Abstract
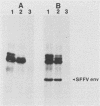
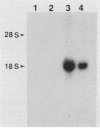
Erythroid cells from mice infected with the polycythemia-inducing strain of Friend spleen focus-forming virus (SFFVP), unlike normal erythroid cells, can proliferate and differentiate in apparent absence of the erythroid hormone erythropoietin (Epo). The unique envelope glycoprotein encoded by SFFV has been shown to be responsible for this biological effect. The recent isolation of an Epo-dependent erythroleukemia cell line, HCD-57, derived from a mouse infected at birth with Friend murine leukemia virus, afforded us the opportunity to study the direct effect of SFFVP on a homogeneous population of factor-dependent cells. The introduction of SFFVP in complex with various helper viruses into these Epo-dependent cells efficiently and reproducibly gave rise to lines which expressed high levels of SFFV and were factor independent. SFFV appears to be unique in its ability to abrogate the factor dependence of Epo-dependent HCD-57 cells, since infection of these cells with retroviruses carrying a variety of different oncogenes had no effect. The induction of Epo independence by SFFV does not appear to involve a classical autocrine mechanism, since there is no evidence that the factor-independent cells synthesize or secrete Epo or depend on it for their growth. However, the SFFV-infected, factor-independent cells had significantly fewer receptors available for binding Epo than their factor-dependent counterparts had, raising the possibility that the induction of factor independence by the virus may be due to the interaction of an SFFV-encoded protein with the Epo receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Scolnick E. M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983 May;46(2):594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Chung S. W., Wolff L., Ruscetti S. K. Transmembrane domain of the envelope gene of a polycythemia-inducing retrovirus determines erythropoietin-independent growth. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7957–7960. doi: 10.1073/pnas.86.20.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. W., Wolff L., Ruscetti S. Sequences responsible for the altered erythropoietin responsiveness in spleen focus-forming virus strain SFFVP-infected cells are localized to a 678-base-pair region at the 3' end of the envelope gene. J Virol. 1987 May;61(5):1661–1664. doi: 10.1128/jvi.61.5.1661-1664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Pierce J. H., Kraus M. H., Di Fiore P. P., Pennington C. Y., Aaronson S. A. Mammalian cell transformation by a murine retrovirus vector containing the avian erythroblastosis virus erbB gene. J Virol. 1986 Oct;60(1):19–28. doi: 10.1128/jvi.60.1.19-28.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. D., Kaminchik J. Modification of erythropoiesis and hormone sensitivity by RNA tumor viruses. Prog Clin Biol Res. 1984;148:141–152. [PubMed] [Google Scholar]
- Klinken S. P. Erythroproliferation in vitro can be induced by abl, fes, src, ras, bas, raf, raf/myc, erb B and cbl oncogenes but not by myc, myb and fos. Oncogene Res. 1988 Sep;3(2):187–192. [PubMed] [Google Scholar]
- Langdon W. Y., Hartley J. W., Klinken S. P., Ruscetti S. K., Morse H. C., 3rd v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Tavitian A., Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988 Jan 21;331(6153):277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Fredrickson T. N., Holmes K. L., Morse H. C., 3rd, Jansen H. W., Patschinsky T., Bister K. Rapid induction of hemopoietic neoplasms in newborn mice by a raf(mil)/myc recombinant murine retrovirus. J Virol. 1985 Jul;55(1):23–33. doi: 10.1128/jvi.55.1.23-33.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. M., Bader J. P., Bassin R. H. Inhibitors of glycosylation reverse retroviral interference. Virology. 1982 May;119(1):185–192. doi: 10.1016/0042-6822(82)90075-7. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K. Employment of a [3H]thymidine-incorporation assay to distinguish the effects of different Friend erythroleukemia-inducing retroviruses on erythroid cell proliferation. J Natl Cancer Inst. 1986 Jul;77(1):241–245. [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S. K., Ruscetti F. W. Apparent Epo-independence of erythroid cells infected with the polycythemia-inducing strain of Friend spleen focus-forming virus is not due to Epo production or change in number or affinity of Epo receptors. Leukemia. 1989 Oct;3(10):703–707. [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Spleen focus-forming virus: relationship of an altered envelope gene to the development of a rapid erythroleukemia. Curr Top Microbiol Immunol. 1984;112:21–44. doi: 10.1007/978-3-642-69677-0_2. [DOI] [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Goldwasser E. Binding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cells. J Biol Chem. 1987 Apr 25;262(12):5554–5562. [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Sawada K. Receptors for erythropoietin in mouse and human erythroid cells and placenta. Blood. 1989 Jul;74(1):103–109. [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scholnick E. M. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979 Jul 15;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- Shoemaker C. B., Mitsock L. D. Murine erythropoietin gene: cloning, expression, and human gene homology. Mol Cell Biol. 1986 Mar;6(3):849–858. doi: 10.1128/mcb.6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro C., Gliniak B., Kabat D. A tagged helper-free Friend virus causes clonal erythroblast immortality by specific proviral integration in the cellular genome. J Virol. 1988 Nov;62(11):4129–4135. doi: 10.1128/jvi.62.11.4129-4135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambourin P., Casadevall N., Choppin J., Lacombe C., Heard J. M., Fichelson S., Wendling F., Varet B. Production of erythropoietin-like activity by a murine erythroleukemia cell line. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6269–6273. doi: 10.1073/pnas.80.20.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E. F., Rettenmier C. W., Look A. T., Sherr C. J. The v-fms oncogene induces factor independence and tumorigenicity in CSF-1 dependent macrophage cell line. 1986 Nov 27-Dec 3Nature. 324(6095):377–380. doi: 10.1038/324377a0. [DOI] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985 Jun 28;228(4707):1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. The spleen focus-forming virus (SFFV) envelope gene, when introduced into mice in the absence of other SFFV genes, induces acute erythroleukemia. J Virol. 1988 Jun;62(6):2158–2163. doi: 10.1128/jvi.62.6.2158-2163.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]