Abstract
Unintended pregnancies are expensive for patients and for society in terms of medical costs, the cost of caring for more children, and the cost to personal and professional goals. Sterilization is the most common contraceptive method utilized by couples in the United States. Given technological advances over the past few decades, male and female surgical sterilization has become a safe, convenient, easy, and highly effective birth control method for the long term. This article reviews current male and female sterilization options.
Key words: Sterilization, Tubal ligation, Transcervical tubal occlusion, Vasectomy
Of the 6.4 million pregnancies that occurred in the United States in 2001, 49% were unintended. Of these 3.1 million unintended pregnancies, 52% (1.6 million) occurred in women who were not using contraception during the month of conception, whereas the remaining 48% were contraceptive failures.1 Although unintended does not always mean unwanted, these pregnancies should nonetheless be considered family-planning defeats. Apart from the emotional burden, unintended pregnancies are expensive for patients and for society in terms of medical cost, the cost of caring for more children, and the cost to personal and professional goals.
The American woman has approximately 35 years of her life to either use contraception or run the risk of becoming pregnant, whereas men must be concerned about contraception for almost their entire lives. When deciding on a contraceptive method, users must consider its side effects (both perceived and actual), regimen, and accessibility. There is a high discontinuation rate for temporary birth control methods with 32% of oral contraceptive users, 32% of combined patch and ring users, up to 70% of Depo-Provera® (Pfizer Inc., New York, NY) injection users, 10% to 13% of progesterone implant users, and about 20% of intrauterine device users discontinuing their birth control method within the first year.2,3 Given technological advances over the past few decades, male and female surgical sterilization has become a safe, convenient, easy, and highly effective birth control method for the long term.
Background
Female or male sterilization is the most common contraceptive method utilized by couples in the United States, with 36% of fertile women using contraception employing this method. According to the National Survey of Family Growth (2002), 10.3 million women (27%) rely on female sterilization for birth control, whereas 3.5 million women (9.2%) rely on vasectomy in their partners for contraception. The next most commonly utilized birth control method among American women is oral contraceptive pills, used by 11.7 million or 30.6% of women using contraception.4
About 700,000 female sterilizations are performed annually, half of which are performed within 48 hours post-partum.5 Sterilization is performed following 10% of all births. Approximately 345,000 female sterilizations are interval procedures that do not occur immediately following pregnancy.6 Approximately 500,000 vasectomies are performed annually for a rate of 9.9 procedures per 1000 men aged 25 to 49.4 Overall, the sterilization rates for men and women have remained constant over the past 40 years, although the surgical methods employed have changed with advances in technology and anesthesia.
Methods
Female Laparoscopic Sterilization
For women who undergo interval sterilization, intra-abdominal access is most frequently obtained through the use of laparoscopy. Given the need for pneumoperitoneum and its associated discomfort, this procedure is usually performed using general anesthesia in an outpatient setting. Almost all patients are candidates for this procedure, except for women with profound medical problems that preclude the use of general anesthesia, even for a short duration. Typically, an umbilical port is used for primary abdominal access with 1 or 2 ancillary ports in the midline or lower quadrants.
Once abdominal access is achieved, both fallopian tubes are identified and occluded under direct visualization. Methods of tubal occlusion include electrosurgical methods using unipolar or bipolar electrocoagulation or mechanical methods such as the Hulka-Clemens spring clip, the Filshie hinged clip, or the Falope or Yoon silastic ring/band (Figure 1). Patients usually have a 48- to 72-hour recovery period with mild abdominal pain due to the incision sites and any residual pneumoperitoneum. Contraception is considered immediate following occlusion of the fallopian tubes.
Figure 1.
