Abstract
Programmed death-1 (PD-1) is a member of the CD28/B7 superfamily that delivers negative signals upon interaction with its two ligands, PD-L1 or PD-L2. The high-resolution crystal structure of the complex formed by the complete ectodomains of murine PD-1 and PD-L2 revealed a 1:1 receptor:ligand stoichiometry and displayed a binding interface and overall molecular organization distinct from that observed in the CTLA-4/B7 inhibitory complexes. Furthermore, our structure also provides insights into the association between PD-1 and PD-L1 and highlights differences in the interfaces formed by the two PD-1 ligands (PD-Ls) Mutagenesis studies confirmed the details of the proposed PD-1/PD-L binding interfaces and allowed for the design of a mutant PD-1 receptor with enhanced affinity. These studies define spatial and organizational constraints that control the localization and signaling of PD-1/PD-L complexes within the immunological synapse and provide a basis for manipulating the PD-1 pathways for immunotherapy.
Keywords: costimulation, coinhibition, inhibitory receptor, T cell activation
T cell activation requires a primary antigen-specific signal that results from the engagement of the T cell receptor (TCR) with antigenic peptide presented in the context of the major histocompatibility complex (MHC). The strength, duration, and course of this response are modulated by antigen-independent signals provided by a number of distinct costimulatory molecules, including members of the CD28/B7 family. Upon binding its ligands, B7–1 and B7–2, the constitutively expressed CD28 delivers stimulatory signals for T cell proliferation, expansion, and differentiation. In contrast, CTLA4 (≈30% sequence identity with CD28) expressed on activated T cells delivers negative signals upon binding to the same B7 ligands (1). The balance between costimulatory and coinhibitory signals is crucial for maximizing immune responses while maintaining immunological tolerance. Both CTLA-4 and CD28 consist of a single immuno- globulin variable (IgV) ectodomain linked to a cytoplasmic tail containing tyrosine-based signaling motifs (1). The IgV domain contains a proline-rich motif (MYPPPY) that is responsible for ligand binding. In vivo both CD28 and CTLA4 exist as covalent homodimers because of an interchain disulfide formed by a conserved cysteine residue in the linker region connecting the IgV domain to the transmembrane segment. The in vivo oligomeric states of the ligands are less characterized, but recent studies demonstrated that B7–1 has high propensity to oligomerize on the cell surface (2). The oligomeric and polyvalent features of these receptors and ligands may contribute to the formation and localization of multicomponent signaling assemblies at the immunological synapse.
Programmed death-1 (PD-1) is a member of the CD28/B7 family that plays an important role in negatively regulating immune responses (1). In contrast to other receptors in this family, upon activation, PD-1 expression is induced not only on T cells but also on B cells and myeloid cells (3). Concomitant with TCR or BCR cross-linking, engagement of PD-1 by its ligands, PD-L1 (4) or PD-L2 (5), induces inhibitory signals through the recruitment of phosphatases, such as SHP-2, to the immunoreceptor tyrosin-based switch motif (ITSM) of the cytoplasmic tail of PD-1, resulting in dephosphorylation of effector molecules involved in downstream TCR or BCR signaling (5, 6). PD-1 signaling plays an important role in inducing and maintaining peripheral tolerance. PD-1 ligands (PD-Ls) on antigen-presenting cells have been shown to inhibit autoreactive T cells and induce peripheral tolerance, whereas those on parenchymal cells prevent tissue destruction by suppressing effector T cells to maintain tolerance (7). The inhibitory role of PD-1 is highlighted by the phenotype of PD-1 deficient mice, which develop various autoimmune diseases, depending on the genetic background (8, 9). In humans, single-nucleotide polymorphisms of the PD-1 gene may be linked to various autoimmune diseases, such as rheumatoid arthritis, SLE, and diabetes, and the PD-1/PD-L pathway is crucial in establishing fetomaternal tolerance and maintaining the integrity of immuno-privileged sites (7). The PD-1/PD-L pathway is frequently exploited as a target for immune evasion by tumor cells (10) and by a wide range of pathogens (11).
