Abstract
For humans alcohol consumption often has devastating consequences. Wild mammals may also be behaviorally and physiologically challenged by alcohol in their food. Here, we provide a detailed account of chronic alcohol intake by mammals as part of a coevolved relationship with a plant. We discovered that seven mammalian species in a West Malaysian rainforest consume alcoholic nectar daily from flower buds of the bertam palm (Eugeissona tristis), which they pollinate. The 3.8% maximum alcohol concentration (mean: 0.6%; median: 0.5%) that we recorded is among the highest ever reported in a natural food. Nectar high in alcohol is facilitated by specialized flower buds that harbor a fermenting yeast community, including several species new to science. Pentailed treeshrews (Ptilocercus lowii) frequently consume alcohol doses from the inflorescences that would intoxicate humans. Yet, the flower-visiting mammals showed no signs of intoxication. Analysis of an alcohol metabolite (ethyl glucuronide) in their hair yielded concentrations higher than those in humans with similarly high alcohol intake. The pentailed treeshrew is considered a living model for extinct mammals representing the stock from which all extinct and living treeshrews and primates radiated. Therefore, we hypothesize that moderate to high alcohol intake was present early on in the evolution of these closely related lineages. It is yet unclear to what extent treeshrews benefit from ingested alcohol per se and how they mitigate the risk of continuous high blood alcohol concentrations.
Keywords: alcohol self-administration, bertam palm, nectar feeding, pollination
Human alcohol (ethanol) use and abuse can be partly linked to genetically inheritable traits (1). It is still unclear whether and how these traits have been shaped by natural selection directly connected to alcohol intake (2). Three principal scenarios are possible: one hypothesis stems from the observation that many psychoactive substances, including alcohol, exert negative effects by interference with neurobiological pathways of normal learning (3, 4). It claims that such detrimental hitch-hiking is possible only because the genetics underlying it evolved in the absence of pure drugs and direct routes of drug administration before the invention of brewing ≈9,000 years ago (3–5). According to another hypothesis, the traits predisposing for maladaptive alcohol-related behaviors evolved under a long selective regime of low alcohol availability in fruit-eating human and prehuman ancestors. Alcohol in ripening fruit indicated a valuable source of nutrients. With harmful alcohol doses generally prevented by the natural availability, genetic traits for increased intake came under positive selection. No similar pressure worked on genes protecting against harmful effects. The time elapsed since the invention of beverages that allowed the consumption of enough alcohol to cause intoxication, resulting in a reversal of selective pressures, was too short to induce adequate evolutionary responses. In this sense, modern alcoholism has been called an evolutionary hangover (6, 7). Independent of past scenarios, a third hypothesis may be suggested, that moderate to high human alcohol consumption could be evolutionarily maintained by positive effects on evolutionary fitness that outweigh any associated health risk in most current settings, despite negative net effects in some others.
For investigating these hypotheses, it would be helpful if comparisons could be drawn with other living mammals that naturally consume alcohol. Because of an ecology that may resemble extinct ancestor species (6), missing links could help to differentiate between past evolutionary scenarios of no, low, or high alcohol availability for human alcohol use. Moreover, they would provide separate cases allowing for comparative studies on the causes and evolutionary consequences of low or high alcohol challenges.
There are numerous anecdotal reports of wild animals showing signs of inebriation, including various mammals (8, 9). Whereas some of these reports substantiate the claim that drugs are available in natural environments at least at times, evidence for their everyday consumption as it occurs in humans is lacking.
We detected chronic alcohol intake by pentailed treeshrews (Ptilocercus lowii) and some other mammals through alcoholic nectar of the bertam palm (Eugeissona tristis) of West Malaysia. We then collected detailed evidence of a mutualistic relationship between mammals (pollinators) and the bertam palm (producer of alcoholic nectar). Using a self-constructed food model and a hair biomarker for chronic alcohol consumption, we also demonstrate that alcohol intake by the pentailed treeshrew reaches levels that are dangerous to other mammals. This finding suggests adaptive benefits inherent to a diet high in alcohol and strong selective pressures for combating the adverse effects seen in humans (cell and organ damage and negative consequences of altered neurobiological performance). The pentailed treeshrew may be ecologically and behaviorally close to extinct species ancestral to primates living >55 mya ago (10, 11). We conclude that exposure to potentially harmful alcohol levels was probably significant early on in the evolution of several mammalian lineages, including treeshrews and primates.
Results
Palm Phenology.
During previous ecological studies on two small mammal species, the pentailed treeshrew (Fig. 1A) and the slow loris (Nycticebus coucang), in the Manjung district, West Malaysia, we had noted frequent feeding on floral nectar of the bertam palm (12).
Fig. 1.