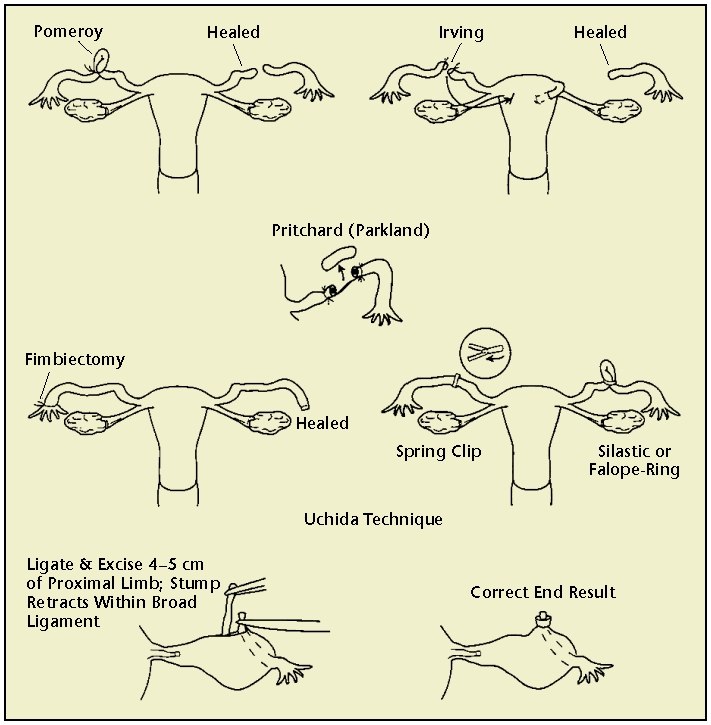
Tubal ligation techniques. Reprinted with permission from Hatcher RA et al.3
Postpartum and Interval Minilaparotomy
Minilaparotomy involves making a 1- to 5-cm incision in the abdomen, locating the fallopian tubes, and bringing them to the incision site in order to cut or block. In the United States, this is most commonly performed immediately after vaginal birth using an infraumbilical incision and regional anesthesia. However, this approach can also be employed in an interval tubal ligation with an incision lower in the abdomen. Because the minilaparotomy incision allows the fallopian tubes to be easily visualized and manipulated by the surgeon, there are multiple ligation methods, some of which become quite involved (Figure 1). The choice of occlusion method depends upon the provider’s training, the patient’s medical history and anatomy, and the availability of supplies.
Cesarean Delivery
Using the same variety of ligation methods as minilaparotomy, tubal ligation can be accomplished at the time of cesarean delivery, adding little additional risk to the operation. However, a cesarean delivery should not be performed for the sole indication of desired postpartum sterilization. The Parkland method of occlusion is the most frequent method of tubal occlusion at the time of cesarean delivery due to ease of surgical technique and excellent success rates.7
Postabortion Tubal Ligation
Tubal sterilization can be performed in the immediate postabortion setting using either minilaparotomy, with the incision higher than in interval tubal ligation based on gestational age, or using laparoscopy. The fallopian tubes will generally be less engorged than in a term pregnancy, but extra care must be taken with hemostasis in the immediate postpartum state. Transcervical sterilization should be delayed at least 6 weeks following a dilation and curettage (D & C) in order to maximize bilateral tubal ostea visualization and microinsert placement.8
Transcervical Sterilization
There has been intermittent interest in transcervical sterilization since the first attempted hysteroscopic electrocoagulation of the fallopian tubes by Schroeder in 1927.9 However, the first transcervical device, Essure® (Conceptus, Inc., Mountain View, CA), was only approved by the European Union in 2001 and by the US Food and Drug Administration (FDA) in 2002. With this nonincisional method of sterilization, a metal microinsert is placed under hysteroscopic guidance into the interstitial portion of each fallopian tube (Figure 2). The insert comes loaded in a single-use delivery system and consists of an inner coil of stainless steel and polyethylene terephthalate (PET) and an outer coil of nickel-titanium (nitinol). The device is placed in the proximal fallopian tube in the wound down state and then deployed to an expanded state that anchors the insert in the tube10 (Figure 2). After placement, the PET fibers stimulate ingrowth around the device over the course of several weeks, resulting in tubal occlusion.11 Tubal occlusion is confirmed 12 weeks following microinsert placement by hysterosalpingogram (HSG; Figure 3). Backup birth control must be used until bilateral tubal occlusion is confirmed by HSG. According to clinical trials, by 3 months 96% of women had both tubes occluded and by 6 months 100% of women had both tubes occluded on HSG evaluation.12
Figure 2.
