Abstract
RIG-I has been implicated in innate immunity by sensing intracellular viral RNAs and inducing type I IFN production. However, we have found a significant RIG-I induction in a biological setting without active viral infection—namely, during RA-induced terminal granulocytic differentiation of acute myeloid leukemias. Here, we present evidence that a significant Rig-I induction also occurs during normal myelopoiesis and that the disruption of the Rig-I gene in mice leads to the development of a progressive myeloproliferative disorder. The initiation of progressive myeloproliferative disorder is mainly due to an intrinsic defect of Rig-I−/− myeloid cells, which are characterized by a reduced expression of IFN consensus sequence binding protein, a major regulator of myeloid differentiation. Thus, our study reveals a critical regulatory role of Rig-I in modulating the generation and differentiation of granulocytes.
Keywords: knockout mice, myelopoiesis, RA-inducible gene I
RA-induced granulocytic differentiation of acute promyelocytic leukemia (APL) represents a successful application of tumor differentiation-inducing therapy in the treatment of human malignancies. It is also an excellent model for studying the cellular and molecular mechanisms controlling RA-induced, and probably physiological cues-regulated myelopoiesis. Previously, we reported the isolation of a number of genes whose mRNA levels were highly up-regulated, along with all-trans-RA (ATRA)-induced terminal granulocytic differentiation of APL cell line NB4 cells in vitro. Among the up-regulated genes, a particularly interesting one was RIG-I, encoding a putative DExD/H box-containing RNA helicase (1). Recently, RIG-I has also been identified in the screening experiment for the molecules that mediate viral RNA-induced type I IFN generation (2). Upon the occupancy of its DExD/H box-containing carboxyl terminal region by foreign invading RNAs, or even RNase L-cleaved small self-RNAs (3), the amino-terminal double-CARD motif of RIG-I binds onto IPS-1/MAVS/VISA/Cardif, resulting in the recruitment and phosphorylation of certain downstream cytoplasmic factors, including IKKγ and IKK-related kinases TBK1/IKKε. These immediate events, in turn, relay signals via phosphorylation cascade and nuclear translocation of transcription factors such as NF-κB and IRF3 to coordinate the formation of IFN-β promoter enhancesome within the nucleus (4, 5). As expected, melanoma differentiation antigen 5 (MDA-5), a close structural and functional analogue of RIG-I, was also found to mediate a viral infection-induced type I IFN transcription through the IPS-1 pathway (6, 7). Notably, MDA-5 activity has been implicated in a biological setting without any signs of active viral infection. The overexpression of full-length of MDA-5, but not that of the CARD domain- or ATPase domain-deleted mutant, induced melanoma cells to apoptosis (8). Now that RIG-I expression can be highly induced by regulatory cues other than type I IFN, such as RA and IFN-γ, we postulated that additional biological functions of RIG-I analogues other than as a sole inducer of type I IFN generation in antiviral immunity may exist. In this study, we provide evidence that a development-associated expression pattern of the Rig-I molecule occurs along with myeloid differentiations. The phenotypic analyses of a Rig-I−/− mouse model further show that Rig-I is an essential negative regulator of physiological myelopoiesis. Moreover, Rig-I exerts this function at least partially through its prompting role on myeloid expression of interferon consensus sequence binding protein (Icsbp), a major transcription factor regulating myeloid cell differentiation. Thus, our work has uncovered an important aspect of Rig-I intrinsic activities different from its involvement with type I IFN induction and will better our understanding of how Rig-I homologues exert versatile roles in basic regulatory mechanism controlling cell growth and survival.
Results
Rig-I Expression Is Up-Regulated Along with Myeloid Differentiation.
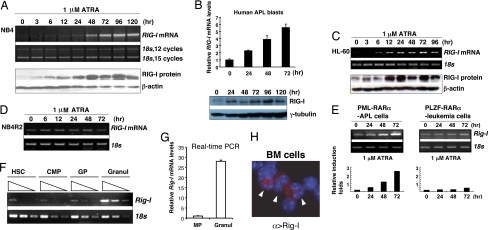
To further characterize a possible correlation between the RIG-I induction and the restoration, at least in part, to the normal differentiation program of malignant myeloid leukemia cells by RA signaling, we screened various myeloid leukemia cells for RIG-I induction kinetics after their exposure to 1 μM ATRA. As shown in Fig. 1 A–C, ATRA selectively induced the up-regulation of RIG-I mRNA and protein levels only upon the terminal granulocytic differentiation of RA-sensitive myeloid leukemia cell lines, including human APL cell line NB4 and the M2 subtype of acute myeloid leukemia cell line HL-60, in addition to primary human and mouse APL cells harboring oncogenic fusion product PML-RARα. ATRA caused no significant induction of RIG-I mRNA level in the ATRA-resistant NB4 derivative NB4R2 cell line or PLZF-RARα–harboring primary murine leukemia cells (Fig. 1 D and E).