Human and mouse PD-1 share ≈60% amino acid identity, whereas the extracellular IgV domain shows only 21% and 16% sequence identity with CD28 and CTLA4, respectively. Consistent with this modest sequence similarity, PD-1 exhibits important differences relative to the other CD28 family members, including the lack of the proline-rich ligand recognition loop and the absence of the cysteine residue responsible for disulfide bond formation (12). Like other B7 homologs, PD-L ectodomains consist of a membrane distal IgV and a membrane proximal IgC domain. PD-L1 and PD-L2 share 34% identity with each other and ≈20% identity with B7–1 and B7–2. The PD-Ls differ in their patterns of expression and affinity for PD-1. PD-L2 exhibits 3-fold higher affinity for PD-1 (13) and is restricted to activated dendritic cells and macrophages. In contrast, PD-L1 is constitutively expressed and up-regulated on antigen presenting cells (APCs), and T cells and a variety of nonhematopoietic cell types (14).
The roles that PD-L1 and PD-L2 play in T cell activation are diverse and both stimulatory and inhibitory functions for these ligands are reported in ref. 15. It was also recently demonstrated that B7–1 can bind T cell-associated PD-L1, resulting in the inhibition of T cell proliferation and cytokine production (16). This finding highlights the complexity of T cell costimulation, because competitive binding interactions between multiple molecules provide mechanisms for linking the PD-1, CTLA-4, and CD28 pathways.
We report the 1.8-Å-resolution structure of the murine PD-1/PD-L2 complex, which reveals a binding interface different from that observed in the CTLA-4/B7–1 and CTLA-4/B7–2 complexes. We also report the 1.77-Å-resolution structure of the isolated PD-L2 IgV domain. The structural and organizational features of the PD-1/PD-L2 complex provide important mechanistic constraints that must be accommodated in models describing signaling associated with the PD-1/PD-L2 and PD-1/PD-L1 complexes. Mutagenesis studies confirm the details of the binding interfaces between PD-1 and the PD-Ls, and suggest the basis for designing novel tools for immunotherapy.
Results
Overall Structure of PD-1/PD-L2 Complex.
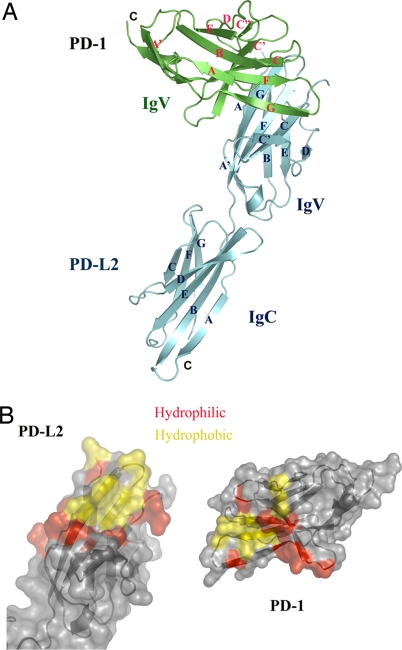
The structure of the PD-1/PD-L2 complex reveals an assembly with 1:1 receptor–ligand stoichiometry and a binding interface formed by the front β-sheets of both the PD-1 and PD-L2 IgV domains (Fig. 1A). Residues from the GFCC′ strands and CC′, CC″, and FG loops of PD-1 contribute to the binding interface and pack against the AGFC strands and the FG loop of the PD-L2 IgV domain, burying a total surface area of 1,915 Å2. Eighteen potential hydrogen bonds are formed by 11 residues contributed by the C, C′, and G-strands and the CC′ and FG loops in PD-1 and 11 residues from the A, C, F, and G strands and the AA′ and FG loops in PD-L2 [supporting information (SI) Table S1 and Fig. S1]. A small hydrophobic core, formed by residues from the C, F, and G strands and the FG loop in PD-1 and residues from the F and G strands and the FG loop in PD-L2, also contributes to the binding interface and buries a surface area of 570 Å2. Additional hydrophobic contacts are formed between residues in the C″ strand and the C″D loop of PD-1 and the A strand in PD-L2 (Fig. 1B and Fig. S1).
Fig. 1.
Structure of the PD-1/PD-L2 complex. (A) Overall structure of the PD-1/PD-L2 complex. Green, PD-1; cyan, PD-L2. The strands of PD-1 and PD-L2 are labeled in red and blue, respectively. (B) Surface representation of PD-1/PD-L2 binding interface. Red, hydrophilic residues in the binding interface; yellow, hydrophobic residues in the binding interface. PD-L2 is in the same orientation as in A; PD-1 is rotated 180° about a vertical axis to reveal the binding surface.