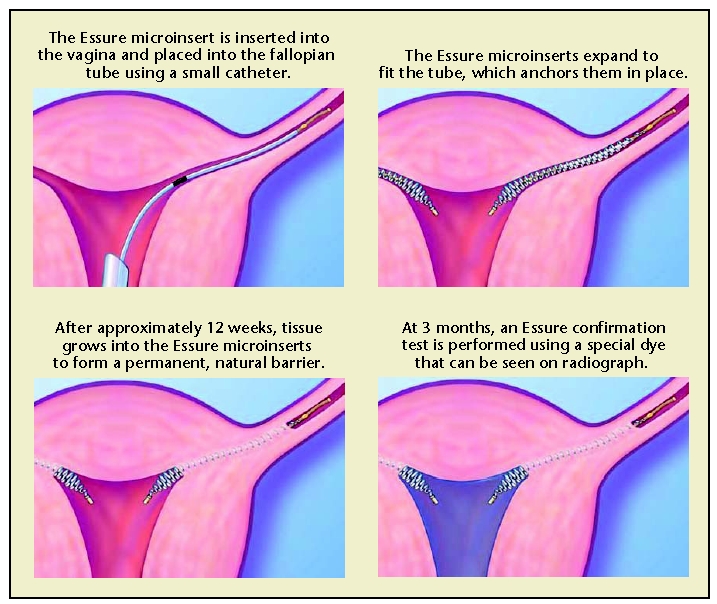
The Essure (Conceptus, Inc., Mountain View, CA) procedure for permanent birth control. Copyright 2006 Conceptus Incorporated. All rights reserved.
Figure 3.
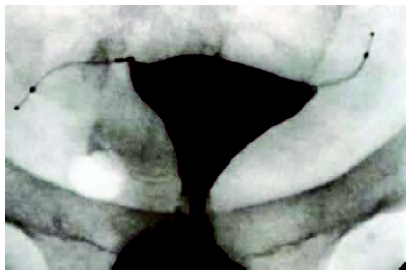
Tubal occlusion is confirmed 12 weeks following Essure (Conceptus, Inc., Mountain View, CA) microinsert placement by hysterosalpingogram. Copyright Conceptus Incorporated. All rights reserved.
Given that transcervical sterilization can be comfortably performed in an office setting under minimal or no anesthesia,13 and, depending on the skill of the physician, can often be performed in under 15 minutes, almost all patients are candidates for this procedure. Presurgical counseling should ensure that a patient is not allergic to nickel. Patients who potentially have a general metal allergy should undergo allergy testing to rule out a nickel allergy prior to the procedure. Patients should also be asked about an allergy to contrast dye because an HSG is required postprocedurally by the FDA protocol. However, studies and surgical experience in Europe indicate that an ultrasound 12 weeks postprocedure is equally effective at confirming sterility.14 Therefore, women in the United States who have a contrast allergy can still undergo transcervical sterilization, but their counseling must inform them that their postprocedural evaluation of the fallopian tubes will have to deviate from the FDA-approved protocol.
Vaginal Approach
Access and ligation of the fallopian tubes can be done through a colpotomy in the posterior vaginal fornix. This approach has fallen out of favor with the development and improvement of laparoscopy and hysteroscopy. International studies have demonstrated that tubal ligation through colpotomy may be less safe and less effective than other techniques.15 Therefore, this approach should be saved for only exceptional cases.
Chemical Exposure
Quinacrine sulfate is a cytotoxic agent known to induce occlusive sclerosis of the intramural portion of the fallopian tubes. In the developing world, where surgical sterilization is difficult to provide safely, the use of intrauterine quinacrine sulfate as a method of female sterilization has been described with varying success.16 To date, this method has not received either FDA or European Union approval. More safety and efficacy studies are needed.
