Abstract
We have assessed the potential of the fusion inhibitory peptide T-1249 for development as a vaginal microbicide to prevent HIV-1 sexual transmission. When formulated as a simple gel, T-1249 provided dose-dependent protection to macaques against high-dose challenge with three different SHIVs that used either CCR5 or CXCR4 for infection (the R5 virus SHIV-162P3, the X4 virus SHIV-KU1 and the R5X4 virus SHIV-89.6P), and it also protected against SIVmac251 (R5). Protection of half of the test animals was estimated by interpolation to occur at T-1249 concentrations of ≈40–130 μM, whereas complete protection was observed at 0.1–2 mM. In vitro, T-1249 had substantial breadth of activity against HIV-1 strains from multiple genetic subtypes and in a coreceptor-independent manner. Thus, at 1 μM in a peripheral blood mononuclear cell-based replication assay, T-1249 inhibited all 29 R5 viruses, all 12 X4 viruses and all 7 R5X4 viruses in the test panel, irrespective of their genetic subtype. Combining lower concentrations of T-1249 with other entry inhibitors (CMPD-167, BMS-C, or AMD3465) increased the proportion of test viruses that could be blocked. In the PhenoSense assay, T-1249 was active against 636 different HIV-1 Env-pseudotyped viruses of varying tropism and derived from clinical samples, with IC50 values typically clustered in a 10-fold range ≈10 nM. Overall, these results support the concept of using T-1249 as a component of an entry inhibitor-based combination microbicide to prevent the sexual transmission of diverse HIV-1 variants.
Keywords: microbicide, SHIV challenge model, entry inhibitors
Vaginal microbicides remain a potential way to protect women against infection by HIV type 1 (HIV-1) during sexual intercourse (1–3). The early generation microbicide candidates, based on detergents or polyanions, have so far failed to confer protection when tested in efficacy trials, and some products have even enhanced HIV-1 transmission (4). The reasons for some failures are known, others are under active investigation, but it has become increasingly apparent that credible microbicide candidates must have highly potent antiretroviral activity and an impeccable safety record and toxicology profile (3, 4). Moreover, to overcome problems of poor compliance, microbicides should be formulated in a way that makes them easy to use, preferably on a once-daily or less frequent basis (3, 5, 6). One such compound is the fusion inhibitory peptide, T-1249, a second-generation derivative of the licensed antiretroviral drug enfuvirtide (T-20). T-1249 was evaluated clinically for its potential as a therapy to treat established HIV-1 infection and was shown to have a strong antiviral effect, with viral load reductions approaching 2.0 logs after once- or twice-daily s.c. injections delivering 150–200 mg per day (7, 8). Moreover, the safety profile for T-1249 was excellent, with only injection-site reactions being a significant concern, something that would not apply to a topically administered formulation.
Although all credible microbicide candidates must be highly potent inhibitors of HIV-1 replication in vitro, an important way to evaluate their potential for protecting women is to test them in one of the rhesus macaque vaginal transmission models. Several inhibitors of HIV-1 fusion with cells have been shown to protect macaques from infection with a simian/human immunodeficiency virus (SHIV) after vaginal application a short time before challenge via the same route. These in vivo validated inhibitors include the CCR5 antagonists PSC-RANTES and CMPD-167, the gp120-binding small-molecule BMS-806, the gp41 peptide fusion inhibitor C52L, and the gp120-binding protein cyanovirin-N (9–11). To see how T-1249 compared with these microbicide compounds, we formulated the peptide in a simple gel and applied it 30 min before vaginal SHIV challenge. We used three different challenge viruses, SHIV-162P3, SHIV-89.6P, and SHIV-KU1, allowing us to make some assessment of the breadth of activity of T-1249 against divergent strains and, more significantly, to determine whether a single inhibitor could protect against viruses that use different coreceptors for infection. Thus, although SHIV-162P3 enters cells only via CCR5, SHIV-89.6P can use both CCR5 and CXCR4, and SHIV-KU1 employs only CXCR4 (12–14). Although most naturally transmitted HIV-1 strains use only CCR5, a small but significant fraction of new infections does involve viruses that also or instead enter cells via CXCR4 (15, 16). Hence, using three challenge viruses with different coreceptor usage profiles allowed us to gauge whether a single microbicide candidate could be protective in a tropism-independent manner.