Most of the residues in the binding interface are conserved (12 of 16 residues from PD-1 and 10 of 14 residues from PD-L2 are identical) between human and mouse sequences (Figs. 2 and 3). Six of the 14 residues involved in binding PD-1 are fully conserved between the two ligands in all species examined, and two additional residues are very similar between the PD-L2 and PD-L1 sequences (E versus D or Q versus E) (Fig. 3). This conservation suggests that both PD-L1 and PD-L2 may form similar assemblies with PD-1. Importantly, the positions of all predicted glycosylation sites in PD-1 and the PD-Ls are consistent with the observed mode of interaction between PD-1 and PD-L2 and that proposed for the PD-1/PD-L1 complex (Figs. 2 and 3).
Fig. 2.
Alignment of the PD-1 ectodomains. The β strands in mouse PD-1 are denoted with arrow segments above the sequence. Red shading, conserved residues; red labeling, residues with similar properties; green triangles, residues bearing potential N-glycans; green asterisks, residues that contribute to binding to PD-L2.
Fig. 3.
Alignment of the extracellular domains of PD-L1 and PD-L2. The β strands in mouse PD-L2 are denoted with arrows above the sequence. Red shading, conserved residues; red labeling, residues with similar properties; triangles, residues bearing potential N-glycans; pink, residues conserved between PD-Ls; green, residues conserved for PD-L2 only; green asterisks, residues that contribute to receptor binding of PD-L2; filled circles, residues forming the interdomain hydrogen bonds between PD-L2 IgV and IgC domains.
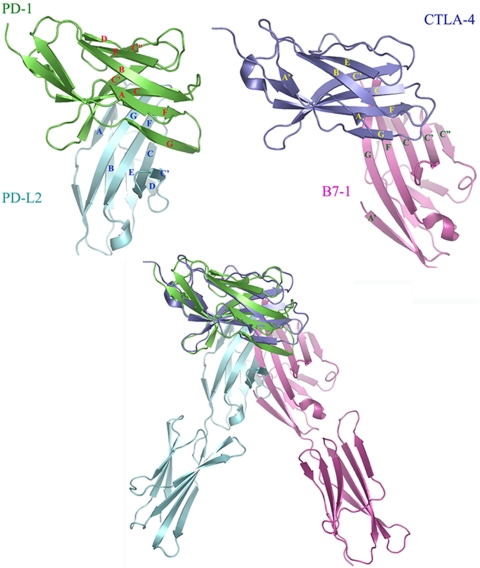
The organization of the PD-1/PD-L2 complex differs considerably from that observed in the CTLA-4/B7–1 and CTLA-4/B7–2 structures, in which the CTLA-4 and the B-7 IgV domains cross at ≈90° as opposed to 60° in the PD-1/PD-L2 complex. The overall difference in organization is highlighted by direct superimposition of CTLA-4 and PD-1 in the two complexes (Fig. 4). The PD-1/PD-L2 interface is formed by residues distributed over the front sheets and associated loops of both molecules, with the approximate center of the interface defined by the interaction of W110 and Y112 in the G-strand of PD-L2 and with a concave surface on the front face of PD-1 formed by the C, F, and G strands (Fig. 5A). In contrast, the majority of the CTLA-4/B7 interfaces arise from contributions of the invariant FG loop (MYPPPY) in CTLA-4, which packs against a concave surface formed by the C,C′ strands and the CC′ and C″D loops on the front sheet of the B7 molecules. These different binding interactions result in a relatively compact PD-1/PD-L2 complex with an end-to-end distance that spans ≈76 Å, compared with the CTLA-4/B7 complexes that span ≈100 Å. Notably, the linker regions connecting the ectodomains and transmembrane segments are longer for PD-1 (20 residues) and PD-L2 (11 residues) than those present in CTLA-4 (6 residues) and B7–1 (9 residues), and could easily allow the PD-1/PD-L2 complex to span an end-to-end distance comparable with the linear dimensions of other pairs of signaling molecules within the immunological synapse (17). Furthermore, based on the structures of the PD-1/PD-L2 complex and the isolated PD-1 and PD-L2 IgV domains, complex formation results in very modest binding-induced structural alterations, which seem insufficient to directly transduce signals across the plasma membrane (see SI Results and Fig. S2).
Fig. 4.