Fig. 1.
RIG-I expression is up-regulated along with myeloid differentiation. (A) NB4 cells were treated with 1 μM ATRA for the indicated time points. Total RNA samples and whole cell lysates were analyzed by one-step RT-PCR and Western blotting assays, respectively. (B) Representative RIG-I induction of fresh human APL blasts by ATRA, as measured by real-time RT-PCR and Western blotting assay. (C) RIG-I induction in HL-60 cells by ATRA was measured by RT-PCR and Western blotting assays. (D) NB4R2 cells, an RA-resistant derivative of NB4 cells, were treated as in A, and total RNA samples were assayed by RT-PCR. (E) Rig-I mRNA induction in the freshly isolated APL blasts from PML-RARα transgenic mice or in the primary leukemia cells of PLZF-RARα transgenic mice by ATRA was assayed by RT-PCR. The relative induction folds of Rig-I mRNA are numerated in the bottom panel. (F–H) Lin−Sca-1+c-Kit++ hematopoietic stem cells (HSCs), Lin−IL-7Rα−Sca-1−c-Kit+ myeloid precursors (MPs), c-Kit+Gr-1lo granulocytic progenitors (GPs), and Gr-1++Mac-1++ mature granulocytes (Granuls) from normal mouse BM were sorted out by FACS. The mRNA level of Rig-I was measured by semiquantitative RT-PCR. (F) Triangle indicates three serial dilutions (1:3) of each RNA sample, which were run for a given cycle of PCR simultaneously. (G) Rig-I mRNA was measured by real-time RT-PCR assay. (H) Cytospin of primary BM cells, including mature granulocytes (arrowheads) was stained with an Alexa Fluor 555-coupled Rig-I antibody and DAPI.
Now that RA-induced granulocytic differentiation of myeloid leukemias may at least partly mimic the normal developmental program, we reasoned that Rig-I might participate in the regulatory mechanism that governs normal myelopoiesis. To address this, we examined the expression profile of Rig-I mRNA and protein during normal myelopoiesis. Of note, the Rig-I mRNA level was not evenly distributed among different stages of hematopoietic cells along with normal myelopoiesis (Fig. 1F), with a significant up-regulation of Rig-I mRNA level (>25-fold) being observed during the physiological maturation of the early myeloid precursors toward the Mac-1++/Gr-1++ granulocytes at the intermediate to late stages (Fig. 1G). Accordingly, strong immunostaining signals of Rig-I protein were detected within the cytoplasm of mature myeloid cells with a ring-shaped or band-shaped nucleus, indicating the identity of granulocytes (Fig. 1H). Thus, the expression of Rig-I was shown to be developmentally regulated along with myeloid differentiation.
Rig-I Deficiency Leads to a Progressive Granulocytosis.