We observed that T-1249 protected macaques against all three SHIV challenge viruses (and also against SIVmac251) when applied at concentrations in the 0.1–1 mM range. Moreover, T-1249 was broadly and potently active in vitro against infection of PBMCs by a panel of HIV-1 isolates from multiple genetic subtypes, and it inhibited multiple HIV-1 Env-pseudotyped viruses from clinical samples with IC50 values ≈10 nM. Overall, judged by its potency and breadth of activity both in vitro and in the macaque model and by its safety profile when administered systemically to humans, T-1249 is a highly credible candidate for development as a vaginal microbicide. Whether it can in fact be developed successfully will depend on the ease and cost of its formulation in ways suitable for practical use in women and of course its safety when delivered vaginally in such formulations.
Results
Activity of T-1249 Against Macaque Challenge Viruses in Vitro.
To assess whether T-1249 was active against the viruses we intended to use in the macaque vaginal challenge model, we tested its ability to inhibit their replication in human and rhesus macaque PBMCs (Table 1). The IC50 values for T-1249 against each of SHIV-162P3 (R5), SHIV-89.6P (R5X4), and SHIV-KU1 (X4) ranged from 0.77 to 3.9 nM, which is consistent with the reported potencies for this peptide against a range of HIV-1 isolates in similar assays (see below). T-1249 was also active against SIVmac251 with an IC50 value of 3.5 nM. There were no differences in the IC50 values obtained when SHIV-KU1 and SHIV-89.6P were tested in both human and macaque PBMCs (Table 1). Of note is that T-1249 was ≈5-fold more potent than the C52L peptide that we had found could protect macaques from SHIV-162P3 vaginal challenge (9). The attachment inhibitor BMS-C was also highly active against all three SHIVs, with IC50 values of 0.2–0.3 nM. BMS-378806, a less potent compound in the same inhibitor class, protected macaques from SHIV-162P3 vaginal challenge (9).
Table 1.
Inhibition of SHIV and SIVmac replication in human and rhesus PBMC
| Virus | CMPD-167 | AMD3465 | CMPD + AMD** | T-1249 | C52L | BMS-C |
|---|---|---|---|---|---|---|
| SHIV-KU1 | >1,000* | 2.2 (7.6) | 1.0 | 0.90 (1.4) | 5.9 | 0.20 |
| SHIV-89.6P | >1,000 | 1.3 (<1.6) | 0.42 | 0.77 (1.9) | 5.3 | 0.30 |
| SHIV-162P3 | 0.30 | >1,000 | 0.80 | 3.9 | 13 | 0.20 |
| SHIVmac | 0.80 | >1,000 | 1.5 | 3.5 | 4.8 | >1,000 |
*Inhibition of replication in pooled human PBMCs is shown as IC50 concentrations (nM) that were derived from four-parameter sigmoid fits by nonlinear regression. The values in parentheses were generated using rhesus PBMCs.
**CMPD-167 and AMD3465 in a 1:5 mix; the total concentration is given.
We confirmed these results by using a second assay, based on the use of cMAGI reporter cells. The IC50 values for T-1249 against SHIV-162P3 (0.99 nM), SHIV-89.6P (2.0 nM), and SIVmac251 (3.8 nM) were similar to those derived in the PBMC-based assay. SHIV-KU1 was not tested in the cMAGI assay, but SIVdeltaB670 and HIV-2 NIH-Z were found to have geometric mean IC50 values of 3.8 nM and 1.6 nM, respectively.
We also validated the coreceptor usage profiles of the SHIV challenge viruses in vitro by performing inhibition experiments with the CCR5 inhibitor CMPD-167 and the CXCR4 inhibitor AMD3465. Only CMPD-167 inhibited SHIV-162P3 replication in PBMCs, whereas SHIV-KU1 and SHIV-89.6P were sensitive only to AMD3465 (Table 1). This data pattern is consistent with previous reports (12–14). SHIV-89.6P therefore behaves in PBMCs as if it were an X4 virus (12). However, SHIV-89.6P can use either CCR5 or CXCR4 to enter indicator cell lines, and some dual-tropic viruses that use only CXCR4 to enter T cells can also use CCR5 for replication in macrophages (17, 18). Our unpublished studies suggest that CMPD-167 or AMD3465 can each inhibit SHIV-89.6P vaginal transmission to macaques, albeit inconsistently. Overall, we designate SHIV-89.6P as an R5X4 virus for the purpose of this report.
T-1249 Protects Macaques from Vaginal Challenge with Three Different SHIVs.