Pollinating small mammal and floral display of the bertam palm. (A) Anesthetized pentailed treeshrew with a radio-collar. (B) A medium-sized inflorescence, cut from a palm. (C) A pencil-shaped, woody flower ≈5 cm in length with its three petals still closed and exuded nectar. Nectar is produced for periods averaging (± SE) 46.0 ± 5.8 days before flowers open to expose pollen-bearing stamens later followed by the exposure of receptive stigmas (female receptive phase of a flower).
The stemless bertam palm grows in dense clusters in many rainforests of West Malaysia. That plant develops very large (1–3 m), erect inflorescences that were difficult to link to any known pollination mode (13) (Fig. 1B). They consist on average of 1,004 ± 230 SD flowers, half of which are male (bearing stamens only) and half hermaphroditic (bearing stamens and one pistil). Both flower types have a similar shape and size and can produce copious nectar for several weeks during bud stage, i.e., before pollen and stigma are exposed. All flowers of an inflorescence develop in synchrony; there is no temporal overlap between nectaring, male stages (pollen exposure), and female stages (stigma receptiveness). The complete succession of floral stages of an inflorescence during which potential food is offered is as follows: 38 days of nectar production followed by 1 day of pollen exposure (nocturnal anthesis) by male flower buds, an intermediate period of 41.8 days during which no floral resources are available for flower visitors, 51 days of nectar production, 2 days of pollen exposure (nocturnal anthesis), and an intermediate period of 20.9 days during which no or very little nectar is produced until stigmas become receptive and fruit develops at hermaphroditic flowers (medians given). This succession and the production and fermentation of the floral nectar of the bertam palm are unusual. During nectar production (Fig. 1C), the inflorescences release a strong alcoholic smell reminiscent of a brewery.
High Alcohol Concentrations in the Nectar.
The odor and frequent frothing of the nectar observed during all field sessions suggested vigorous fermentation, which we confirmed by direct measurements of the alcohol concentration in the nectar and the identification of the yeast species responsible. The 3.8% (vol/vol) maximum alcohol concentration that we recorded is comparable to that of beer. Among natural food, only the fruit pulp of one wild plant species has been found to occasionally contain higher concentrations (14). With 0.6% and 0.5% the mean and median alcohol concentrations of freshly exuded nectar are lower (Fig. 2), but still within the range known to produce interference with major molecular targets of alcohol in the brain (15, 16) and to elicit attractive behavioral responses in humans (17). The yeast community, listed in supporting information (SI) Table S1, is unusual for nectar and contains a number of hitherto unknown species. The dominant members are typical of communities found in rotting fruit and necrotic tissue of succulent plants (18) or those found in spontaneous industrial fermentations (19).
Fig. 2.
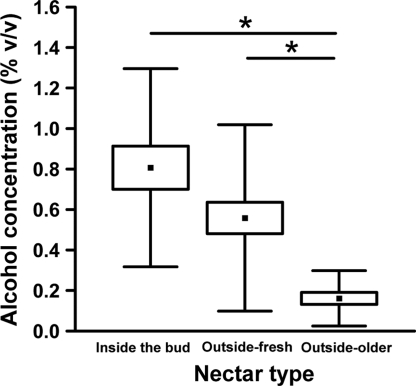
Alcohol concentrations of nectar of the bertam palm. The three nectar stages differ significantly (P < 0.001). Nectar inside the buds (n = 21) has significantly higher alcohol concentrations than nectar that has been clinging outside to the buds for >30 min (“older” nectar, n = 21; P < 0.001). Nectar that accumulated ≤30 min at the buds' outer surface (“fresh” nectar, n = 35) has significantly higher alcohol concentrations than older exuded nectar (P < 0.001). Nectar inside the buds tends to have a higher alcohol content than freshly exuded nectar (P = 0.203). Boxes and whiskers show means, SE, and SD respectively. Significant pairwise differences between the different nectar types are indicated by asterisks.
Some of the more unusual aspects of the floral display of the bertam palm facilitate nectar fermentation and thus indicate that high alcohol is an integral part of sexual reproduction of the palm. One of these is the exceptionally long duration of nectar production. We suspect inoculation with yeast starts minutes after the nectar exudes from the bud, through vector insects such as drosophilids, nitidulid beetles, and various bees that are frequently observed in the vicinity of the flowers. All of the 70 samples of nectar droplets collected on different flowers during different seasons contained yeast. Fermentation within a few hours after infection with yeast is facilitated by the unusual hard and woody external structures of the flower buds. Nascent nectar first fills the cavity enclosed between the reproductive organs and the closed petals, later to leak out through fine fissures present between the petals. Yeast was also evident inside the flower buds examined.
A comparison of alcohol concentration of nectar inside the buds, freshly exuded nectar, and older nectar clinging to the bud surface showed that the highest concentrations occurred inside the buds (Fig. 2). Once exuded and available to animals visiting flowers, the nectar rapidly loses its alcohol content through evaporation and possibly the action of less fermentative yeast that consume ethanol as a carbon source. The flower buds function as brewing chambers, providing an environment conducive to fermentation by the yeast community and protection of the community from complete depletion by nectar-licking animals.