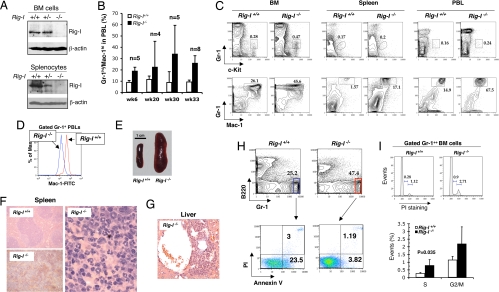
Recently, we reported a Rig-I−/− mouse model developed by removing its exon 4 to exon 8 region (encoding the most part of the second CARD domain and DExD/H domain) (9). In this model, Rig-I protein was absent in both bone marrow (BM) and spleen tissues of adult Rig-I−/− mice (Fig. 2A). The undetectable expression of a possible truncated Rig-I protein (Δaa142-405) could be ascribed either to the promoter interference from an inserted Neor-expressing cassette in the reverse transcriptional direction toward the Rig-I allele (9) and/or to an extremely unstable status of such a mutant protein. In accordance with a highly expressed level of Rig-I in normal myeloid cells, which might imply a role of Rig-I in either involving developmental regulation or conducting mature cellular activity, the analyses of adult Rig-I−/− mice revealed an apparently perturbed granulopoiesis. An apparent granulocytosis in peripheral blood leukocytes (PBLs) appeared as soon as 6 weeks after birth and progressed with the aging (Fig. 2B). Notably, the percentage of Gr-1loc-Kit+ granulocytic progenitors within BM was doubled (Fig. 2C) in association with Rig-I deficiency, although those for common myeloid precursors, granulocytic and monocytic precursors, and megakaryocytic and erythroid precursors were not significantly altered (data not shown). The grossly increased production of the intermediate to later stages of Mac-1++/Gr-1++ granulocytes was ubiquitously evident in BM, spleen, and PBLs (Fig. 2C). An unsaturated Mac-1 expression in Rig-I−/− granulocytes in comparison with that measured in the wild-type counterparts (Fig. 2D), in addition to the enlarged cellular and nuclear sizes of mutant granulocytes (data not shown), indicates a certain extent of myeloid differentiation hindrance caused by the loss of Rig-I function. In striking contrast, the B-cell production, especially at its final maturation stages, was significantly hampered in both BM and spleen (data not shown), which was in line with the previous finding of reduced B-cell amounts in Peyer's patches of Rig-I−/− mice (9). The in vitro colony assay of measuring B-cell production suggested that the reduced B-cell level might be partly related to a defect in the commitment and/or differentiation of the hematopoietic stem/progenitor cells toward B-cell lineage (data not shown). On the other hand, the decreased production of erythroid cells in BM, as shown by a reduced Ter119+ cell compartment size, was compensated for by an increased activity of erythropoiesis in spleen (data not shown), suggesting an occurrence of extramedullary hematopoiesis. The thymic and BM CD4+/CD8+ T cell ratios and the percentage of CD41+ megakaryocytes in BM seemed undisturbed (data not shown), although an abnormality in peripheral T cell activation was identified (9).
Fig. 2.
Rig-I deficiency leads to a progressive granulocytosis. (A) Rig-I expression levels within whole BM cell and spleen cell lysates from Rig-I+/+, Rig-I+/−, or Rig-I−/− mice were measured by Western blotting assay. (B) Onset and progress of the abnormal Gr-1hi/Mac-1hi granulocytic production in PBLs (mean ± SD). (C) Representative results of analyzing the percentages of c-Kit+Gr-1lo granulocytic progenitors (GPs) and Gr-1++Mac-1++ granulocytes in BM, spleen, and PBLs of Rig-I+/+ or Rig-I−/− mice at the age of 6 months. Notably, Mac-1 signal strength of Rig-I−/− myeloid cells is lower than that in wild-type counterparts. (D) Comparison of Mac-1 expression strengths in Rig-I+/+ and Rig-I−/− peripheral blood granulocytes. (E) Rig-I deficiency resulted in severe splenomegaly in aged mice. A representative enlarged spleen frequently found in old Rig-I−/− mice (≥9 months) is shown. (F) Hematoxylin-eosin staining of splenic sections shows the disrupted splenic nodules by the infiltrating granulocytic cells (Right and Lower Left) in Rig-I−/− mice in comparison with the normal splenic nodular structure. (G) Rig-I−/− granulocytes invaded hepatic tissues. An infiltration focus of Rig-I−/− granulocytes into the perivascular compartment of liver is shown. (H) Rig-I−/− granulocytes survived better than their normal counterparts. The freshly isolated primary BM cells were simultaneously stained with Gr-1, B220, Annexin V, and propidium iodide (PI). One set of representative flow cytometric data of gated B220− Gr-1++ myeloid cells from Rig-I−/− or Rig-I+/+ mice is shown. (I) Cell cycle statuses of Gr-1++ BM myeloid cells were measured by PI staining after permeabilization. The average lengths for indicated cell cycling phases are expressed as mean ± SD (n = 5, S phase P < 0.05).