The above studies demonstrate that T-1249 is highly active in vitro against all three SHIV challenge viruses, irrespective of their coreceptor usage profile, and against SIVmac251. Although the in vitro activity is in the low nanomolar range, our previous experience with the C52L gp41 peptide and other inhibitors of attachment or fusion suggested that much higher peptide concentrations would need to be applied vaginally for protection under in vivo conditions. We therefore formulated T-1249 in HMC gel and applied it vaginally to macaques at a range of concentrations between 2 μM and 2 mM, followed by challenge 30 min later with one of the test SHIVs (or, in one experiment, SIVmac251). The outcome of challenge was determined by measuring plasma viremia at weekly intervals, starting 1 week after the challenge. As in our previous studies, we defined protection from infection as the absence of detectable plasma viremia (assay threshold of 125 RNA copies per ml) at all time points from 1 to 10 weeks (9, 19).
Dose-dependent protection was observed for T-1249 against all three SHIVs (Fig. 1). Protection of half of the animals was estimated by linear interpolation to occur at T-1249 concentrations of ≈40–130 μM for the three challenge viruses (Fig. 1). Transmission of the R5 virus SHIV-162P3 was apparently the most sensitive to complete block by T-1249, whereas complete protection against the X4 SHIV-KU1 and the dual-tropic SHIV-89.6P viruses may require higher T-1249 concentrations, although the variability of the dose–responses and the low numbers of animals preclude certainty on these points. Of note is that if there are any differences in the dosage requirements for protection in vivo, they are not predicted by the in vitro activities of the inhibitor against the three challenge viruses, assessed by using PBMCs as target cells (Table 1). Nonetheless, at the higher T-1249 concentrations tested, complete protection was achieved against all three SHIVs (100 μM for SHIV-162P3; 600 μM for SHIV-KU1; 2 mM for SHIV-89.6P). We also found that T-1249 at 200 μM protected three of three rhesus macaques challenged with the R5 virus SIVmac251; approximately half of the animals were protected at T-1249 concentrations of ≈100 μM (data not shown). Hence, T-1249 can protect macaques from vaginal challenge with four different, highly divergent viruses that span the relevant range of coreceptor usage profiles.
Fig. 1.
Protection against SHIV vaginal challenge by vaginally applied T-1249. T-1249 was formulated in HMC gel and applied vaginally to macaques at the concentrations indicated on the x axis 30 min before challenge with SHIV-162P3 (A), SHIV-89.6P (B), or SHIV-KU1 (C). The proportion of the test animals that became infected at each T-1249 concentration is shown on the y axis. The figure in parentheses adjacent to each data point records the number of animals used to derive the recorded value.
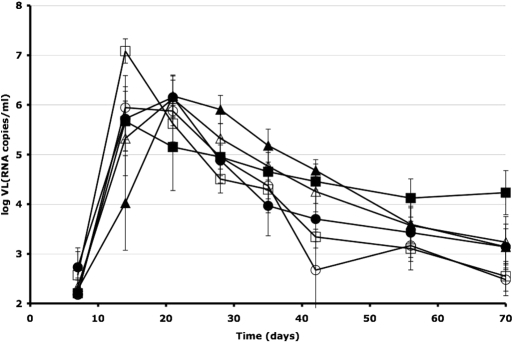
An analysis of mean viral loads (VL) in macaques that became infected despite receiving T-1249 showed no significant difference from control animals (unpaired two-tailed t test, P > 0.5) for any of the challenge viruses (Fig. 2). The cumulative viral loads were calculated as the areas under the curve (AUC) for the log (viral copy number) over time from day 7 to day 70. The mean AUC values (±SEM) were: 250 ± 15 (controls) and 270 ± 26 (treated) for SHIV-162P3; 260 ± 14 (controls) and 280 ± 26 (treated) for SHIV-89.6P; and 280 ± 18 (controls) and 290 ± 9.4 (treated) for SHIV-KU1. However, one monkey that received 60 μM T-1249 before SHIV-89.6P challenge had a low VL (RNA copies/ml), with a peak of only log(VL) = 3.5, much lower than the mean peak log(VL) of 6.5 ± 0.89 among the other animals infected with this virus (data not shown). Whether this animal was partially protected by T-1249 or whether it was naturally able to suppress SHIV-89.6P replication to low levels is unknown.
Fig. 2.
