lens drug-related toxicity. Assay Drug Dev Technol. 2006;4:695–707. doi: 10.1089/adt.2006.4.695. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255:12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Warner CH, Bobo W, Warner C, Reid S, Rachal J. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74:449–456. [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: a Guide for the Laboratory use of Zebrafish (Danio rerio) 1995University of Oregon Press: Eugene; 3rd edn. [Google Scholar]
- Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol. 2002;53:157–171. doi: 10.1002/neu.10123. [DOI] [PubMed] [Google Scholar]
- Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behav Processes. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin JP, Hutchinson TH. Validation of a larval zebrafish locomotor assay for assessing the seizure liability of early-stage development drugs. J Pharmacol Toxicol Methods. 2008;57:176–187. doi: 10.1016/j.vascn.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Witten PE, Hansen A, Hall BK. Features of mono- and multinucleated bone resorbing cells of the zebrafish Danio rerio and their contribution to skeletal development, remodeling, and growth. J Morphol. 2001;250:197–207. doi: 10.1002/jmor.1065. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. J Comp Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Xu X, Scott-Scheiern T, Kempker L, Simons K. Active avoidance conditioning in zebrafish (Danio rerio) Neurobiol Learn Mem. 2007;87:72–77. doi: 10.1016/j.nlm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Yang T, Snyders D, Roden DM. Drug block of I(kr): model systems and relevance to human arrhythmias. J Cardiovasc Pharmacol. 2001;38:737–744. doi: 10.1097/00005344-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- Zeddies DG, Fay RR. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J Exp Biol. 2005;208:1363–1372. doi: 10.1242/jeb.01534. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Wang SY, Leclair OU, Danilova NP. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]