Remarkably, a chronic myeloid leukemia (CML)-like phenotype developed after the age of 9 months, with hugely enlarged spleens infiltrated by swarms of granulocytes (Fig. 2 E and F), and in half of them, even with granulocytic foci invading into hepatic tissue (Fig. 2G). The percentage of mice with a CML-like phenotype reached up to ≈90% of all Rig-I−/− littermates at the age of 16 months. The measurement on freshly isolated BM cells indicated that Rig-I deficiency resulted in not only an increased cell proliferation capacity but also an enhanced survival ability of mutant granulocytes (Fig. 2 H and I). Taken together, these results demonstrate a developmentally regulatory role of Rig-I in promoting the terminal differentiation of granulocytes but curbing the scale of late granulocytosis.
Rig-I Deficiency-Associated Granulopoiesis Is Ascribed to an Autonomous Defect of Hematopoietic Cells.
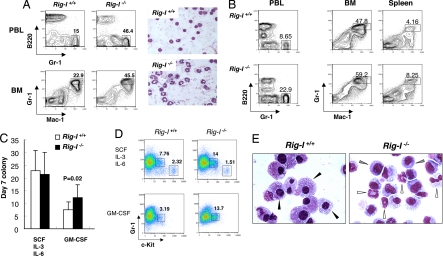
It was previously reported that Rig-I−/− mice are frequently associated with the occurrence of colitis, with an onset of ≈2 weeks later than the biased granulocytic generation in PBLs (Fig. 2B) (9). To examine whether Rig-I deficiency-related granulocytosis is simply a secondary response to this accompanying colitis or a reflection of a possible abnormality in the hematopoietic microenvironment, we did hematopoietic transplantation studies of Rig-I−/− early hematopoietic cells into wild-type syngeneic recipients and traced their differentiation potentials toward myeloid compartment in vivo. As shown in Fig. 3 A and B, in 7 weeks after tail vein injection, an apparent granulocytosis was recapitulated through the transplantation of 5-fluorouracil (5-FU)-treated Rig-I−/− BM cells, not only within primary recipients but also within secondary recipients when no sign of colitis was observed yet (data not shown), suggesting that the Rig-I deficiency-related granulocytosis could be mainly attributed to an intrinsic defect of hematopoietic cells.
Fig. 3.
Rig-I deficiency-associated granulopoiesis is ascribed to an autonomous defect of hematopoietic cells. (A) Total of 1 × 106 pooled BM cells from Rig-I+/+ or Rig-I−/− mice at 6 months of age (n = 3) were transplanted into the lethally irradiated syngeneic mice (n = 7). The regeneration of BM or PBL granulocytes was measured 7 weeks later. The color images on the right show May-Grünwald-Giemsa–stained cytospins of PBLs from primary recipients. The DNA-PCR analysis of PBLs showed that >90% of leukocytes were derived from donors (data not shown). (B) Bone marrow cells from the primary recipients were harvested and further transplanted into the secondary recipients. A representative sample of flow cytometric analysis of granulocytes in PBLs, BM, and spleen was performed 7 weeks later (n = 6). (C) Primary colony-forming potentials of Rig-I+/+ or Rig-I−/− BM myeloid progenitors responded to the stimulations by different cytokines as indicated (mean ± SD) (GM-CSF colony, P = 0.02). (D) Percentages of c-Kit+Gr-1lo granulocytic progenitors (GPs) within the retrieved colony cells. (E) May-Giemsa staining of macrophages (black arrowheads), granulocytes (white arrowheads) and immature myeloid cells (gray arrowheads) were generated from primary Rig-I+/+ and Rig-I−/− BM myeloid progenitors stimulated by GM-CSF.
We then isolated Rig-I−/− BM cells and tested their in vitro responses to the stimulations of hematopoietic cytokines in terms of the myeloid differentiation and proliferation potentials. As shown in Fig. 3 C and D, although the combination of stem cell factor (SCF), IL-3, and IL-6 stimulated the growth of similar numbers of colonies from Rig-I+/+ and Rig-I−/− BM cells, more myeloid colonies were generated in Rig-I−/− background by the stimulation of myeloid-specific cytokine GM-CSF. Moreover, in both conditions, the retrieved colony cells of Rig-I−/− background retained a higher percentage of c-Kit+Gr-1lo granulocytic progenitors. The morphological examinations of GM-CSF–stimulated colony cells further showed that the generation of foamy macrophages in Rig-I−/− background was significantly reduced in BM cells (60 ± 5% versus 11 ± 3%); instead, the granulocytes or certain immature myeloid cells were produced (Fig. 3E). Taken together, these data indicate an intrinsic activity of Rig-I in regulating the myeloid differentiation and proliferation potentials of hematopoietic progenitors.