Alcoholic Nectar Attracts Mammal Pollinators.
We found that the openly available nectar attracts a vast array of different animal species, mainly insects and tree-climbing mammals weighing <1 kg. Video surveillance of inflorescences revealed frequent nondestructive visits by seven species of small mammals (Movies S1 and S2). The common treeshrew (Tupaia glis; mean body mass, 157 g) and the plantain squirrel (Callosciurus notatus; 250 g) were regular visitors during the day, dusk, and dawn. However, visitation frequencies more than doubled during the night (Fig. S1). Nocturnal visitors included a range of murids: the gray tree rat (Lenothrix canus; 159 g), the Malayan wood rat (Rattus tiomanicus; 80 g), and the chestnut rat (Niviventer bukit; 77 g). Other important nocturnal visitors were the slow loris (681 g), a prosimian primate, and the pentailed treeshrew (47 g). Bats do not visit inflorescences, probably because all aerial parts of the palm bear spines, which can cause injuries to flying membranes. Small birds were observed at inflorescences only on two occasions. Most inflorescences received nocturnal visits by nonflying mammals during the nectar production period and the pollen exposure (84% of the inflorescences monitored during 4–14 nights of nectar production; 80% of the inflorescences monitored during 1 night of pollen exposure). The average number of nocturnal visits to individual inflorescences was 3.0 during the nectar production period and 1.9 during pollen exposure. During visits, mammals move up and down an inflorescence and lick off available nectar or pollen. Slow lorises and pentailed treeshrews cling to the inflorescences by grasping the protruding spine-tipped flowers and climb rather slowly. However, we did not notice any motor incoordination or other behavioral signs of inebriation in the animals (Movies S1 and S2).
In contrast to most plants, which show prominent seasonality in flowering and fruiting, all inflorescence stages of the bertam palm are available year-round, making the alcoholic nectar a nonseasonal food source. Individual mammals in high-density bertam palm areas on average consumed bertam palm nectar for 138 min (pentailed treeshrews) and 86 min (slow lorises) per night. They spent more time consuming bertam palm nectar than any other food source. Frequent shifts between inflorescences suggested a role in pollination of the palm. To be effective pollinators, mammals have to transport pollen grains that stick to their body from one flower to the receptive female structures (stigmas) of another. Consistent with this requirement, we recorded mammals during the period when stigmas are receptive (see SI Text). Selective exclusion of animals the size of a 20-g mouse and larger from inflorescences during that period resulted in a statistically significant decline of 50% in fruit set (P = 0.048; one-tailed Wilcoxon test). Although visited by insects, the floral display of the palm does not resemble any insect flower syndrome. Instead, the bertam palm has sturdy structures, broad accessibility of floral rewards, and copious strong-smelling nectar in common with the plant species that have been found to be visited by nonflying mammals (20).
Alcohol Doses Obtained by Mammals.
Evidence for alcohol presenting a biological challenge to the consuming mammals is provided by comparing the probabilities of exceeding a standard reference intake value. We used the amount of alcohol ingested over 12 h that would cause blood alcohol concentration to exceed 0.05% (wt/vol) in humans as a benchmark for deleterious effects. The time period of 12 h represents the activity period of small mammals feeding on nectar of the bertam palm. Humans with higher intake usually show adverse effects and are considered legally intoxicated in most countries. Calculations according to a common standard in legal medicine (21) yielded a corresponding total amount of ingested pure alcohol of 115 ml or nine small glasses of wine per 12 h for a human (woman) of average weight. Note that health experts recommend a lower maximum for women of two such drinks a day (four for men; pentailed treeshrews and slow lorises of both sexes visited bertam palm flowers; see SI Text). For cross-species comparisons, however, the standard measure of alcohol intake used was g alcohol/kg body mass, which corresponds in this case to a dose of 1.4 g/kg irrespective of the actual blood alcohol levels achieved. Intoxication causing impaired reaction times is a life-threatening condition, especially for small mammals with natural predators. Exceeding this dose should translate into selective pressure toward mechanisms that protect against such effects, for example, a higher metabolic tolerance of alcohol.
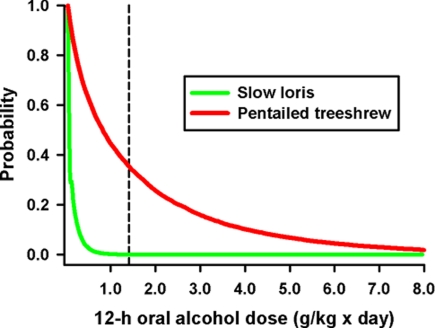
Field measurements of nectar feeding by radio-collared animals and nectar renewal patterns, together with the data on alcohol content, allowed us to derive likelihood estimates of daily alcohol intake from nectar of the bertam palm for pentailed treeshrews and slow lorises. For pentailed treeshrews the analysis yields a probability to exceed the deleterious effect benchmark of 36% (Fig. 3). In other words, with metabolic mechanisms to eliminate alcohol similar to humans and an assumed homogeneity of drinking patterns across the population (assumed only for illustrative purposes), a pentailed treeshrew would be intoxicated every third night. In contrast, the likelihood of achieving doses of bertam nectar intoxicating to humans for the much larger slow lorises, which spend less time ingesting bertam palm nectar, is low (< 0.001%; Fig. 3).