Comparison of the PD-1/PD-L2 and the CTLA-4/B7–1 complexes. (Upper Left) IgV domains of PD-1 (green) and PD-L2 (cyan) in the PD-1/PD-L2 complex. (Upper Right) IgV domains of CTLA-4 (blue) and B7–1 (magenta) in the CTLA-4/B7–1 complex. (Lower) Overlay of the entire PD-1/PD-L2 and CTLA-4/B7–1 complexes by superimposition of PD-1 and CTLA-4.
Fig. 5.
Mapping the binding interface between PD-1 and PD-L2. (A) The core of the binding interface: electron density of residues W110 through Y114 from the G strand of PD-L2 contacting the front concave surface of PD-1 formed by the C, F, and G strands. (B) Receptor binding interface of PD-L2. Mutation of those residues shown in red results in significantly reduced or no binding. Other residues predicted to form the PD-L2 binding site based on the structure of the complex are shown in magenta. Mutation of those residues shown in gray had little or no effect on binding to PD-1. (C) Binding of cell surface expressed PD-L2 mutants (DW110 indicates deletion of residue W110) to PD-1 Ig (0.5 and 5 μg/ml), detected by flow cytometry. Data are shown as percentages of MFI values of wild-type PD-L2 binding to PD-1. (D) Ligand binding surface of PD-1. Mutation of those residues shown in red results in significantly reduced or no binding to either of the ligands. Mutation of those residues shown in blue decreases binding to PD-L1 only, but not to PD-L2. Mutation of A99 (green) increases affinity for both ligands. Other residues contributing to the ligand-binding site based on the structure of the complex are shown in magenta. Mutation of those residues shown in gray had little or no significant effect on binding to PD-L2. (E) Binding of PD-1 mutants to PD-L1 (blue) and PD-L2 (purple) Ig. Data are shown as percentages of MFI values of wild-type PD-1 binding to either PD-L1 or PDL-L2.
PD-L2 Ectodomain Structure.
The PD-L2 monomer exhibits a rod-like shape, with two Ig domains (IgV and IgC domain) joined by a short linker region (Fig. S3). The PD-L2 IgV domain exhibits classic two-layer β-sandwich topology, with front and back sheets composed of the A′GFCC′ and ABED strands, respectively. This IgV domain possesses the hallmark intersheet disulfide linking the B and F strands that is a characteristic feature of many IgV domains, including all known PD-L1 and PD-L2 sequences. In the mouse PD-L2 structure, there is one additional disulfide bond linking the F strand and the BC loop, which is not present in human PD-L2 or other B7 family ligands. The PD-L2 IgV shares considerable structural similarity with the B7–1 and B7–2 IgV domains (RMSD of 1.59 Å and 2.33 Å). Notably, the PD-L2 IgV domain has a much shorter C′ strand than B7–1 and B7–2, and the C″ strand, which is typical of conventional IgV domains, is missing in the PD-L2 IgV domain. In addition, the A strand present in PD-L2 IgV domain is absent in the B7–1 and B7–2 IgV domains (Fig. S4).
The front and back sheets of the PD-L2 IgC domain are composed of the GFC and ABED strands, respectively. Before the current PD-L2 structure, human B7–1 was the only member of the B7 family whose full ectodomain structure was available (18, 19), and the IgC domains of these proteins superimpose with an RMSD of 1.55 Å (Fig. S3).
The overall rod-like architecture of PD-L2 is stabilized by specific interactions between the IgV and IgC domains. The IgV domain ends at A121 and is followed by a five residue stretch (122SYMRI126) that links the two Ig domains. The interdomain interface is stabilized by a series of hydrophilic and hydrophobic interactions: K120 from the end of the G strand of the IgV domain forms an ionic interaction with E201 from the G strand of the IgC domain; the side chain of S122 and main chain of A121 from the linker form potential hydrogen bonds with the side chain of E201 and main chain of Y148, respectively; V94 and V119 from the IgV domain and Y148 from the IgC domain contribute hydrophobic interactions (Fig. S3B). These residues are identical between all PD-L2 species, and five of seven residues are conserved between PD-L2 and PD-L1, suggesting that all PD ligand ectodomains adopt an approximately similar organization (Fig. 3).
Despite the absence of sequence conservation, the interdomain linkers of PD-L2 and B7–1 are similar in length (five versus six residues) and share similar main chain conformations (Fig. S3C). The PD-L2 IgV-IgC interface buries 689 Å2 of surface area, which is comparable with the 670 Å2 of buried surface area in B7–1. Superimposition of the N-terminal IgV domains of PD-L2 and B7–1 results in a significant deviation of the membrane-proximal IgC domains, which can be described as a rotation of ≈30° around the long axis of the molecule (Fig. S3C).