Mean viral loads in control macaques and nonprotected T-1249 recipients. The plasma viral loads (VL) of all of the infected animals described in Fig. 1 are plotted on the y axis as mean log(VL) (RNA copies per milliliter) ± SEM over time (x axis) for control (open symbols) and test animals that were not protected by T-1249 at the various test doses (filled symbols). The challenge viruses were SHIV-162P3 (circles), SHIV-89.6P (squares), and SHIV-KU1 (triangles). The numbers of animals in the groups were: SHIV-162P3, 3 controls and 5 T-1249-treated; SHIV-89.6P, 2 controls and 7 T-1249-treated; SHIV-KU1, 4 controls and 4 T-1249-treated. The AUCs did not differ between the control and T-1249 groups for any of the challenge viruses.
Breadth of T-1249 Activity Against HIV-1 Strains from Multiple Genetic Subtypes in Vitro.
Although T-1249 is clearly active against all three SHIV challenge viruses in vitro and in vivo, the Env proteins from these viruses are all based on HIV-1 subtype B. A practical microbicide must be able to counter viruses from multiple genetic subtypes, particularly the non-B viruses that predominate in geographic areas where heterosexual transmission is highest. To gauge the cross-subtype activity of T-1249, we determined its ability to inhibit infection of PBMCs by primary HIV-1 isolates of different tropisms and from various subtypes. Moreover, because a truly effective microbicide formulation might need to include more than one entry inhibitor with complementary mechanisms of action, we tested T-1249 in combination with the small-molecule CCR5 inhibitor CMPD-167, the small-molecule CXCR4 inhibitor AMD3465, and the gp120-binding attachment inhibitor BMS-C. The design of these experiments was identical to those we have described previously: assessment of the proportions of test isolates inhibited by >90% at three different inhibitor concentrations, alone and in combination (Tables 2–4) (20).
Table 2.
Relative frequency of HIV-1 replication inhibition by >90% in R5 viruses (n = 29)
| Conc. | CMPD-167, % | BMS-C, % | T-1249, % | CMPD + BMS-C, % | CMPD + T-1249, % | BMS-C + T-1249, % | Triple combination, % |
|---|---|---|---|---|---|---|---|
| Low* | 0 | 10 | 0 | 14 | 3 | 17 | 21 |
| High† | 52 | 41 | 41 | 86 | 100 | 76 | 100 |
| Top‡ | 100 | 72 | 100 | 100 | 100 | 100 | 100 |
*CMPD-167, 0.1 nM; all others, 1 nM, alone and in combinations.
†CMPD-167, 1 nM; all others, 10 nM, alone and in combinations.
‡CMPD-167, 100 nM; all others, 1 μM, alone and in combinations.
Table 3.
Relative frequency of HIV-1 replication inhibition by >90% in X4 viruses (n = 12)
| Conc. | AMD3465, % | BMS-C, % | T-1249, % | AMD + BMS-C, % | AMD + T-1249, % | BMS-C + T-1249, % | Triple combination, % |
|---|---|---|---|---|---|---|---|
| Low* | 8 | 33 | 0 | 25 | 33 | 25 | 58 |
| High† | 67 | 58 | 25 | 92 | 100 | 92 | 100 |
| Top‡ | 100 | 58 | 100 | 100 | 100 | 100 | 100 |
*AMD3465, 5 nM; all others, 1 nM, alone and in combinations.
†AMD3465, 50 nM; all others, 10 nM, alone and in combinations.
‡AMD3465, 5 μM; all others, 1 μM, alone and in combinations. At this high concentration of AMD3465, nonspecific effects on some R5 virus infections were also seen.
Table 4.
Relative frequency of HIV-1 replication inhibition by >90% in R5X4 viruses (n = 7)
| Conc. | AMD3465, % | CMPD-167, % | AMD + CMPD-167, % | BMS-C, % | T-1249, % | CoR* + BMS-C, % | CoR + T-1249, % | BMS-C + T-1249, % | Quadruple combination, % |
|---|---|---|---|---|---|---|---|---|---|
| Low† | 0 | 0 | 0 | 29 | 0 | 29 | 14 | 43 | 71 |
| High‡ | 29 | 14 | 43 | 86 | 0 | 100 | 100 | 100 | 100 |
| Top§ | 71 | 14 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
*CoR, mixture of AMD3465 with CMPD167.
†AMD3465, 5 nM; CMPD-167, 0.1 nM; all others, 1 nM, alone and in combinations.
‡AMD3465, 50 nM; CMPD-167, 1 nM; all others, 10 nM, alone and in combinations.
§AMD3465, 5 μM; CMPD-167, 100 nM; all others, 1 μM, alone and in combinations. At this high concentration of AMD3465, nonspecific effects on R5 virus infection were also seen.