Fig. 3.
Quantification of the alcohol challenge to wild mammals. The probabilities of daily alcohol doses by pentailed treeshrews and slow lorises from ingestion of fermenting nectar of the bertam palm. Each point on the cumulative distribution function curve in the graph gives the probability (y axis) of achieving or surpassing a dose (x axis). The dashed line depicts the benchmark dose for human legal intoxication (1.4 g/kg) after 12 h of drinking.
Uncertainty that has not been incorporated in our estimates of chronic exposure to alcohol from ingested natural food stems from two untested assumptions: we assumed that the animals do not feed selectively with respect to nectar alcohol content, and that their feeding pattern does not influence nectar renewal. However, preference for high or low alcohol is possible (driving alcohol consumption up or down) and the removal of one nectar droplet from a bud surface by a licking animal may result in nectar with a higher alcohol concentration leaking from the bud (driving alcohol consumption up).
Hair Biomarker Levels.
We used hair analysis techniques as a second and independent method to estimate alcohol intake to account for uncertainties associated with alcohol intake. Ingested alcohol is distributed in the body and broken down or conjugated to other molecules by a number of different enzymes and pathways. Enzymes that break down alcohol include alcohol dehydrogenase (ADH), which oxidize alcohol to form acetaldehyde. Enzymes that conjugate alcohol to other molecules include UDP-glucuronosyltransferases (UGTs), which produce ethyl glucuronide (EtG). EtG when synthesized, for example, in the liver enters into growing hair shafts where it remains for long periods, even post mortem. EtG present in hair is a widely used biomarker for chronic alcohol consumption in humans (22). We tested hair sampled from live-trapped animals present in our study site. For comparison, hair samples of wild mammals from other temperate and tropical regions and alcohol-naïve laboratory-raised mammals were also tested for EtG content.
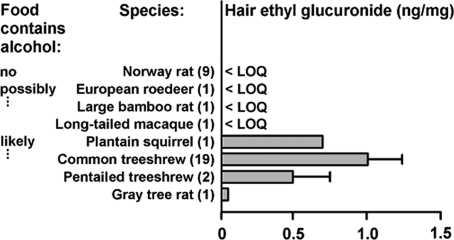
Pentailed treeshrews, common treeshrews, one plantain squirrel, and one gray tree rat with access to bertam palm inflorescences had much higher hair EtG concentrations than alcohol-naïve laboratory rats. In contrast, other wild mammals potentially feeding on fermenting material showed no similarly elevated EtG (Fig. 4).
Fig. 4.
Hair concentrations of EtG. EtG is a direct metabolite of ingested alcohol present in hair; hair EtG is correlated with chronic alcohol consumption. Species ingesting food likely containing alcohol are adult mammals from our study site that had access to fermenting nectar of the bertam palm: female pentailed treeshrews, male and female common treeshrews, female plantain squirrel, and male gray tree rat. For comparison, we collected hair samples from one adult male long-tailed macaque (Macaca fascicularis; body mass, 5 kg), and one adult large bamboo rat (Rhizomys sumatrensis; 2 kg) of unknown sex in other areas in Malaysia. We also obtained a hair sample from one wild adult male European roedeer (Capreolus capreolus; 16 kg) from Germany. Occasional alcohol consumption by these animals was possible as they may feed on rotting plant material. As alcohol-naïve negative controls, we used Norway rats of the out-bred WISTAR stock maintained on commercial food pellets (V 1536–000; ssniff Spezialdiaeten). Bars and whiskers represent means + SE. Numbers in brackets give the sample sizes. The limit of quantitation (LOQ) was 0.017 ng/mg. Concentrations above this limit indicate excessive drinking in humans (23).
Discussion
The Function of Alcohol in Plant–Animal Relationships.
Alcohol and other psychoactive substances available in natural settings have a huge potential for mediating plant–animal relationships. Although some drugs may ecologically function as a chemical defense against herbivores, the role of others may be that of an attractant (6). Alcohol may be enhancing the attractiveness of inflorescences of the bertam palm to mammal pollinators. This attraction and, consequently, a higher probability of pollinator arrival during crucial phases, i.e., pollen exposure and stigma receptiveness, may be achieved, for example, by facilitation of spatial learning during the earlier long period of nectar production and improved spatial memory (24, 25). Positive health effects of consumed floral resources on pollinators, as opposed to impaired cognition and behavioral deficits, are expected in an evolved system where individual pollinators repeatedly visit inflorescences. In agreement with this, mammals on bertam palm inflorescences did not exhibit odd behavior indicative of a drunken state, but moved as efficiently on the spiny substrate as they would on other supports.
