Abstract
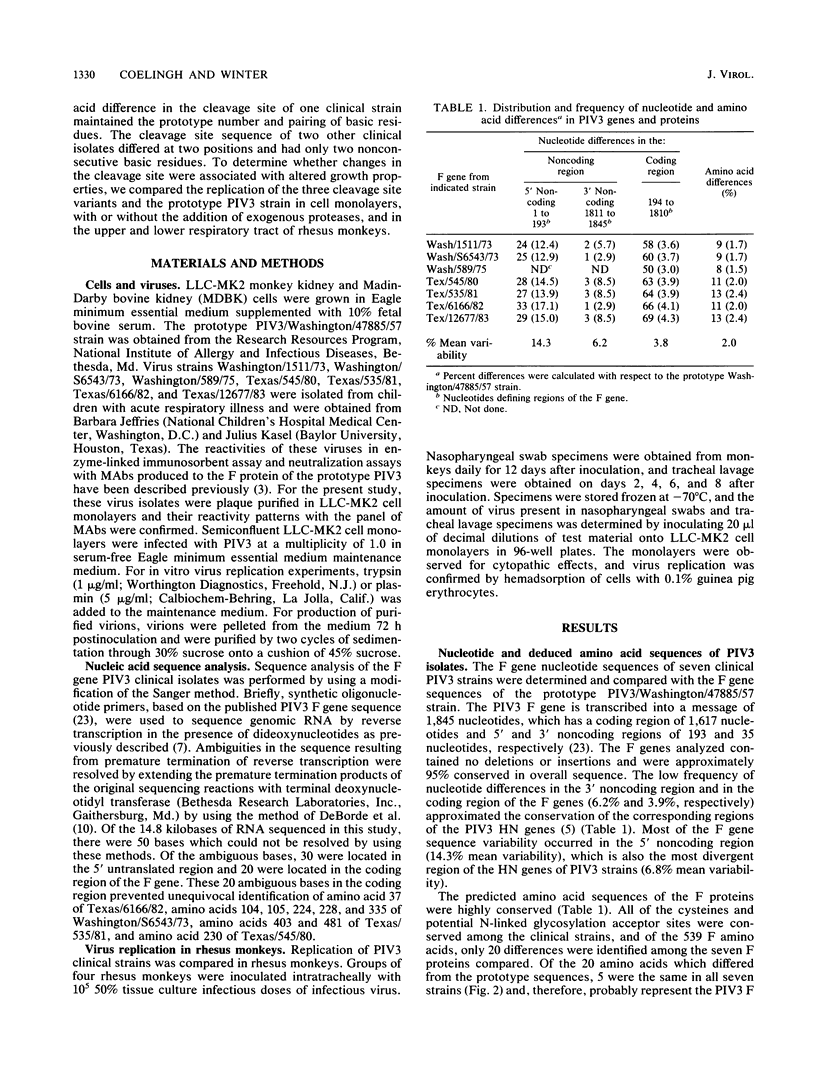
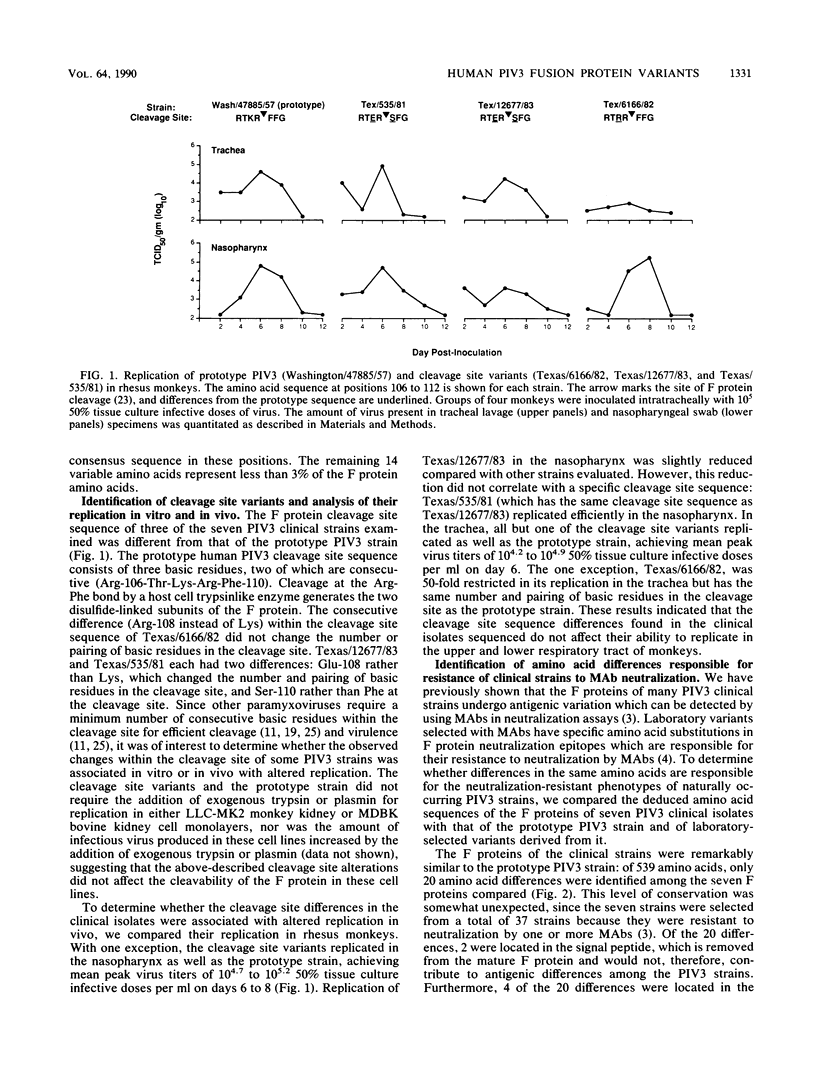
Many human parainfluenza type 3 virus (PIV3) strains isolated from children with respiratory illness are resistant to neutralization by monoclonal antibodies (MAbs) which recognize epitopes in antigenic site A or B of the fusion (F) protein of the prototype 1957 PIV3 strain. The F protein genes of seven PIV3 clinical isolates were sequenced to determine whether their neutralization-resistant phenotypes were associated with specific differences in amino acids which are recognized by neutralizing MAbs. Several clinical strains which were resistant to neutralization by site A or B MAbs had amino acid differences at residues 398 or 73, respectively. These specific changes undoubtedly account for the neutralization-resistant phenotype of these isolates, since identical substitutions at residues 398 or 73 in MAb-selected escape mutants confer resistance to neutralization by site A or B MAbs. The existence of identical changes in naturally occurring and MAb-selected neutralization-resistant PIV3 strains raises the possibility that antigenically different strains may arise by immune selection during replication in partially immune children. Three of the seven clinical strains examined had differences in their F protein cleavage site sequence. Whereas the prototype PIV3 strain has the cleavage site sequence Arg-Thr-Lys-Arg, one clinical isolate had the sequence Arg-Thr-Arg-Arg and two isolates had the sequence Arg-Thr-Glu-Arg. The different cleavage site sequences of these viruses did not affect their level of replication in either continuous simian or bovine kidney cell monolayers (in the presence or absence of exogenous trypsin or plasmin) or in the upper or lower respiratory tract of rhesus monkeys. We conclude that two nonconsecutive basic residues within the F protein cleavage site are sufficient for efficient replication of human PIV3 in primates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANOCK R. M., PARROTT R. H., COOK K., ANDREWS B. E., BELL J. A., REICHELDERFER T., KAPIKIAN A. Z., MASTROTA F. M., HUEBNER R. J. Newly recognized myxoviruses from children with respiratory disease. N Engl J Med. 1958 Jan 30;258(5):207–213. doi: 10.1056/NEJM195801302580502. [DOI] [PubMed] [Google Scholar]