Vasectomy
The vasectomy procedure involves identification, localization, and occlusion of the bilateral vas deferens in order to prevent sperm from entering the ejaculate. It is almost exclusively performed with local lidocaine anesthesia. The vas deferens can be localized through 1 midline or 2 small scrotal incisions. These incisions can be made with a scalpel. More commonly, a no-scalpel technique is utilized that reaches the vas deferens through a scrotal puncture site. This approach is used more frequently worldwide due to fewer infectious and hematoma complications, less pain during the procedure, and earlier resumption of sexual activity following the procedure.17 Occlusion of the vas deferens is performed with ligation, excision, clips, clamps, sutures, cautery, or a combination of these techniques. The addition of fascial interposition increases effectiveness.18
Discomfort in the scrotum usually lasts for 2 to 3 days and is alleviated with a nonsteroidal anti-inflammatory drug. Men are encouraged not to ejaculate for 2 days. Importantly, sterility is not immediate following vasectomy. Therefore, the patient should return for semen analysis 3 months after the procedure to ensure absence of sperm. The couple should use a backup birth control method in the interval between vasectomy and semen analysis proven sterility. Azoospermia results in 60% to 80% of men after 12 weeks or after 20 ejaculations, with variability in results depending on the age of the man and the type of occlusion used.19
Efficacy
Tubal Ligation
The efficacy of tubal ligation has been most extensively studied in the US Collaborative Review of Sterilization (CREST) study. This study followed 10,685 sterilized women for up to 14 years following their tubal ligation. The findings demonstrated that tubal ligation is highly effective, though effectiveness varies by the ligation method employed and by the patient’s age, race, and ethnicity.7 The cumulative 10-year probability of pregnancy following tubal ligation was 18.5 per 1000 procedures (95% confidence interval [CI], 15.1–21.8). Postprocedural pregnancy rates were highest following laparoscopic Hulka-Clemens clip sterilization (36.5 pregnancies per 1000 procedures) and lowest following unipolar coagulation and postpartum partial salpingectomy (each 7.5 pregnancies per 1000 procedures; Figure 4). Sterilization failure occurred more commonly in women who underwent the procedure at a younger age due to increased fertility in these women.7
Figure 4.
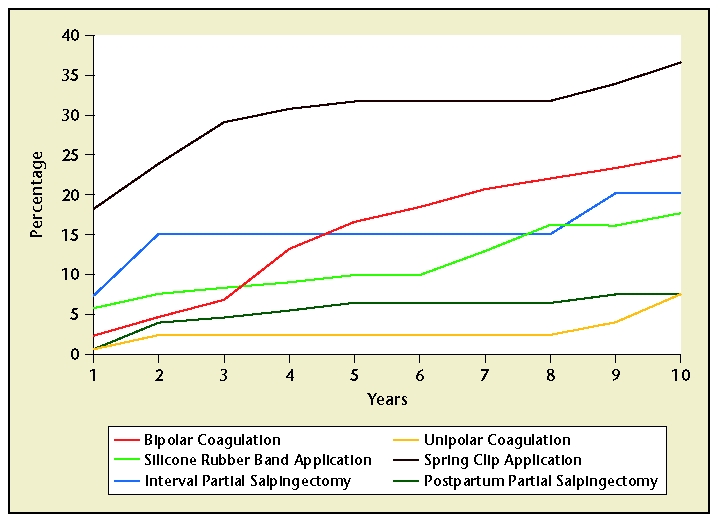
Efficacy of tubal ligation in the 10 years following the procedure. However, effectiveness varies with the ligation method employed. Data from Peterson HB et al.7
Transcervical Tubal Occlusion
Essure is the only transcervical occlusion device currently available in the United States. “As of October 15, 2004, 643 women with bilateral placement contributed to effectiveness time, 194 in the phase II study and 449 in the Pivotal Trial. In total, the 643 trial participants contributed 28,290 months of follow-up time with no (0) pregnancies reported.”20 In a more recent case report, 2 pregnancies were reported in roughly 130,000 patients with microinsert hysteroscopic sterilization and bilateral occlusion confirmed by HSG (although both cases were the result of perforation/ misplacement rather than device failure).21 If these data could be applied to the CREST data, transcervical tubal occlusion with a confirmatory HSG would represent the most effective of all female or male sterilization techniques.
Vasectomy
According to CREST data, the cumulative probability of pregnancy per 1000 vasectomies was 7.4 (95% CI, 0.2–14.6) during the first year following the procedure and 11.3 (95% CI, 2.3–20.3) after 5 years.22 Studying the effectiveness of vasectomy by monitoring pregnancy rates is complicated by the fact that some of the reported pregnancies may not be attributable to the patients who underwent vasectomy. However, even with this added inaccuracy, vasectomy is highly effective.
Health Benefits
Tubal sterilization has demonstrated protection against the development of ovarian cancer in several welldesigned studies.23,24 The Nurses’ Health Study reports a 67% risk reduction of epithelial ovarian cancer in sterilized compared with nonsterilized women.25 Women with the BRCA1 mutation are also found to have a 60% risk reduction of ovarian cancer following sterilization.26
A second noncontraceptive health benefit of female sterilization is the observed reduced risk of pelvic inflammatory disease following tubal ligation.27,28 Women and their partners need to be counseled that sterilization does not protect against the acquisition of sexually transmitted diseases, and that barrier methods are still necessary. However, tubal ligation appears to protect against the pelvic ascent of those infections.
