Abstract
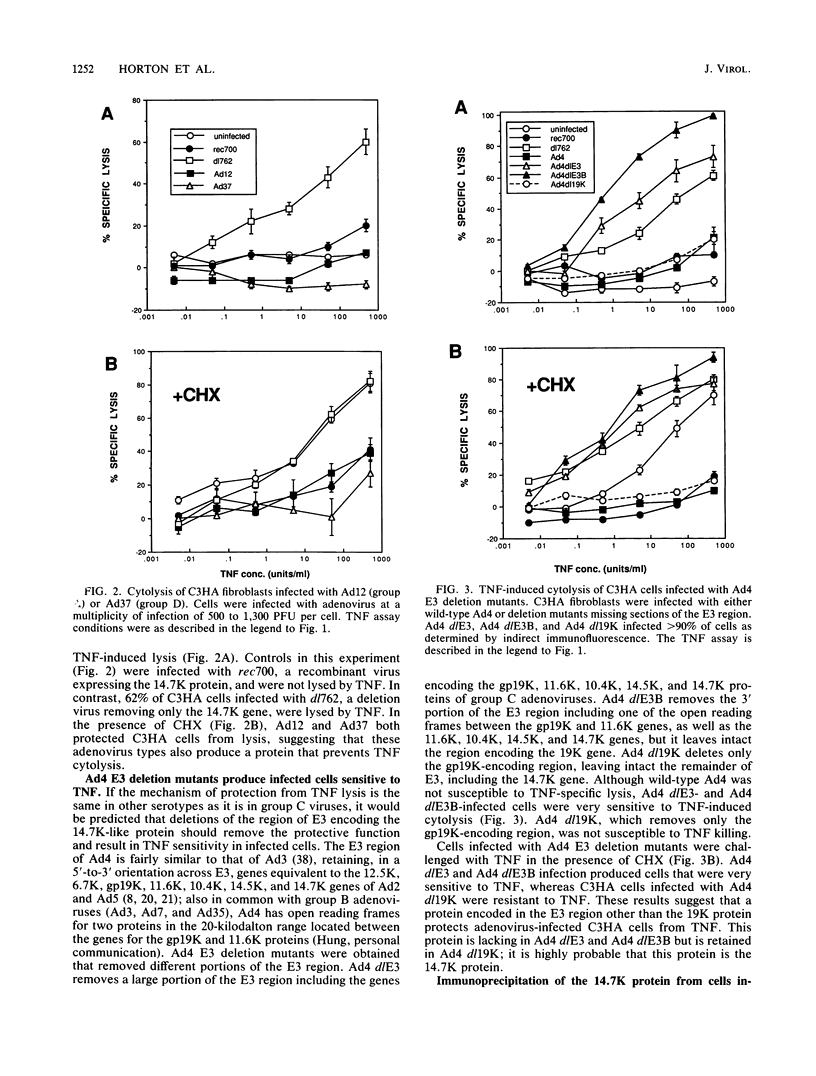
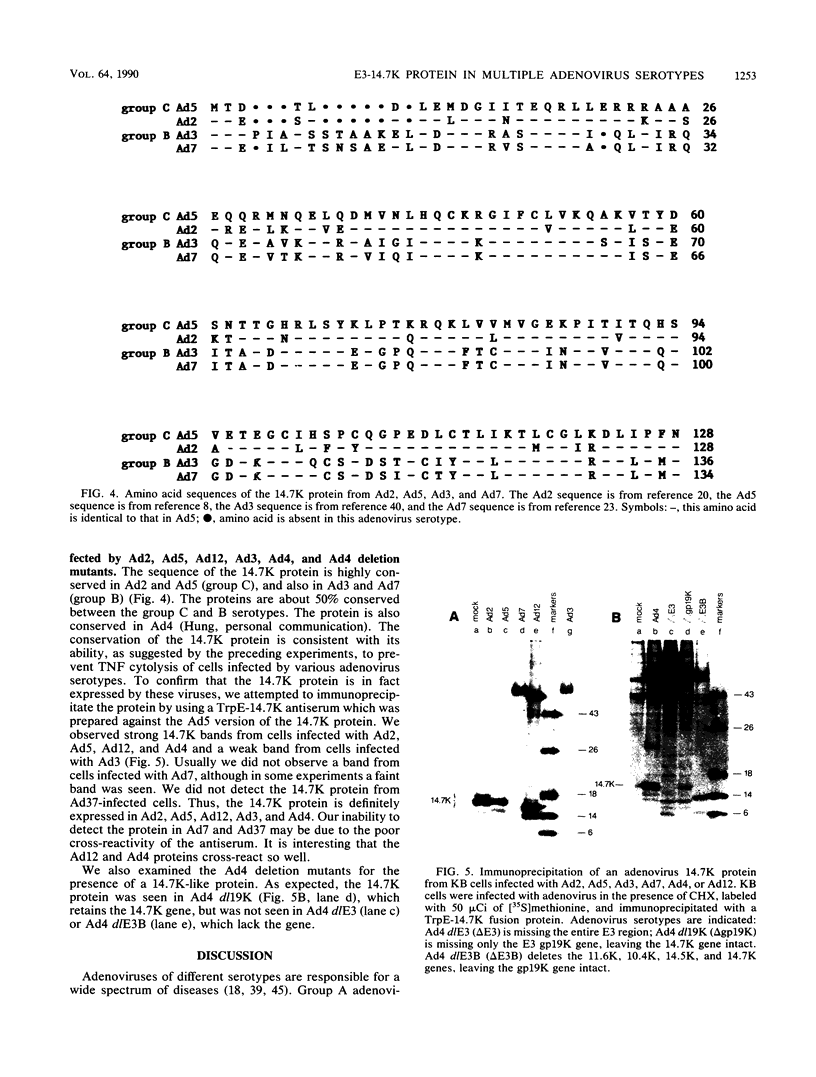
A 14.7-kilodalton protein (14.7K protein) encoded by the E3 region of group C adenoviruses has been shown to protect virus-infected fibroblasts from lysis by tumor necrosis factor (TNF) (L.R. Gooding, L.W. Elmore, A.E. Tollefson, H.A. Brady, and W.S.M. Wold, Cell 53:341-346, 1988). In this study we show that adenoviruses of other groups are also protected from TNF-induced cytolysis. Representative serotypes of groups A, B, D, and E produce a protein analogous to the 14.7K protein found in human group C adenoviruses. Deletion of this protein in group C viruses permits virus infection to induce cellular susceptibility to TNF killing. As with group C adenoviruses, cells infected with wild-type adenoviruses of other serotypes are not killed by TNF and are protected from lysis induced by TNF plus cycloheximide. However, cells are susceptible to TNF-induced lysis when infected with adenovirus type 4 mutants from which the 14.7K gene has been deleted. Although all known adenovirus serotypes infect epithelial cells, adenoviruses cause several diseases with various degrees of pathogenesis. Our findings suggest that the 14.7K protein provides a function required for the in vivo cytotoxicity of many adenoviruses independent of the site of infection or degree of pathogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Hsu Y. R., Toth E., Stebbing N. The antiviral activity of recombinant human tumor necrosis factor-alpha. J Interferon Res. 1987 Feb;7(1):103–105. doi: 10.1089/jir.1987.7.103. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Tollefson A. E., Brady H. A., Hoffman B. L., Wold W. S. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989 Apr 7;57(1):135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Holskin B., Strickler J., Gorniak J., Clark M. A., Johnson P. J., Mitcho M., Shalloway D. Induction by E1A oncogene expression of cellular susceptibility to lysis by TNF. Nature. 1987 Dec 10;330(6148):581–583. doi: 10.1038/330581a0. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Duerksen-Hughes P., Wold W. S., Gooding L. R. Adenovirus E1A renders infected cells sensitive to cytolysis by tumor necrosis factor. J Immunol. 1989 Dec 15;143(12):4193–4200. [PubMed] [Google Scholar]
- Flomenberg P. R., Chen M., Horwitz M. S. Characterization of a major histocompatibility complex class I antigen-binding glycoprotein from adenovirus type 35, a type associated with immunocompromised hosts. J Virol. 1987 Dec;61(12):3665–3671. doi: 10.1128/jvi.61.12.3665-3671.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flomenberg P. R., Chen M., Horwitz M. S. Sequence and genetic organization of adenovirus type 35 early region 3. J Virol. 