- Coelingh K. V., Tierney E. L. Identification of amino acids recognized by syncytium-inhibiting and neutralizing monoclonal antibodies to the human parainfluenza type 3 virus fusion protein. J Virol. 1989 Sep;63(9):3755–3760. doi: 10.1128/jvi.63.9.3755-3760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Ward C. W. Structure and diversity of influenza virus neuraminidase. Curr Top Microbiol Immunol. 1985;114:177–255. doi: 10.1007/978-3-642-70227-3_5. [DOI] [PubMed] [Google Scholar]
- DeBorde D. C., Naeve C. W., Herlocher M. L., Maassab H. F. Resolution of a common RNA sequencing ambiguity by terminal deoxynucleotidyl transferase. Anal Biochem. 1986 Sep;157(2):275–282. doi: 10.1016/0003-2697(86)90626-3. [DOI] [PubMed] [Google Scholar]
- Glickman R. L., Syddall R. J., Iorio R. M., Sheehan J. P., Bratt M. A. Quantitative basic residue requirements in the cleavage-activation site of the fusion glycoprotein as a determinant of virulence for Newcastle disease virus. J Virol. 1988 Jan;62(1):354–356. doi: 10.1128/jvi.62.1.354-356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne J. D., Albrecht P. Sensitive plaque neutralization assay for parainfluenza virus types 1, 2, and 3 and respiratory syncytial virus. J Clin Microbiol. 1981 Apr;13(4):730–737. doi: 10.1128/jcm.13.4.730-737.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Protease activation mutants of Sendai virus: sequence analysis of the mRNA of the fusion protein (F) gene and direct identification of the cleavage-activation site. Virology. 1987 Jan;156(1):84–90. doi: 10.1016/0042-6822(87)90438-7. [DOI] [PubMed] [Google Scholar]
- Itoh M., Shibuta H., Homma M. Single amino acid substitution of Sendai virus at the cleavage site of the fusion protein confers trypsin resistance. J Gen Virol. 1987 Nov;68(Pt 11):2939–2944. doi: 10.1099/0022-1317-68-11-2939. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Naeve C. W., Webster R. G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology. 1984 Dec;139(2):303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Merson J. R., Hull R. A., Estes M. K., Kasel J. A. Molecular cloning and sequence determination of the fusion protein gene of human parainfluenza virus type 1. Virology. 1988 Nov;167(1):97–105. doi: 10.1016/0042-6822(88)90058-x. [DOI] [PubMed] [Google Scholar]