Confirmation of the PD-1/PD-L Binding Interfaces: PD-L2 mutants.
Our structure predicts that PD-1 binds on the front face of PD-L2, making the greatest number of contacts with the G strand, and additional contacts with the C and F strands. On this basis, PD-L2 mutants (E28A in the AA′ loop and W110A, ΔW110, D111A, Y112A, K113A, and Y114A in the G strand) were generated to map the receptor-binding surface of PD-L2 (Fig. 5B). Several mutants, such as D111A and K113A, within the proposed PD-1/PD-L2 interface abolished binding to PD-1-Ig (Fig. 5C). Mutation or deletion of W110, which resides in the middle of the interface, results in significantly reduced binding, and the Y114A mutation earlier also diminishes binding to PD-1. In addition, mutagenesis data have shown that the R101S, L103A and I105A mutations in the F strand of PD-L2 resulted in decreased affinity for PD-1 (20), consistent with the crystallographically observed binding interface.
Our data highlight the importance of the G strand of PD-L2 in contacting PD-1 (Figs. 3 and 5A). Of particular note is W110, located in the core of the binding interface, which forms the largest numbers of contacts with different residues from PD-1. Indeed, deletion of this residue or substitution with Ala reduces binding to PD-1 to <20% and 40% of the wild type, respectively. W110 is conserved in all known PD-L2 sequences, but is substituted with alanine in all PD-L1 sequences (Fig. 3). Residues D111 through K113 are conserved in all known PD-L sequences, suggesting that they are important for receptor recognition by both PD-L1 and PD-L2, consistent with idea that PD-L1 and PD-L2 form similar assemblies with PD-1. This similarity is further supported by earlier data showing that the I117A and K127A mutations in PD-L1, corresponding to PD-L2 residues L103 and K113 (F and G strands, respectively), result in decreased binding to PD-1. Notably, mutations of E60A and C115A in PD-L1 corresponding to Q60 (C strand) and R101 (F strand) in PD-L2 show >120% wild-type binding to PD-1, suggesting that these residues are also within the binding interface in the PD-1/PD-L1 complex (20).
The unique presence of W110 in PD-L2 and its important role in binding to PD-1 suggests that this residue might be a determinant for the higher affinity that PD-L2 exhibits toward PD-1 (Fig. S5). However, other differences exist, including a 14-residue insertion in PD-L1 after the C′ strand, which could also modulate receptor–ligand affinity in the PD-1/PD-L1 complex (Fig. 3). Nine of these residues are conserved in all known PD-L1 sequences, and it was reported that mutations in this segment affect binding of PD-L1 to PD-1 (20).
PD-1 Mutants.
Our previous mutagenesis studies (12) provided a gross description of the PD-1 residues that contribute to the binding interface. Based on the current structure, additional PD-1 mutants were examined by SPR and flow cytometry (Fig. 5D). Mutants such as K45A (C′ strand), I93A (F strand), I101A and E103A (G strand) exhibited unmeasurable or extremely weak binding to both PD-L1 and PD-L2, suggesting that the side chains of these residues are involved in recognition of both ligands (Fig. 5E). Notably, the A99L mutation of PD-1 results in higher binding not only to PD-L1 (12), but also to PD-L2, consistent with an ≈2-fold lower Kd for PD-L1 and an ≈3-fold lower Kd to PD-L2 (Fig. S5).
Our structure-based mutagenesis studies also allowed for epitope mapping of several monoclonal antibodies to PD-1. Notably, clone J43 showed reduced binding to the PD-1 mutants P97A, K98A, and A99L, suggesting that it recognizes an epitope involving residues 97PKA99 on the FG loop. Consistent with the current structure, this antibody has been reported to block the binding of both PD-L1 and PD-L2 Ig to PD-1-transfected cells and has been used extensively in in vivo models of immunological relevance (21). Other monoclonal antibodies to PD-1, such as RMP1–14 (blocking) and RMP1–30 (nonblocking), showed binding to all of the mutants tested, suggesting that they recognize different epitopes that are not within the ligand binding interface (data not shown).