The results of these experiments were very similar to what we observed using a different gp41-based fusion inhibitory peptide, C52L, in experiments of the same design (20). The principal difference was that T-1249 was more effective than C52L at intermediate concentrations, alone and in combination with the other inhibitors. At the highest of the three concentrations tested (1 μM), T-1249 inhibited all 29 R5 viruses, all 12 X4 viruses, and all 7 R5X4 viruses in the test panel, irrespective of their genetic subtype. At a 100-fold lower concentration (10 nM), T-1249 was active against 41% of R5 viruses, but this hit rate rose to 100% when the CCR5 inhibitor CMPD-167 was also present (at 1 nM). Because 1 nM CMPD-167 was only able to block 52% of the R5 viruses, the merits of combining two, complementary inhibitors as a device to deal with HIV-1 sequence diversity is clear. A similar, although less complete, benefit was seen when T-1249 was combined with BMS-C at intermediate concentrations against both R5 and X4 viruses and when it was combined with AMD3465 against X4 viruses.
It was notable that T-1249 was less effective at intermediate concentrations against R5X4 viruses (0% hit) than against R5 (41% hit) or X4 (25% hit) viruses. Whether this finding is relevant to the possibly reduced potency of T-1249 against the R5X4 SHIV-89.6P challenge virus in vivo is a point to consider. Nonetheless, combining intermediate concentrations of T-1249 and the two coreceptor-specific inhibitors, or with BMS-C alone, increased the hit rate to 100%. All of these findings with R5X4 viruses were similar to what was observed with the C52L peptide (20).
The cMAGI assay was used to assess further the breadth of activity of T-1249, in this case against 118 HIV-1 isolates from within subtype B. The geometric mean IC50 value (+SD) for these viruses was 1.6 + 3.0 nM. Thus, T-1249 is consistently active against HIV-1 in vitro in the low nanomolar concentration range, as was also noted above for other primate immunodeficiency viruses.
In the commercial PhenoSense entry assay (Monogram Biosciences), T-1249 had a narrow spectrum of potency against a large panel of HIV-1 Env-pseudotyped viruses with differing coreceptor tropisms, all derived from the plasmas of treatment-naïve individuals, most of whom were infected with subtype B viruses (Table 5). For example, the IC50 values for T-1249 against 393 R5 viruses fell within an ≈10-fold range, much narrower than the >100-fold range observed with the less potent fusion inhibitor, T-20. Similar results were obtained with 22 X4 viruses and with 221 dual-tropic viruses (Table 5). The activity of T-1249 against the latter viruses was independent of whether CCR5-expressing or CXCR4-expressing U87-CD4 target cells were used in the PhenoSense entry assay.
Table 5.
T-1249 and T-20 activities in the PhenoSense assay against HIV-1 Env-pseudotyped viruses derived from plasmas of treatment-naïve individuals
| Tropism | T-1249 IC50, nM* | T-20 IC50, nM* |
|---|---|---|
| R5 (n = 393)† | 9.1 (12) | 55 (110) |
| X4 (n = 22) | 17 (16) | 120 (140) |
| Dual-tropic‡ (R5X4/R5 + X4) (n = 221) | 5.1 (6.9) | 33 (66) |
| Dual-tropic on CCR5+ cells§ | 6.3 (7.5) | 34 (61) |
| Dual-tropic on CXCR4+ cells§ | 4.2 (15) | 32 (160) |
*The half-maximal inhibitory concentrations (IC50) are given as geometric means with the upward standard deviation shown in parentheses.
†n denotes the number of pseudotyped viruses tested.
‡Some dual-tropic viruses can use both CCR5 and CXCR4 for entry (R5X4), others may constitute a mixture of R5 viruses and X4 viruses (R5 + X4).
§The dual-tropic viruses were tested both on U87-CD4/CCR5 cells and on U87-CD4/CXCR4 cells.
Overall, these experiments demonstrate that T-1249 has substantial cross-subtype activity against genetically and phenotypically divergent HIV-1 isolates in vitro, irrespective of the coreceptor used. The studies also reiterate the advantages of using more than one entry inhibitor when attempting to counter global HIV-1 sequence diversity.