Reduced Expression of Icsbp Associates with Rig-I Deficiency-Related Granulocytosis.
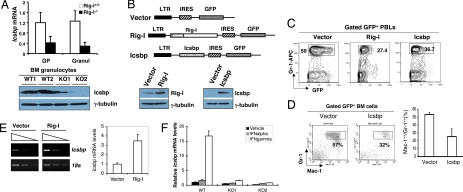
Orchestrated activities of myeloid transcription factors that are subject to the modulation of numerous environmental cues govern normal granulocytic development. The gene-targeting procedures of these transcription factors in mice greatly perturb normal myelopoiesis. In certain cases, they produce the phenotypes of biased granulocytosis similar to what we observed in Rig-I−/− mice (10–15). To understand the molecular mechanisms underlying the consequence of Rig-I loss on granulopoiesis modulation, we started by comparing the mRNA expression profile of Rig-I+/+ granulocytes with that of Rig-I−/− granulocytes to search for the known granulopoiesis-regulatory mechanism that could be affected by Rig-I deficiency. The results revealed that among some 200 genes with altered expression, the mRNA and protein levels of myeloid-specific transcription factor Icsbp were mostly deregulated in Rig-I−/− myeloid cells (Fig. 4A), whereas those for Pu.1, C/ebpα, C/ebpβ, C/ebpγ, C/ebpε, and their target genes, including Gm-csfr, P8, and G-csfr, were not affected [supporting information (SI) Fig. S1]. It is significant in considering that Icsbp deficiency has been shown to lead to a similar phenotype of biased granulocytosis (12, 13). To further examine a possible Rig-I and Icsbp functional connection, we performed an Icsbp rescue experiment in which the in vivo granulocytic differentiation potentials of 5-FU–treated Rig-I−/− BM cells were measured after they had been transduced with either empty retroviral vector MigR1, or Rig-I cDNA, or Icsbp cDNA (Fig. 4B) and subsequently transplanted into lethally irradiated wild-type littermates. As shown in Fig. 4C, the reintroduction of Icsbp cDNA, like that of Rig-I cDNA, reduced granulocytosis of PBLs 4 weeks after transplantation. Furthermore, restoration of Icsbp activation not only decreased the granulocytosis of BM tissue in 8 weeks after transplantation but also drove Rig-I−/− granulocytes to further differentiation, as indicated by the elevated expression levels of maturation-related antigen Mac-1 in Icsbp cDNA-transduced Gr-1++/Mac-1++ granulocytes (Fig. 4D).
Fig. 4.
Reduced Icsbp expression is associated with Rig-I deficiency-caused granulocytosis. (A) Relative mRNA levels and protein levels of Icsbp in granulocytes of Rig-I+/+ or Rig-I−/− mice were determined by the real-time PCR method (n = 3, mean ± SD, P < 0.01) or by Western blotting assay (representative results from two Rig-I−/− mice were shown). (B) (Upper) Bicistronic structures of MigR1 empty vector, MigR1–Rig-I, and MigR1-Icsbp. (Lower) Western blotting assay confirmed the expected expression of Rig-I or Icsbp protein after the transduction. (C and D) Retroviral transduction of primitive Rig-I−/− BM cells either by Rig-I cDNA or Icsbp cDNA inhibited the generation of mature granulocytes in vivo. (C) Percentages of PBL Gr-1++ granulocytes in the indicated recipients were examined 4 weeks posttransplantation, after their receiving genetically modified Rig-I−/− BM cells (GFP+, n = 5). (D) Percentages of Gr-1++/Mac-1++ cells within the transduced BM cells (GFP+) of indicated recipients 8 weeks after transplantation, P < 0.01. (E) Rig-I−/− BM cells were transduced in vitro with the empty vector or Rig-I cDNA in the presence of 50 ng/ml of SCF, 10 ng/ml of IL-6 and IFN-γ, 6 ng/ml of IL-3, and 5% FCS. Icsbp mRNA levels were measured by semiquantitative RT-PCR assay (as in Fig. 1E) and real-time RT-PCR assay. (F) Rig-I+/+ or Rig-I−/− myeloid cells were stimulated with either IFN-α or IFN-γ for 12 h, and Icsbp mRNA levels of indicated cells were measured by real-time PCR assays.