In total, these mutagenesis experiments validate the crystallographically observed PD-1/PD-L2 interface and strongly support the similarity of the assemblies formed by PD-1 with both PD-L1 and PD-L2. These studies have also unexpectedly identified a mutant PD-1 receptor with novel biochemical properties that exhibits high affinity binding.
Interaction Between PD-1 and PD-Ls Is Sufficient to Drive Their Enrichment at a “Pseudosynapse.”
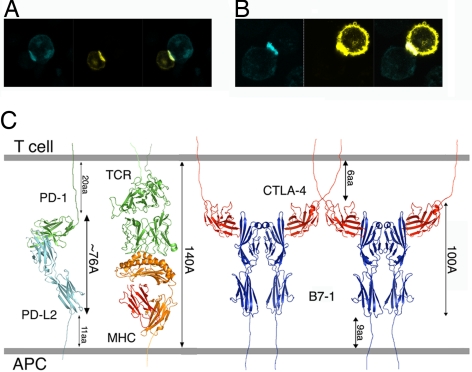
To further study the interaction between PD-1 and its ligands in the context of a cell–cell interface, an artificial pseudosynapse system was exploited. Both receptor and ligand were expressed on the cell membrane as either C-terminal CFP or YFP fusion proteins. Cells expressing either receptor or ligand were mixed, and the localization of the intercellular receptor–ligand complex was examined by confocal microscopy. A pattern of localization at the cell–cell interface that is characterized by increased intensity for both fluorophores is consistent with the accumulation of both receptor and the ligand at the pseudosynapse (Fig. 6 A and B). Our data show that nonimmune CHO cells expressing PD-1 can form stable conjugates with cells expressing either PD-L1 or PD-L2. These results suggest that the diffusive movement of costimulatory molecules driven by their mutual affinity could make important mechanistic contributions to the initiation and maturation of synapse formation.
Fig. 6.
PD-1/PD-L interaction at the cell–cell interface. Noncovalent interactions between PD-1 and PD-Ls are sufficient to drive their enrichment at a pseudosynapse. (A and B) PD-1 and PD-L1 (A) or PD-L2 (B) expressed in CHO cells are recruited to the cell–cell contact area and form conjugates that are analogous to the immunological synapse. (Left) PD-1-CFP-expressing cells in blue. (Center) PD-L1-YFP or PD-L2-YFP-expressing cells in yellow. (Right) Overlay of the CFP and YFP images. (C) Model of the PD-1/PD-L2 complex in the immunological synapse. A number of receptor–ligand assemblies have dimensions that are compatible with colocalization to the central zone of the immunological synapse.
Discussion
High-resolution crystallographic analysis revealed that the PD-1/PD-L2 complex is distinct from the CTLA-4/B7 inhibitory complexes in both overall organization and the detailed atomic interactions responsible for binding and specificity. Mutagenesis studies validated the basic features of this model and, in combination with primary sequence considerations, indicate that PD-L1 and PD-L2 form similar complexes with PD-1. These structural models provide several constraints that must be accommodated by any detailed mechanistic model describing signaling through the PD-1 pathway and the integration of these signals into the overall immune response.
Primary sequence considerations, including the lack of a proline-rich ligand recognition loop and the absence of a cysteine residue in the linker segment connecting the IgV and transmembrane domains, identify PD-1 as a unique member of the CD28/B7 family. In CTLA-4, the MYPPPY sequence motif in the FG loop contributes a large fraction of the contacts responsible for binding the B7 ligands. Within this motif, the three consecutive prolines adopt an unusual high-energy cis-trans-cis backbone conformation that provides the geometric complementarity required for efficient recognition of B7–1 and B7–2 (19, 22). It is notable that this same detailed FG loop conformation is present in the unliganded CTLA-4, indicating that CTLA-4 is poised in a preformed productive binding mode before ligand engagement (23). CD28 contains this same FG loop motif that is critical for B7 binding and the CD28 structure also exhibits the unique cis-trans-cis backbone conformation (24), suggesting that CTLA-4 and CD28 bind the B7 ligands with similar geometries. In contrast to these receptors, the FG loop of PD-1 possesses only a single proline and only the base of the loop contacts PD-L2. In addition, the apex of the FG loop, which corresponds to the highly rigid MYPPPY loop in CTLA-4 and CD28, displays considerable disorder in both the bound and unbound forms of PD-1 and makes no contacts, probably because of the absence of the C″ strand and the shortness of the C′ strand in PD-L2. Furthermore, our structural and mutagenesis results demonstrate that the residues involved in ligand recognition are more fully distributed over the front sheet of PD-1 than in CTLA-4 and other family members.