1988 Nov;62(11):4431–4437. doi: 10.1128/jvi.62.11.4431-4437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford G. E., Loewenstein J., Yamin A., Gallily R. Correlation of macrophage-mediated tumor-cell lysis with the production of macrophage cytolytic factor (CF). Preliminary characterization of a factor inhibiting CF production. Int J Cancer. 1986 Jan 15;37(1):73–79. doi: 10.1002/ijc.2910370113. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm-Beauchamp U., Horswood R. L., Pernis B., Wold W. S., Chanock R. M., Prince G. A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by T lymphocytes generated against syngeneic SV40 transformants: studies employing recombinants within the H-2 complex. J Immunol. 1979 Mar;122(3):1002–1008. [PubMed] [Google Scholar]
- Green M., Mackey J. K., Wold W. S., Rigden P. Thirty-one human adenovirus serotypes (Ad1-Ad31) form five groups (A-E) based upon DNA genome homologies. Virology. 1979 Mar;93(2):481–492. doi: 10.1016/0042-6822(79)90251-4. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Mullis K. G., Engler J. A. Characterization of the early region 3 and fiber genes of Ad7. Virology. 1988 Dec;167(2):545–553. [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Nucleotide sequence of the EcoRI D fragment of adenovirus 2 genome. Nucleic Acids Res. 1980 May 24;8(10):2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Galibert F. Nucleotide sequence of the EcoRI E fragment of adenovirus 2 genome. Nucleic Acids Res. 1981 Mar 11;9(5):1229–1240. doi: 10.1093/nar/9.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973 Sep;12(3):643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M., Baglioni C. Tumor necrosis factor induces synthesis of two proteins in human fibroblasts. J Biol Chem. 1986 Jul 25;261(21):9565–9567. [PubMed] [Google Scholar]
- Kirstein M., Fiers W., Baglioni C. Growth inhibition and cytotoxicity of tumor necrosis factor in L929 cells is enhanced by high cell density and inhibition of mRNA synthesis. J Immunol. 1986 Oct 1;137(7):2277–2280. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Possible requirement of internalization in the mechanism of in vitro cytotoxicity in tumor necrosis serum. Cancer Res. 1981 Dec;41(12 Pt 1):4885–4890. [PubMed] [Google Scholar]
- Lewis A. M., Jr, Levin M. J., Wiese W. H., Crumpacker C. S., Henry P. H. A nondefective (competent) adenovirus-SV40 hybrid isolated from the AD.2-SV40 hybrid population. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1128–1135. doi: 10.1073/pnas.63.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Morin J. E., Lubeck M. D., Barton J. E., Conley A. J., Davis A. R., Hung P. P. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini D. L., Dubovi E. J., Clyde W. A., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984 Jul;150(1):92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Nilsson T., Peterson P. A. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9665–9669. doi: 10.1073/pnas.83.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawle F. C., Tollefson A. E., Wold W. S., Gooding L. R. Mouse anti-adenovirus cytotoxic T lymphocytes. Inhibition of lysis by E3 gp19K but not E3 14.7K. J Immunol. 1989 Sep 15;143(6):2031–2037. [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Lunn R. M., Richardson N. K., Hellermann G. R., Smith L. J., Old L. J. Tumor necrosis factor and IFN induce a common set of proteins. J Immunol. 1988 Aug 15;141(4):1180–1184. [PubMed] [Google Scholar]