The Quantification of Alcohol Intake.
High EtG levels found in pentailed treeshrews strongly imply high chronic alcohol intake in line with daily intake doses through palm nectar suggested by our food model. Still higher concentrations in common treeshrews and the plantain squirrel and elevated concentrations in the gray tree rat cannot be pinned down to any particular dietary source (e.g., because some input data for our food model are lacking). They may be caused by intake from other potential sources like other plant saps and fruit (6). However, for common treeshrews, plantain squirrels, and gray tree rats just like for pentailed treeshrews, EtG concentrations point to potentially risky alcohol consumption. Although the observed behavior of the two treeshrew species and the squirrel suggests moderate to high, but not excessive, drinking, EtG concentrations in their hair are extremely high according to human standards where similar values indicate life-threatening chronic drinking and strong behavioral deficits (23). This mismatch may be the result of differences in metabolic pathways that eliminate alcohol from the body. Mammals that feed on bertam palm nectar may eliminate a significantly larger proportion of ingested alcohol via the glucuronic acid conjugation pathway than humans, where the pathway has minor influence.
Evolutionary Time Scale of Mammalian and Bertam Palm Interaction.
How far back does chronic alcohol intake by small mammal fauna in West Malaysia date? The Indomalesian genus Eugeissona is the only extant member of an isolated palm lineage of Asian origin that diverged from other palm clades at the basis of the palm radiation, likely during the Cretaceous period (> 65 mya) (26). Copious amounts of fermenting nectar may occur in all six extant species of Eugeissona, but we are not aware of any previous study of flower ecology on any of these palms. The limited morphological and ecological diversification in this lineage may indicate constant ecological conditions over an evolutionary period. An ancient origin of gray tree rats, pentailed treeshrews, and slow lorises in the region, all considered to have retained many ancestral morphological and anatomical features of their respective orders, is supported by Miocene fossils that are 9 million to 12 million years (myr) old (27–29). Treeshrews (order Scandentia) are closely related to primates and flying lemurs (order Dermoptera) (11). Incidentally, the chronically alcohol consuming pentailed treeshrew is considered to be the morphologically least-derived living descendent of early ancestors of primates living >55 mya (10, 11). The conserved morphology of the pentailed treeshrew may be the result of a relatively stable ecology that included a diet of alcoholic nectar (30). Therefore, the symbiotic pollination relationship between the bertam palm and nonflying small mammals may be >55 myr old. The pentailed treeshrew is the earliest offshoot of the main treeshrew lineage (11), but our data show that in extant members of both lineages chronic alcohol consumption occurs. Hence, alcohol intake through sugar-rich plant products like nectar, sap, and fruit probably was a recurrent, and in some lineages (e.g., treeshrews) even a constant, feature for tens of millions of years.
Alcohol as an Evolutionary Factor.
Nectar and other sugar-rich plant saps (e.g., phloem sap) susceptible to fermentation are part of the natural diet of many extant primates, including monkeys and indigenous human populations of traditional cultures. However, direct human precursor species similar to living great apes probably were much less likely to consume high alcohol doses than treeshrews because of a much larger body size, a much lower reliance on plant saps, and a lower alcohol concentration in fruit, their main food. Nonetheless, alcohol intake in a living model for ancestral primates speaks against the claim that the sensitivity of basic biochemical pathways of normal learning to ingested alcohol could only evolve in the absence of dietary alcohol. The alcohol intake estimates obtained from our food model also refute the suggestion that any naturally available alcohol does not allow for doses that would inebriate humans. Thus, the idea of an evolutionary adaptive maintenance of alcohol-related behaviors in most current settings, including many human drinking situations, is indirectly supported. Accordingly, we suggest that future studies look into two aspects that are crucial for achieving a positive evolutionary fitness balance of alcohol consumption: adaptive benefits inherent to alcohol use and protective mechanisms against alcohol pathologies.
Adaptive benefits to humans have been linked to the psychoactive properties of alcohol, e.g., a reduction in anxiety and stress (31), and other medical properties (32, 33). Up to date, they remain controversial (34, 35). It is now possible to examine whether treeshrews in real-life drinking situations, e.g., when an unavoidable stressor is present, incur similar positive effects and achieve a positive net balance on health.
We conjecture that treeshrews cope with the risks of chronic high oral alcohol intake through an increase in effectiveness of the glucuronidation pathway of alcohol detoxification that keeps concentrations of alcohol in the blood and the brain low (high metabolic tolerance). If substantiated, this idea would support the view that along and across phylogenetic lineages, different evolutionary solutions to alcohol challenges are possible. For example, in wild fruit flies, like maybe in treeshrews, metabolic tolerance is positively correlated with alcohol exposure through higher activities in ADH and aldehyde dehydrogenase (ALDH) (36), which catalyze consecutive steps in the oxidative alcohol breakdown. Higher metabolic tolerance is opposite to lowered metabolic tolerance to alcohol mediated by ADH and ALDH that protects against alcoholism in some East Asian people (37).