- Natali A., Oxford J. S., Schild G. C. Frequency of naturally occurring antibody to influenza virus antigenic variants selected in vitro with monoclonal antibody. J Hyg (Lond) 1981 Oct;87(2):185–190. doi: 10.1017/s0022172400069394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Shaughnessy M. A., Lamb R. A. Analysis of the relationship between cleavability of a paramyxovirus fusion protein and length of the connecting peptide. J Virol. 1989 Mar;63(3):1293–1301. doi: 10.1128/jvi.63.3.1293-1301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Orlich M., Klenk H. D., Wang M. L., Skehel J. J., Wiley D. C. Studies on the adaptation of influenza viruses to MDCK cells. EMBO J. 1984 Dec 20;3(13):3329–3332. doi: 10.1002/j.1460-2075.1984.tb02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Pritzer E., Khoshnan M. A., Yamakawa M., Kuroda K., Klenk H. D., Rott R., Seto J. T. Characterization of a pantropic variant of Sendai virus derived from a host range mutant. Virology. 1988 Aug;165(2):577–583. doi: 10.1016/0042-6822(88)90601-0. [DOI] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- Wang M. L., Skehel J. J., Wiley D. C. Comparative analyses of the specificities of anti-influenza hemagglutinin antibodies in human sera. J Virol. 1986 Jan;57(1):124–128. doi: 10.1128/jvi.57.1.124-128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Determination of the number of nonoverlapping antigenic areas on Hong Kong (H3N2) influenza virus hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology. 1980 Jul 15;104(1):139–148. doi: 10.1016/0042-6822(80)90372-4. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Jorgensen E. D., Murphy B. R. Antigenic and structural properties of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3: sequence analysis of variants selected with monoclonal antibodies which inhibit infectivity, hemagglutination, and neuraminidase activities. J Virol. 1987 May;61(5):1473–1477. doi: 10.1128/jvi.61.5.1473-1477.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Murphy B. R. Nucleotide and deduced amino acid sequence of hemagglutinin-neuraminidase genes of human type 3 parainfluenza viruses isolated from 1957 to 1983. Virology. 1988 Jan;162(1):137–143. doi: 10.1016/0042-6822(88)90402-3. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Tierney E. L., London W. T., Murphy B. R. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988 Apr;157(4):655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C., Murphy B. R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985 Jun;143(2):569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K., Tierney E. L. Antigenic and functional organization of human parainfluenza virus type 3 fusion glycoprotein. J Virol. 1989 Jan;63(1):375–382. doi: 10.1128/jvi.63.1.375-382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