- Severinsson L., Peterson P. A. Abrogation of cell surface expression of human class I transplantation antigens by an adenovirus protein in Xenopus laevis oocytes. J Cell Biol. 1985 Aug;101(2):540–547. doi: 10.1083/jcb.101.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signäs C., Akusjärvi G., Pettersson U. Region E3 of human adenoviruses; differences between the oncogenic adenovirus-3 and the non-oncogenic adenovirus-2. Gene. 1986;50(1-3):173–184. doi: 10.1016/0378-1119(86)90322-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tevethia S. S. Differential effect of adenovirus 2 E3/19K glycoprotein on the expression of H-2Kb and H-2Db class I antigens and H-2Kb- and H-2Db-restricted SV40-specific CTL-mediated lysis. Virology. 1988 Aug;165(2):357–366. doi: 10.1016/0042-6822(88)90580-6. [DOI] [PubMed] [Google Scholar]
- Tollefson A. E., Wold W. S. Identification and gene mapping of a 14,700-molecular-weight protein encoded by region E3 of group C adenoviruses. J Virol. 1988 Jan;62(1):33–39. doi: 10.1128/jvi.62.1.33-39.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. Preparations of lymphotoxin induce resistance to their own cytotoxic effect. J Immunol. 1984 May;132(5):2464–2469. [PubMed] [Google Scholar]
- Wang E. W., Scott M. O., Ricciardi R. P. An adenovirus mRNA which encodes a 14,700-Mr protein that maps to the last open reading frame of region E3 is expressed during infection. J Virol. 1988 Apr;62(4):1456–1459. doi: 10.1128/jvi.62.4.1456-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Faha B., Stillman B. Regulation of adenovirus gene expression in human WI38 cells by an E1B-encoded tumor antigen. Mol Cell Biol. 1986 Nov;6(11):3763–3773. doi: 10.1128/mcb.6.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Cladaras C., Deutscher S. L., Kapoor Q. S. The 19-kDa glycoprotein coded by region E3 of adenovirus. Purification, characterization, and structural analysis. J Biol Chem. 1985 Feb 25;260(4):2424–2431. [PubMed] [Google Scholar]
- Wold W. S., Cladaras C., Magie S. C., Yacoub N. Mapping a new gene that encodes an 11,600-molecular-weight protein in the E3 transcription unit of adenovirus 2. J Virol. 1984 Nov;52(2):307–313. doi: 10.1128/jvi.52.2.307-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold W. S., Deutscher S. L., Takemori N., Bhat B. M., Magie S. C. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA region E3 of adenovirus. Virology. 1986 Jan 15;148(1):168–180. doi: 10.1016/0042-6822(86)90412-5. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]