These differences in the binding interface result in a considerable difference in the overall organizations of the PD-1/PD-L2 and CTLA-4/B7 complexes. In particular, the PD-1/PD-L2 assembly is significantly more compact than the CTLA-4/B7 complexes, displaying end-to-end distances that span ≈76 and 100 Å, respectively. The dimensions of receptor–ligand complexes provide a convenient mechanism for the sorting and localization of signaling molecules within the immunological synapse, with small signaling molecules going to the central zone and larger adhesion molecules residing in the peripheral zone. Notably, the apparently small size of the PD-1/PD-L complexes is potentially compensated by the long linker segment connecting the PD-1 IgV domain to the transmembrane segment, allowing for appropriate colocalization with the pMHC/TCR complex and other costimulatory receptor/ligand pairs (Fig. 6C).
Another important feature of PD-1 is its oligomeric state. Both CTLA-4 and CD28 exist as disulfide-linked homodimers. In addition, B7–1 exhibits a considerable propensity to form noncovalent oligomers in solution and on the plasma membrane (2, 18). The higher order oligomeric states of these receptors and ligands afford the opportunity to assemble multicomponent signaling complexes with specific stoichiometries and geometries. For example, the crystal structures of the CTLA-4/B7 ectodomain complexes (19, 22) revealed that both receptor and ligand form unusual dimer interfaces that place their respective binding sites distal to the dimer interface, resulting in receptors and ligands that are bivalent. The bivalency of both CTLA-4 and the B7s supports the formation of a periodic, alternating arrangement of CTLA-4 and B7 homodimers, characterized by an ≈100-Å repeat between receptors that extends throughout the crystal. This periodic network provides a model for the assembly of these molecules at the T cell-APC interface and offers a mechanism for the localized enrichment of signaling molecules at the central zone of the immunological synapse. Although the organization of these assemblies are driven by interactions involving the ectodomains, these same constraints are imposed on the noncovalently associated cytoplasmic signaling and scaffolding proteins that are responsible for propagating and amplifying extracellular cues. In contrast to CTLA-4 and CD28, which are disulfide-linked homodimers, PD-1 lacks the extracellular equivalent of Cys-122 in CTLA-4 and, consequently, exists as a monomer in solution, in the crystalline state, and on the cell surface (12).
The monomeric state of PD-1 precludes the types of multivalent assemblies that may be associated with the localization and assembly of CTLA-4-containing complexes. Instead, our structural and cell-based data suggest that simple diffusive processes allow for the engagement of PD-1 and the PD-Ls in interacting cells and for the subsequent enrichment of PD-1/PD-L complexes at the central zone of the immunological synapse. Our demonstration of PD-1/PD-L complex formation in pseudoconjugates, which occurs in the absence of primary or costimulatory signals, supports an important role for diffusion within the plasma membrane for recognition, engagement, and synapse development. These mechanistic considerations are consistent with the recent report that the degree of enrichment of PD-1 at the immunological synapse depends on the affinity and availability of its ligands (25).
Finally, our mutagenesis studies identified a high affinity A99L mutant PD-1 receptor with twofold and threefold enhanced affinities for PD-L1 and PD-L2, respectively. It is notable that Belatacept, a modified CTLA-4-Ig containing two point mutations, possesses only a modest twofold increase in affinity for the B7 ligands relative to the wild type but remarkably exhibits a 10-fold enhancement in biological potency (26). In clinical trials Belatacept has demonstrated equivalent efficacy with fewer side effects than existing immunosuppresants for renal transplantation (27). By analogy, soluble (Ig-fusion) forms of the high affinity A99L PD-1 receptor could represent a superior reagent for the therapeutic modulation of the PD-/PD-L pathways.
In summary, our data demonstrate a unique 1:1 PD-1/PD-L2 assembly that exhibits distinct structural and organizational features compared with the CTLA-4/B7 inhibitory complexes. Structure-based mutagenesis studies have confirmed the unique ligand binding interfaces proposed for the PD-1/PD-L complexes and resulted in the generation of a high-affinity mutant with potential therapeutic value.
Experimental Procedures
Expression, Crystallization, Data Collection, and Structure Determination.