This study has found chronic alcohol consumption by certain mammals within an ecological context suggestive of a benefitial effect of an alcoholic diet. Some of the mechanisms involved in maintaining a positive evolutionary fitness balance of alcohol consumption may eventually help combat human pathologies related to alcohol abuse.
Materials and Methods
We collected field data during 12 sessions totaling 38 months between May 1995 and March 2006 in a lowland primary forest in Segari Melintang Forest Reserve, State of Perak, West Malaysia (4° 18′ N, 100° 34′ E) (12).
Expanded methods for some components are in SI Text.
Floral Display.
We counted the flowers of 49 randomly selected inflorescences. Floral life histories were determined with census walks along an established 3.4-km transect line. During initial surveys at the beginning of each field session we marked all inflorescences with flower buds already secreting nectar or with fully grown buds shortly before nectar production. We conducted regular censuses of marked inflorescences at least until staminate anthesis and beyond until the predetermined end of the field session. During censuses we checked flowers for exposed nectar and pollen and receptive stigmas (newly exposed and sticky surface that stains Peroxtesmo KO peroxidase test paper; Macherey-Nagel). The total number of target inflorescences censused in all field sessions was 122.
Quantification of the Nectar Ethanol Concentration.
To quantify the concentration of alcohol in the nectar of the palm directly in the forest during two different sessions we used an amperometric biosensor-based handheld device (SensLab). During one field session we determined the alcohol content in nectar from five randomly chosen flowers each from 20 randomly chosen inflorescences to obtain the distribution of nectar ethanol concentration as encountered by flower visiting mammals. We used this data set in the food intake model. The separate data set shown in Fig. 2 was collected during another field session 5 months later specifically to understand the time course of fermentation.
Video Monitoring of Inflorescences.
We video-monitored inflorescences of the bertam palm with two night-vision video camcorders (DCR-TRV14E; Sony) in time-lapse mode following schemes outlined in SI Text. We determined more visual details of foraging behavior from analysis of close-up recordings made in continuous mode (uninterrupted recordings for 1 h each on tapes). Altogether, we recorded 531 mammal visits in 147 camera nights and 22 camera days (time-lapse mode) and an additional 51 during 115 h of continuous nocturnal recordings, 60% by murids, 18% by pentailed treeshrews, 9% by slow lorises, 10% by common treeshrews, and 2% by plantain squirrels. Consumption of floral rewards was confirmed in 93% of all visits. The maximum number of visits by nectar-feeding mammals at an inflorescence in a single night was 26, in a single day it was 9. The average length (± SD) of visits at nectar producing inflorescences was 5 ± 5 min for pentailed treeshrews, 8 ± 5 min for slow lorises, 5 ± 7 min for murids, 3 ± 2 min for common treeshrews, and 4 ± 2 min for plantain squirrels.
Contribution of Mammals to Fruit Set.
To quantify the importance of mammals for fruitset in the bertam palm we selected 14 pairs of neighboring inflorescences. Before stigmas became exposed, we enclosed one member of each pair in a wire cage with a mesh size of 20 × 20 mm. These cages excluded small mammals, while allowing potential insect pollinators free access to receptive flowers. Fruits and persistent flowers were counted 3–6 months after caging. The one-tailed Wilcoxon test used to test for differences in the percentage of fruit is deemed appropriate in cases when visitation patterns and floral structures strongly suggest nonflying mammal pollination as in the bertam palm.
Trapping, Radio Tracking, and Behavioral Observation.
Trapping, tracking, and observation techniques have been described (38). We captured 11 life pentailed treeshrews and 33 life slow lorises. We fitted eight pentailed treeshrews and six slow lorises (adults and subadults) from high-density bertam palm areas with radio collars (Biotrack) and tracked them for 1–9 months. Moreover, we measured the body mass of 18 gray tree rats, 4 chestnut rats, 13 Malayan wood rats, 19 common treeshrews, and 5 plantain squirrels. Animals were handled in a humane manner following standard guidelines (39). All methods were approved by the Economic Planning Unit at the Prime Minister's Department in Kuala Lumpur (permits UPE: 40/200/19 SJ. 179, SJ. 1074, and SJ. 1290).
Ethanol Ingestion Model.