To confirm that an autonomous defect accounts for the reduced Icsbp expression in Rig-I−/− hematopoietic cells, we isolated c-Kit+ progenitor cells from Rig-I−/− BM and transduced them in vitro with either empty MigR1 vector or MigR1–Rig-I retroviruses. As shown in Fig. 4E, in the presence of 5% FCS, 10 ng/ml of IFN-γ, and other hematopoietic cytokines supporting myeloid differentiation, the transduction of Rig-I cDNA, but not of vector, significantly elevated the Icsbp mRNA level in transduced myeloid cells (GFP+), suggesting that the expression level of Rig-I within myeloid cells is a critical factor determining the expression level of Icsbp. Nevertheless, Icsbp mRNA levels in both Rig-I+/+ and Rig-I−/− myeloid cells were not up-regulated by the stimulation of type I IFN even at high concentration (Fig. 4F), suggesting that this intrinsic defect in Rig-I–deficient myeloid cells that results in the reduced expression of Icsbp may not be due to a feasible defective production of type I IFN within hematopoietic microenvironment.
Discussion
In the current model of innate immunity, RIG-I analogues are depicted as a kind of molecules with two basic functional domains: a C-terminal part, behaving as a default N-terminal CARD inhibitory domain whose inhibitory function can be zeroed by sensing and binding to multiple forms of cytoplasmic viral RNAs, and an N-terminal CARD domain, acting as a signal transducer through its direct binding with mitochondrial membrane-anchored IPS-1/MAVS/VISA/Cardif. Although this model does well in explaining the roles of RIG-I analogues in inducing type I IFN transcription upon certain viral infections, it hardly explains their involvement in the differentiation and/or apoptosis-induction processes of malignancies in the absence of viral infection and foreign RNA transfection. In this study, we provide further in vivo evidence supporting that Rig-I intrinsic activities other than sensing foreign RNAs exist, as evident by its crucial role in maintaining the homeostasis of late granulopoiesis by negatively regulating the proliferation and survival of granulocytes. Theoretically, this activity might be exploited by innate immunity machinery, after the up-regulation of RIG-I by type I IFN signaling, to inhibit the survival of viral infected host cells, thus limiting the propagation and spreading of viral particles.
It is notable that a previously reported Rig-I gene-targeting procedure led most of Rig-I−/− mice to die before birth (16). The massive cell death, as observed in embryonic hepatic tissue, actually provided evidence supporting the notion that Rig-I might harbor an intrinsic role in regulating cellular development and survival in the absence of viral infection. However, the newer Rig-I−/− mice we used grow up to adulthood, which provides us an opportunity to study the possible influence of Rig-I deficiency on the development of adult tissues, such as hematopoiesis. This striking phenotype discrepancy between these two Rig-I−/− models could be basically due to the fact that in these two models, two different regions of Rig-I gene in two different mouse strains were targeted. Interestingly, like our Rig-I−/− models, the gene-targeting mice of Mda-5 and Ips-1 (6, 17), a Rig-I analogue and a direct partner of the Rig-I signal transduction pathway in activating the transcription of type I IFN induction, respectively, both also lived up to adulthood.
Previously, we reported that Rig-I deficiency results in the frequent occurrence of a colitis-like phenotype (70%), with the underlying mechanisms not fully understood. Although it is known that colitis is usually associated with anemia rather than with a progressive granulocytosis, which would evolve to a CML-like phenotype, we cannot currently completely exclude the possibility that the accompanying inflammation might, more or less, contribute to the severity of granulocytosis in some mice. However, it is evident that one intrinsic defect in Rig-I−/− myeloid cells contributes to the initiation and development of granulocytosis, because the apparent granulocytosis preceded colitis by ≈2 weeks and two rounds of transplantation of Rig-I−/− BM cells into the normal host environment consistently recapitulated the phenotype of granulocytosis (Fig. 3 A and B). Furthermore, in vitro colony assays also revealed an intrinsic defect of Rig-I−/− myeloid progenitors in term of their proliferation and differentiation potentials, as stimulated by myeloid cytokines (Fig. 3 C–E).