Discussion
Here, we have shown that the vaginal application of gel-formulated T-1249 can protect rhesus macaques from infection by three different SHIV challenge viruses, by SIVmac251. The protection we observed was dose-dependent and, at the higher concentrations, robust, in that all of the test animals resisted infection. The fully protective dose varied among the challenge viruses but was in the approximate range 0.1–1 mM for all three SHIVs. Whether there truly is a virus-dependent difference in the protective dose remains uncertain; the dual-tropic virus SHIV-89.6P may perhaps require a higher concentration than the other two. Much higher concentrations of T-1249 were required to protect macaques (millimolar range) than to inhibit infection of target cells by the same viruses in vitro (nanomolar range). This observation mirrors what has been found in all previous tests of various fusion/entry inhibitors as vaginal microbicides and is most probably attributable to the pharmacology of delivering inhibitors to the sites where they are active (1–4, 9–11, 19, 22).
We found no evidence for partial protection by T-1249, in that cumulative viral loads (AUC values) were indistinguishable between treated and control infected animals for all three SHIVs. One T-1249 treated, SHIV-89.6P-infected animal did have a very low viral load, although it could have arisen by chance. We also did not observe partial protection in an earlier study of other fusion and entry inhibitors (9). Presumably, once an infection becomes established locally within mucosal tissues, it amplifies systemically to an extent that renders irrelevant any microbicide-mediated reduction in the initial transmission of virions (21). However, in earlier experiments using the CCR5 inhibitor CMPD-167 (19) and the neutralizing antibody b12 (22) we did find lower viral loads (measured cumulatively or as peaks, respectively) among treated animals than controls. In the latter studies we used the SHIV-162P4 challenge virus (22), in the present and previous one (9), we used SHIV-162P3, a variant of the same R5 SHIV-162P virus but with a different passage history. Whether the use of different challenge viruses accounts for the different outcomes will require further investigation.
Although all three SHIVs were comparably sensitive to T-1249 in a PBMC-based assay in vitro, we did observe indications that the peptide was less effective against a set of dual-tropic HIV-1 isolates than against R5 or X4 viruses (Tables 2–4). Similar findings were made in an earlier study of the sequence-related C52L peptide (20). However, the replication of dual-tropic viruses in macrophages was not abnormally insensitive to the T-20 fusion inhibitor compared with R5 or X4 strains (17), and there were no major differences in the IC50 values for T-1249 against R5, X4, and R5X4 Env-pseudotyped viruses in the PhenoSense entry assay (Table 3). Irrespective of these quantitative issues, T-1249 can clearly protect macaques in a coreceptor-independent manner, which distinguishes it from, for example, a CCR5-directed inhibitor such as CMPD-167, PSC-RANTES, or Maraviroc. Moreover, our in vitro studies show that T-1249 has substantial breadth of activity across the HIV-1 genetic subtypes, again independently of coreceptor tropism. These properties combine to make T-1249 a promising microbicide candidate, a view supported by its extensive safety record and antiviral potency when delivered systemically to HIV-1-infected humans (by s.c. injection) during clinical trials (7, 8).
Whether T-1249 could be developed as a practical microbicide will depend on whether it can be successfully formulated at a feasible cost (3). We reconstituted T-1249 in a simple HMC gel; more sophisticated formulations would probably improve its delivery to the vaginal environment and might reduce the concentration needed for protection. Nonetheless, even concentrations in the 0.1–1 mM range are not necessarily prohibitive because they would translate to the use of ≈2–20 mg of peptide in each application. The long-term future of microbicides, however, is likely to require the use of long-lasting deliver systems, such as semisolid gels or vaginal rings that gradually release inhibitors at active concentrations (5, 6). Whether a peptide like T-1249 can be formulated in this way remains to be determined.
Although T-1249 is protective against different challenge viruses in a coreceptor-independent manner and does have broad, subtype-independent activity in vitro, protecting against HIV-1 transmission in the real world is extraordinarily challenging, not least because of the ever-increasing sequence diversity of circulating viruses. An effective microbicide formulation is, therefore, likely to require the coformulation of more than one entry inhibitor (2, 3). The advantages of entry inhibitor combinations are shown by our in vitro studies, where increased effects of T-1249 with BMS-C, CMPD-167, and/or AMD3465 were observed at intermediate concentrations of each inhibitor, reinforcing what we described using the C52L peptide (9, 20). Given the probable difficulties and costs of formulating high concentrations of any one inhibitor, a superior strategy could be to coformulate intermediate, but still partially effective, amounts of two different inhibitors with complementary mechanisms of action (e.g., any two of T-1249, CMPD-167, and BMS-C). Again, we do not yet know whether the different formulation chemistries of the various inhibitors will allow such combination products to be produced. Practical issues such as these need now to be addressed because the use of inhibitor combinations could be an important step toward an effective microbicide.