The model was: DE = C × T × ER × LT × EC × ED/BM, where DE was the daily oral alcohol dose to mammal species, C was the concentration of alcohol in bertam palm nectar, T was the time spent on ingesting bertam palm nectar per day, ER was the rate at which flower buds are emptied during visits, LT was the lapse time to the last mammalian visit, EC was the renewal rate of encountered nectar crop at a single bud, ED was the alcohol density, and BM was the body mass of the consumer species. Apart from ED, which was set constant, the distributions of all input variables of the model were empirically determined. The data sets for C, T, and BM and the methods of their collection have been described. ER was determined from close-up recordings made in continuous mode of visits by pentailed treeshrews and slow lorises at nectar-producing inflorescences. Probing of individual flower buds by the animals was clearly discernible when playing back the recordings on a television screen. LT was extracted from the interval videorecordings. EC is the nectar renewal minus the nectar that is consumed by arthropods. We measured EC by determining the volume of nectar renewed at flower buds, from which we had removed all nectar at a known time beforehand (on average 96 min earlier) and from which mammals, but not arthropods were scared away by the presence of a researcher. We used a probabilistic first-order, 1D Monte Carlo method (40) to propagate uncertainty provided in the natural variability of the input variables in the daily ingested dose estimate. Table S2 shows how the model was parameterized during 10,000 iterations and gives the units of measurements.
Calculation of the Benchmark Value.
The formula used was Widmark's (21): A = r × BM (BACt + β × t) × 10, where A is the total amount of alcohol ingested (g), r is the human distribution factor (liter/kg), BM is the body mass (kg), β is the average rate of ethanol metabolism (g/dl/h), and BACt is the blood alcohol concentration after time t (g/dl). Specifically, we used the formula to calculate the amount of ingested ethanol that would make a woman of 65 kg after 12 h drinking and no solid food intake have BAC reach 0.05 g/dl. We set r = 0.55 and β = 0.017, which is the recommended value for moderate drinkers (16).
Hair Analyses.
Hair samples for EtG analyses were 30–300 mg of tail or body hair. EtG measurements were performed with a gas chromatograph 6890 with an Ultra 2 capillary column (12 m × 0.2 mm × 0.33 μm film thickness) and a mass selective detector 5973 (Hewlett-Packard). The temperature was programmed from 70°C (2 min hold) to 280°C with 20°C/min. The injector temperature was 260°C; the helium carrier gas flow rate was 1 ml/min. The mass spectrometer was operated in the NCI mode with methane as the carrier gas (flow of 40%). The method for extraction of the hair samples has been described in detail (23).
Supplementary Material
Acknowledgments.
F.W. and A.Z. thank H. Preuschoft for fostering their interest in nocturnal mammals and K. S. Chai and B. L. Lim for help in Malaysia. Financial assistance came from the Schimper Foundation (F.W.), Bundesministerium für Bildung und Forschung Grant FKZ 01GS0475 (to R.S.), the Deutsche Forschungsgemeinschaft (H. Preuschoft), and the Daimler-Benz AG.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801628105/DCSupplemental.
References
- 1.Goldman D, Oroszi G, Ducci F. The genetics of addictions: Uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 2.Han Y, et al. Evidence of positive selection on a class I ADH locus. Am J Hum Genet. 2007;80:441–456. doi: 10.1086/512485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addiction: A neuro-evolutionary perspective and new “self report” animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- 4.Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- 5.McGovern PE, et al. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci USA. 2004;101:17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley R. Evolutionary origins of human alcoholism in primate frugivory. Q Rev Biol. 2000;75:3–15. doi: 10.1086/393255. [DOI] [PubMed] [Google Scholar]
- 7.Dudley R. Fermenting fruit and the historical ecology of ethanol ingestion: Is alcoholism in modern humans an evolutionary hangover? Addiction. 2002;97:381–388. doi: 10.1046/j.1360-0443.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- 8.Janzen DH. Why fruits rot, seeds mold, and meat spoils. Am Nat. 1977;111:691–713. [Google Scholar]
- 9.Siegel RK. Intoxication: Life in Pursuit of Artificial Paradise. New York: Dutton; 1989. [DOI] [PubMed] [Google Scholar]
- 10.Bloch JI, Silcox MT, Boyer DM, Sargis EJ. New Paleocene skeletons and the relationship of plesiadapiforms to crown-clade primates. Proc Natl Acad Sci USA. 2007;104:1159–1164. doi: 10.1073/pnas.0610579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janecka JE, et al. Molecular and genomic data indentify the closest living relative of primates. Science. 2007;318:792–794. doi: 10.1126/science.1147555. [DOI] [PubMed] [Google Scholar]