Our data also suggest that the reduced expression level of Icsbp, is at least partly underlying the mechanism responsible for the biased granulocytosis in Rig-I–deficient mice. However, although the critical role of Icsbp in determining the fates of myeloid differentiation has been well established, the environmental cues or upstream transcriptional regulatory mechanism are not understood. Hence, it is currently not known how Rig-I deficiency affects the expression regulation of Icsbp in myeloid cells. Coincidently, the expression levels of several other potential regulators of myelopoiesis, such as Klf4 and Trail, were also altered in Rig-I−/− myeloid cells. It is interesting to further investigate whether a shared upstream regulatory pathway that is modulated by Rig-I activity exists. However, the in vitro Rig-I rescue experiment (Fig. 4E) demonstrates that Icsbp expression reduction is ascribed to an autonomous defect of Rig-I−/− myeloid cells via a mechanism different from Rig-I–mediated type I IFN generation. It could be postulated that Rig-I deficiency might lead to a lower level of type I IFN within the microenvironment that governs granulocytic differentiation, but this is not likely to be the major reason causing Icsbp reduction in myeloid cells, because our data and those of others suggest that type II, but not type I, IFNs are upstream regulators of Icsbp expression (18, 19). The possible effect of the Rig-I–Icsbp pathway on the in vivo proliferation of leukemia-initiating cells is worthy of investigation in the near future.
Materials and Methods
Mice and Cells.
Rig-I KO mice and littermates (129S3) were bred in the animal facility of Shanghai Jiao-Tong University with sterilized water and food. Bone marrow samples of APL patients were obtained with informed consent.
Flow Cytometry.
All of the fluorescence-labeled antibodies and the Annexin V detection kit were purchased from BD PharMingen. The flow cytometry data were collected by a FACSCalibur machine (Becton Dickinson) and analyzed by FlowJo software. The stained cells were sorted out with a MoFlo high-speed cell sorter (DakoCytomation) installed in the Shanghai Institute of Hematology.
RT-PCR.
RNA samples were isolated by using TRIzol (Invitrogen) or the RNeasy Mini Kit (QIAGEN GmbH). The real-time PCR reagents (SYBR green) were purchased from Applied Biosystems and performed by following the manufacturer's protocol. The primer sequences for real-time PCR are listed as follows: mouse Rig-I, forward: CCACCTACATCCTCAGCTACATGA, reverse: TGGGCCCTTGTTGTTCTTCT; human RIG-I, forward: GGACGTGGCAAAACAAATCAG, reverse: GCAATGTCAATGCCTTCATCA; and mouse Icsbp, forward: CACCAACCAGTTCATCCGAGA, reverse: TGTCAGGTAGGCGGCTCTG. The primer sequences for semiquantitative reverse transcriptase PCR are listed as follows: mouse Rig-I, forward: ATTCAGGAAGAGCCAGAGTGTC, reverse: GTCTTCAATGATGTGCTGCAC; human RIG-I, forward: TGATCGATTCCATCACTATCCATC, reverse: CCTGTACAATCTCTTCGAATCCTG; and mouse Icsbp, forward: GTTCGTGAAGCGGCTGTGCC, reverse: CTCTTGCCCGCTTCCTCCAC.
Western Blot Analysis.
The Icsbp antibody was purchased form Santa Cruz Biotechnologies. The γ-tubulin antibodies were purchased from Sigma. In Figs. 1A and 2A, a polyclonal RIG-I antibody was a generous gift from Tadaatsu Imaizumi (Hirosaki University School of Medicine, Hirosaki, Japan). In all other cases, RIG-I antibody was isolated from rabbit serum immunized by purified human RIG-I protein.
Immunofluorescence.
Bone marrow cells were stained with a RIG-I C15 antibody (Santa Cruz Biotechnologies), washed, and then restained with Alexa Fluor 555-coupled donkey anti-goat antibody (Molecular Probes).
Colony Assay.
Bone marrow cells were cultivated in 0.9% methylcellulose semisolid culture media supplemented with 15% FCS and appropriate amount of cytokines (R&D). The cultures, set up in triplicate in 24-well plates, were incubated in a humidified chamber in the conditions of 5% CO2 and 37°C for 7 days before scoring.
Histological Methods.
Tissues were sequentially fixed in 4% formaldehyde/PBS, paraffin-embedded, sectioned, and stained with hematoxylin-eosin. Otherwise, 2 × 105 cells were cytospined onto glass slides and stained with May-Grünwald and Giemsa solutions (Fluka).
Rig-I cDNA, Icsbp cDNA, MigR1 Retroviral Vector, and Preparation of Retroviruses.