Materials and Methods
Inhibitors.
The production, in vitro properties, and clinical performance of the T-1249 fusion inhibitor have all been described in ref. 9. The small-molecule CCR5 inhibitor CMPD-167 has also been described, including evaluation of its activity against SHIV-162P3 transmission to macaques after vaginal administration (9). BMS-C is a more potent member of the same structural family of compounds as the gp120-binding attachment inhibitor BMS-378806 that protects macaques against vaginal transmission (23). AMD3465 is a small-molecule inhibitor that binds to CXCR4 (24).
Rhesus Macaque Challenge Studies.
These studies were carried out essentially as described (9, 11, 19, 20). SHIV-162P3 was obtained from National Institutes of Health AIDS Research and Reference Reagent Program; SHIV-KU1 and SHIV-89.6P were expanded in CEMX174 cells to make challenge stocks. The properties and vaginal transmissibility of these strains have been described elsewhere (for SHIV-KU1, ref. 13; for SHIV-89.6P, ref. 10; and for SHIV-162P3, refs. 9 and 25).
T-1249 was formulated in hydroxyethyl cellulose (HEC) gel by dissolving T-1249 powder in sterile water adjusted to pH 9 with NaOH (to disrupt aggregates). The pH was then adjusted to pH 7.2 with acetic acid, and the solution was passed through a 0.2-μm filter. HEC was then added with stirring, and the gel was transferred into syringes. The gels were then degassed by centrifugation (1,000 × g, 20 min).
Activity of T-1249 and Other Inhibitors in Vitro in a PBMC-Based Replication Assay.
The assay has been described, as have the composition and source of the HIV-1 primary isolates from different genetic subtypes (20). Briefly, the R5 test panel (n = 29) comprised 4 viruses from subtype A, 4 from subtype B, 6 from subtype C, 4 from subtype D, 3 from subtype F, 4 from subtype G, and 4 from CRF01_AE; the X4 panel (n = 12) contained 3 subtype B viruses, 2 subtype C viruses, 4 subtype D viruses, and 3 from CRF01_AE; the R5X4 panel (n = 7) included 1 virus from subtype A and 6 from subtype B (20). Half-maximal inhibitory concentrations were calculated by fitting a four-parameter sigmoid function through nonlinear regression (Prism, GraphPad) with the minimum constrained to 0% normalized inhibition and the maximum to 100%; all fits gave R2 >0.8.
cMAGI Cell Infectivity Assay.
The antiviral activity of T-1249 was determined against SHIV-162P3, SHIV-89.6P, SIVmac251, SIVdeltaB670, HIV-1 NIH-Z, and 118 HIV-1 primary isolates, by using a MAGI/cMAGI cell infectivity assay (26, 27). T-1249 was resuspended at ≈1 mg/ml in DPBS and serially diluted to the final concentrations used in the experiments. The assay is based on a single cycle of infection. To ensure that secondary rounds of infection did not complicate the analysis, additional T-1249 was applied to every coculture 24 h after infection at a concentration high enough to block reinfections. Seventy-two hours after infection, the cells were fixed with formaldehyde and glutaraldehyde and stained with X-Gal. The nuclei of infected cells were counted by using a charge-coupled device detector. The IC50 values recorded are the T-1249 concentrations required to inhibit viral infection by 50%.
PhenoSense Entry Assay.
The PhenoSense entry assay was used to assess the coreceptor usage and T-20 and T-1249 sensitivities of HIV-1 derived from patient plasma (28, 29). In brief, viral RNA was extracted from plasma samples, followed by cDNA synthesis. Env-specific primers were then used to amplify a 2.5-kb fragment including the entire ORF of the HIV-1 envelope; amplicons were subcloned, and representative expression vector/envelope sequence libraries were prepared in Escherichia coli. Pseudotyped virus stocks were produced in human embryonic kidney cell cultures by cotransfection of the expression libraries and an Env-deficient HIV-1 vector carrying a luciferase reporter gene. Env-pseudotyped virus stocks were harvested after 48 h and assessed for susceptibility to T-20 and T-1249 and their ability to infect U87 cells expressing CD4 and either the CCR5 or CXCR4 coreceptor (29).
Normalized IC50 values for T-20 and T-1249 were derived by multiplying the sample IC50 by the ratio of a standard reference value (determined by Monogram Biosciences from multiple assay runs) to the reference value obtained concurrently with the patient sample. The IC50 values for dual-mixed (R5X4 or R5 + X4) strains were the geometric means of the values derived from the two cell lines.