- 12.Wiens F, Zitzmann A, Hussein NA. Fast food for slow lorises: Is low metabolism related to secondary compounds in high-energy plant diet? J Mammal. 2006;87:790–798. [Google Scholar]
- 13.Whitmore TC. Palms of Malaya. Kuala Lumpur: Oxford Univ Press; 1977. [Google Scholar]
- 14.Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr Comp Biol. 2004;44:315–323. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- 15.Sundstrom-Poromaa I, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field M, Duka T. Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology (Berl) 2002;59:325–334. doi: 10.1007/s00213-001-0923-z. [DOI] [PubMed] [Google Scholar]
- 18.Starmer WT, Schmedicke RA, Lachance MA. The origin of the cactus-yeast community. FEMS Yeast Res. 2003;3:441–448. doi: 10.1016/S1567-1356(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 19.Lachance MA. Yeast communities in a natural tequila fermentation. Antonie Van Leeuwenhoek. 1995;68:151–160. doi: 10.1007/BF00873100. [DOI] [PubMed] [Google Scholar]
- 20.Carthew SM, Goldingay RL. Nonflying mammals as pollinators. Trends Ecol Evol. 1997;12:104–108. doi: 10.1016/s0169-5347(96)10067-7. [DOI] [PubMed] [Google Scholar]
- 21.Brick J. Standardization of alcohol calculations in research. Alcoholism Clin Exp Res. 2006;30:1276–1287. doi: 10.1111/j.1530-0277.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 22.Pragst F, Balikova MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. 2006;370:17–49. doi: 10.1016/j.cca.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Yegles M, et al. Comparison of ethyl glucuronide and fatty acid ethyl ester concentrations in hair of alcoholics, social drinkers, and teetotalers. Forensic Sci Int. 2004;145:167–173. doi: 10.1016/j.forsciint.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 24.White NM. Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1999;91:921–950. [PubMed] [Google Scholar]
- 25.Tomie A, et al. Effects of ethanol on Pavlovian autoshaping in rats. Psycho-pharmacology (Berl) 1998;139:154–159. doi: 10.1007/s002130050700. [DOI] [PubMed] [Google Scholar]
- 26.Baker WJ, Dransfield J, Hedderson TA. Phylogeny, character evolution, and a new classification of the calamoid palms. Syst Bot. 2000;25:297–322. [Google Scholar]
- 27.Jaeger J-J, Tong H, Buffetaut E, Ingavat R. The first fossil rodents from the Miocene of northern Thailand and their bearing on the problem of the origin of the Muridae. Rev Paleobiol. 1985;4:1–7. [Google Scholar]
- 28.MacPhee RDE, Jacobs LL. In: Vertebrates, Phylogeny, and Philosophy: A Tribute to George Gaylord Simpson. Flanagan KM, Lillegraven JA, editors. Laramie: Univ. Wyoming; 1986. pp. 131–161. [Google Scholar]
- 29.Ni XJ, Qiu ZD. The micromammalian fauna from the Leilao, Yuanmou hominoid locality: Implications for biochronology and paleoecology. J Hum Evol. 2002;42:535–546. doi: 10.1006/jhev.2001.0540. [DOI] [PubMed] [Google Scholar]
- 30.Sussman RW, Raven PH. Pollination by lemurs and marsupials: Archaic coevolutionary system. Science. 1978;200:731–736. doi: 10.1126/science.200.4343.731. [DOI] [PubMed] [Google Scholar]
- 31.Pohorecky LA. Stress and alcohol interaction: An update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 32.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: Meta-analysis of effects on lipids and haemostatic factors. Br Med J. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conigrave KM, et al. A prospective study of drinking patterns in relation to risk of type 2 diabetes among men. Diabetes. 2001;50:2390–2395. doi: 10.2337/diabetes.50.10.2390. [DOI] [PubMed] [Google Scholar]
- 34.Jackson R, Broad J, Connor J, Wells S. Alcohol and ischaemic heart disease: Probably no free lunch. Lancet. 2005;366:1911–1912. doi: 10.1016/S0140-6736(05)67770-7. [DOI] [PubMed] [Google Scholar]
- 35.Waltman C, Blevins LS, Jr, Boyd G, Wand GS. The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. J Clin Endocrinol Metab. 1993;77:518–522. doi: 10.1210/jcem.77.2.8393888. [DOI] [PubMed] [Google Scholar]
- 36.Fry JD, Bahnck CM, Mikucki M, Phadnis N, Slattery WC. Dietary ethanol mediates selection on aldehyde dehydrogenese activity in Drosophila melanogaster. Integr Comp Biol. 2004;44:275–283. doi: 10.1093/icb/44.4.275. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal DP. Genetic polymorphisms of alcohol metabolizing enzymes. Pathol Biol (Paris) 2001;49:703–709. doi: 10.1016/s0369-8114(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 38.Wiens F, Zitzmann A. Social structure of the solitary slow loris Nycticebus coucang (Lorisidae) J Zool (London) 2003;261:35–46. [Google Scholar]
- 39.Animal Care and Use Committee. Guidelines for the capture, handling, and care of mammals as approved by the American Society of Mammalogists. J Mammal. 1998;79:1416–1431. [Google Scholar]
- 40.Regan HM, Hope BK, Ferson S. Analysis and portrayal of uncertainty in a food-web exposure model. Hum Ecol Risk Assess. 2002;8:1757–1777. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.