Complementary DNA for Rig-I and Icsbp was amplified from normal murine RNA samples by Pfu DNA Polymerase (Stratagene). MigR1 retroviral vector was obtained from Warren Pear (University of Pennsylvania, Philadelphia). For the preparation of retroviruses, the ecotropic packaging cells EcoPack2–293 (Clontech) were transiently transfected with MigR1, MigR1-Rig-I, or MigR1-Icsbp plasmid plus Superfect (Qiagen).
Transduction of Rig-I or Icsbp cDNA into Rig-I−/− Primitive BM Cells and BM Transplantation.
Primitive BM cells were enriched by pretreating the donor mice with 5-FU (Amersham Pharmacia and Upjohn) at a concentration of 150 mg/kg for 4 days before BM cell harvesting. 5-FU–treated BM cells were incubated in MyeloCult M5300 (StemCell Technologies) supplemented with 6 ng of IL-3, 10 ng of IL-6, and 100 ng of SCF per milliliter (R&D Systems) and were infected by spinoculation. Six to 8 h after the second infection, the nonadherent cells were harvested and washed in PBS once. A total of 1 × 105 of live nonadherent cells were injected into the tail vein of each syngeneic recipient that had received split 10-Gy whole-body γ-ray irradiation. For the in vitro Rig-I rescue experiment, Rig-I−/− c-Kit+ BM cells were sorted out with a MoFlo high-speed cell sorter and infected with vector or Rig-I retrovirus as previously described. Then, the GFP+ cells were sorted out and incubated in RPMI medium 1640 containing 5% FCS supplemented with 10 ng/ml of IFNγ for 12 h.
Supplementary Material
Acknowledgments.
We thank Drs. Zheng-Yi Wang (Shanghai Institute of Hematology) and Stephen G. Emerson (University of Pennsylvania) for strong support to this study. This work was supported by Chinese National Key Basic Research Project Grant 973, 204CB518606, Chinese National High Tech Program Grant 863, 2006AA02A405, the Samuel Waxman Cancer Research Foundation Co-Principal Investigator Program, National Scientific Foundation of China Grant 30572110, Science and Technology Committee of Shanghai Municipal Government Major Research Projector Grant 06dj14002, Shanghai Pu-Jiang Project Grant 05PJ14059, and E-Institutes of Shanghai Municipal Education Commission Grant E03003.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804895105/DCSupplemental.
References
- 1.Liu TX, et al. Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood. 2000;96:1496–1504. [PubMed] [Google Scholar]
- 2.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 3.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWhirter SM, Tenoever BR, Maniatis T. Connecting mitochondria and innate immunity. Cell. 2005;122:645–647. doi: 10.1016/j.cell.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Zhao T, et al. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 6.Gitlin L, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berghall H, et al. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 2006;8:2138–2144. doi: 10.1016/j.micinf.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Kang DC, et al. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Rig-I(−/−) mice develop colitis associated with downregulation of Galphai2. Cell Res. 2007;17:858–868. doi: 10.1038/cr.2007.81. [DOI] [PubMed] [Google Scholar]
- 10.Passegue E, Wagner EF, Weissman IL. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119:431–443. doi: 10.1016/j.cell.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbauer F, et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU. 1. Nat Genet. 2004;36:624–630. doi: 10.1038/ng1361. [DOI] [PubMed] [Google Scholar]
- 12.Holtschke T, et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 13.Turcotte K, et al. A mutation in the Icsbp1 gene causes susceptibility to infection and a chronic myeloid leukemia-like syndrome in BXH-2 mice. J Exp Med. 2005;201:881–890. doi: 10.1084/jem.20042170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CK, et al. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 15.Kimura A, et al. SOCS3 is a physiological negative regulator for granulopoiesis and granulocyte colony-stimulating factor receptor signaling. J Biol Chem. 2004;279:6905–6910. doi: 10.1074/jbc.C300496200. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Kumar H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egwuagu CE, et al. Interferon-gamma induces regression of epithelial cell carcinoma: Critical roles of IRF-1 and ICSBP transcription factors. Oncogene. 2006;25:3670–3679. doi: 10.1038/sj.onc.1209402. [DOI] [PubMed] [Google Scholar]
- 19.Kanno Y, et al. The genomic structure of the murine ICSBP gene reveals the presence of the gamma interferon-responsive element, to which an ISGF3 alpha subunit (or similar) molecule binds. Mol Cell Biol. 1993;13:3951–3963. doi: 10.1128/mcb.13.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.