Acknowledgments.
We thank Richard Colonno (Bristol-Myers Squibb, Wallingford, CT), Jacqueline Fine (Merck Research Laboratories, Rahway, NJ), and Gary Bridger (AnorMed, Langley, Canada) for providing reagents. We appreciate the technical assistance of Janell LeBlanc, Maryjane Dodd, and Kelsi Rasmussen. We also thank David Heilman and Jie Di of Trimeris for their contributions to the T-1249 gel formulations and the Trimeris Virology Department for helping to determine the activity of T-1249 in vitro against clinical isolates. Viruses were provided by the National Institutes of Health AIDS Research and Reference Reagent Program (SHIV-162P3) or by Preston Marx (Tulane National Primate Research Center) (SHIV-KU1 and SHIV-89.6P). This work was funded by National Institutes of Health Grants U19 AI65413, R01 AI41420, and RR000164, and by a Bristol-Myers Squibb Unrestricted Biomedical Research Grant in Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 2.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 3.Klasse PJ, Shattock R, Moore JP. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 2006;3:1501–1507. doi: 10.1371/journal.pmed.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Wijgert JH, Shattock RJ. Vaginal microbicides: Moving ahead after an unexpected setback. AIDS. 2007;21:2369–2376. doi: 10.1097/QAD.0b013e3282ef83fd. [DOI] [PubMed] [Google Scholar]
- 5.Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC120 as an HIV microbicide. Int J Pharm. 2006;325:82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56:954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 7.Lalezari JP, et al. T-1249 retains potent antiretroviral activity in patients who had experienced virological failure while on an enfuvirtide-containing treatment regimen. J Infect Dis. 2005;191:1155–1163. doi: 10.1086/427993. [DOI] [PubMed] [Google Scholar]
- 8.Eron JJ, et al. Short-term safety and antiretroviral activity of T-1249, a second-generation fusion inhibitor of HIV. J Infect Dis. 2004;189:1075–1083. doi: 10.1086/381707. [DOI] [PubMed] [Google Scholar]
- 9.Veazey RS, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 10.Tsai CC, et al. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 11.Lederman MM, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J Virol. 2000;74:6893–6910. doi: 10.1128/jvi.74.15.6893-6910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joag SV, et al. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: Intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J Virol. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harouse JM, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors: Central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- 16.Margolis L, Shattock R. Selective transmission of CCR5-utilizing HIV-1: The “gatekeeper” problem resolved? Nat Rev Microbiol. 2006;4:312–317. doi: 10.1038/nrmicro1387. [DOI] [PubMed] [Google Scholar]
- 17.Yi Y, et al. Entry coreceptor use and fusion inhibitor T20 sensitivity. J Acquir Immune Defic Syndr. 2008;47:285–292. doi: 10.1097/QAI.0b013e31816520f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi Y, Shaheen F, Collman RG. Preferential use of CXCR4 by R5X4 human immunodeficiency virus type 1 isolates for infection of primary lymphocytes. J Virol. 2005;79:1480–1486. doi: 10.1128/JVI.79.3.1480-1486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veazey RS, et al. Use of a small-molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian/human immunodeficiency virus infection. J Exp Med. 2003;198:1551–1562. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketas TJ, et al. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology. 2007;364:431–440. doi: 10.1016/j.virol.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 22.Veazey RS, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 23.Guo Q, et al. Biochemical and genetic characterizations of a novel human immunodeficiency virus type 1 inhibitor that blocks gp120–CD4 interactions. J Virol. 2003;77:10528–10536. doi: 10.1128/JVI.77.19.10528-10536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatse S, et al. AMD3465, a monomacrocyclic CXCR4 antagonist and potent HIV entry inhibitor. Biochem Pharmacol. 2005;70:752–761. doi: 10.1016/j.bcp.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Veazey RS, et al. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005;11:1293–1294. doi: 10.1038/nm1321. [DOI] [PubMed] [Google Scholar]
- 26.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu QX, et al. Evolution of the human immunodeficiency virus type 1 envelope during infection reveals molecular corollaries of specificity for coreceptor utilization and AIDS pathogenesis. J Virol. 2000;74:11858–11872. doi: 10.1128/jvi.74.24.11858-11872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwick MB, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coakley E, Petropoulos CJ, Whitcomb JM. Assessing chemokine co-receptor usage in HIV. Curr Opin Infect Dis. 2005;18:9–15. doi: 10.1097/00001432-200502000-00003. [DOI] [PubMed] [Google Scholar]