All materials for crystallization were expressed in Escherichia coli and refolded from inclusion bodies. Crystals of murine PD-1 in complex with PD-L2 or PD-L2 IgV alone were obtained by sitting drop vapor diffusion at 293K. All structures were solved by molecular replacement and refined by standard methods, resulting in Rwork/Rfree values of 18.8%/22.6% and 19.0%/22.3% for the PD-1/PD-L2 complex and the isolated PD-L2 IgV structures, respectively (Table S2). Details of expression, purification and structure determination are provided in SI Experimental Procedures.
Mutagenesis of PD-1 and PD-L2 and Transfection into HEK293T Cells.
Mutants of PD-1 for bacterial expression were designed by PCR-based mutagenesis, expressed in E. coli, and refolded as described in ref. 12. For mammalian cell surface expression, the PD-1 and PD-L2 mutants were transfected into HEK293T cells. Details are provided in SI Experimental Procedures.
Binding Assays.
SPR experiments were performed with a Biacore X optical biosensor at 25°C, using immobilized murine PD-L1-Ig or PD-L2-Ig fusion proteins. For flow cytometry, 293T cells were transiently transfected with PD-1 or PD-L2 and, as appropriate, incubated with PD-1, PD-L1, or PD-L2 Ig fusion proteins. Details are provided in SI Experimental Procedures.
Pseudoconjugate Assays.
PD-1, PD-L1, and PD-L2 were expressed as C-terminal fusion proteins of CFP or YFP. CHO cells were transiently transfected with PD-1-CFP, PD-L1-YFP, or PD-L2-YFP, using FuGENE (Roche). Conjugates were prepared by incubating cells expressing PD-1-CFP with cells expressing PD-L1-YFP/PD-L2-YFP and analyzed by using laser-scanning confocal microscopy. Details are provided in the SI Experimental Procedures.
Supplementary Material
Acknowledgments.
We thank the staff of the X29 beam lines at the National Synchrotron Light Source, R. Toro for assistance with the crystallization robot, and X. Zang for critical reading of the manuscript. This work was supported by National Institute of Health Grant AI07289 (to S.G.N. and S.C.A.); a postdoctoral fellowship from Cancer Research Institute (to E.L.-M.); and Albert Einstein Cancer Center Grant P30CA013330; and the Flow Cytometry, the Structural Biology Core Facilities, and the Analytical Imaging Facility.
Note.
While this manuscript was under review, the crystal structure of the complex between human PD-L1 and mouse PD-1 was reported (28). The structure-based alignment of the PD-L IgV domains (Fig. 2D) was manually edited based on the reported PD-L1 structure.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and reflections have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3BP5 and 3BOV).
See Commentary on page 10275.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804453105/DCSupplemental.
References
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Edidin M, Almo SC, Nathenson SG. Different cell surface oligomeric states of B7–1 and B7–2: Implications for signaling. Proc Natl Acad Sci USA. 2005;102:15569–15574. doi: 10.1073/pnas.0507257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 4.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 10.Zang X, Allison JP. The B7 family and cancer therapy: Costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 13.Youngnak P, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–677. doi: 10.1016/s0006-291x(03)01257-9. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 15.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 16.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz JC, Zhang X, Nathenson SG, Almo SC. Structural mechanisms of costimulation. Nat Immunol. 2002;3:427–434. doi: 10.1038/ni0502-427. [DOI] [PubMed] [Google Scholar]
- 18.Ikemizu S, et al. Structure and dimerization of a soluble form of B7–1. Immunity. 2000;12:51–60. doi: 10.1016/s1074-7613(00)80158-2. [DOI] [PubMed] [Google Scholar]
- 19.Stamper CC, et al. Crystal structure of the B7–1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, et al. Molecular modeling and functional mapping of B7–H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7–2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 23.Ostrov DA, Shi W, Schwartz JC, Almo SC, Nathenson SG. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 2000;290:816–819. doi: 10.1126/science.290.5492.816. [DOI] [PubMed] [Google Scholar]
- 24.Evans EJ, et al. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- 25.Pentcheva-Hoang T, Chen L, Pardoll DM, Allison JP. Programmed death-1 concentration at the immunological synapse is determined by ligand affinity and availability. Proc Natl Acad Sci USA. 2007;104:17765–17770. doi: 10.1073/pnas.0708767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen CP, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 27.Vincenti F, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 28.Lin DY, et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci USA. 2008;105:3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.