Disadvantages
Short-Term Complications
Although serious surgical complications are rare, due to the invasive nature of tubal ligation, infection (1% of total cases), minor or major bleeding (0.6%–1%), and anesthesia-related events (1%–2%) are reported.29 The most recent estimates on the risk of death from female sterilization suggest rates of 1 to 2 deaths per 100,000 procedures. The anesthesia risk, although low, can be reduced further through increased used of local and regional over general anesthesia. However, because the surgical and anesthesia risks of sterilization, particularly in women, are significantly lower than the risk of pregnancy, almost all women are candidates for these minimally invasive procedures. Surgical complications are minimized through the use of prudent patient and technique selection. Women undergoing laparoscopic sterilization who have diabetes, obesity, previous abdominal or pelvic surgery, or receive general anesthesia are at greatest risk of surgical complications.30
Transcervical sterilization has revolutionized gynecological practices through the implementation of office-based sterilization under local anesthesia, thereby removing the risks of both invasive laparoscopic incisions and general anesthesia. Nonetheless, good judgment must be practiced to ensure patients have their procedure performed in higher acuity settings when significant concomitant comorbidities are present. Although no major complications are associated with transcervical sterilization, short-term complications have been reported. These include unsuccessful bilateral placement of the microinserts (5% of total patients),20 microinsert expulsion (2.2%), perforation (1.5%), pelvic cramping on the day of the procedure (29.6%), and back pain in the first year of microinsert use (9%), according to early studies.8
No major complications are associated with vasectomy. Minor complications include infection (1%–6%), bleeding (1.6%–4.6%), granuloma formation (1%–40%), and epididymitis (0.4%–6%).29 In addition, an entity called postvasectomy pain syndrome has been described with as many as 15% of previously asymptomatic men reporting scrotal pain 7 months after vasectomy.31
Long-Term Complications
Patient regret following the procedure is the most common long-term complication of sterilization, with rates reported anywhere from 0.9% to 26% for female sterilization32 and less than 5% for male sterilization.33 According to the CREST study, the cumulative probability of expressing regret following tubal sterilization was 12.7% (95% CI, 11.2–14.3).32 Several patient characteristics have been determined to be predictors of regret. Young age at the time of sterilization is the strongest predictor of future regret. Women under the age of 30 at the time of the procedure were twice as likely as women older than 30 to report regretting having the procedure performed (Figure 5).32 Women who are sterilized postpartum also report higher rates of regret than those patients who received interval tubal ligations.34,35 Divorce and/or remarriage subsequent to sterilization, being poor (eg, Medicaid patients), or being of Hispanic origin also predicts higher rates of regret32 following female sterilization. Risk factors for regret following vasectomy include marital instability, age younger than 31, financial instability, and having no or very young children at the time of the procedure.36–38 Furthermore, CREST data demonstrate that women regretting their partners’ vasectomies (6.1%; 95% CI, 3.6–8.6) is similar to that of women regretting their own tubal sterilizations (7%; 95% CI, 5.8–8.1).38
Figure 5.
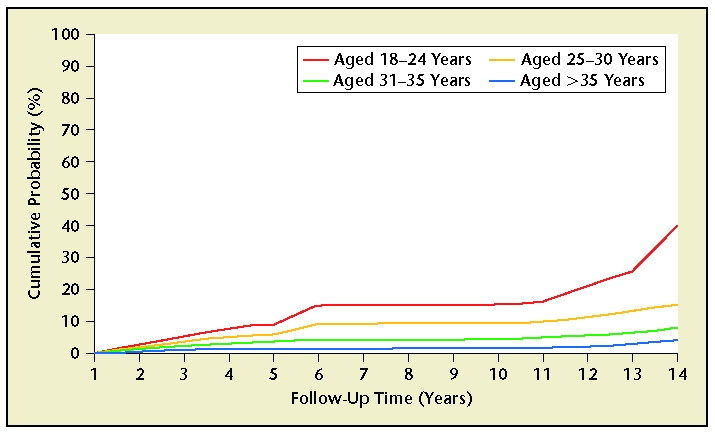
According to the Collaborative Review of Sterilization (CREST) study, the cumulative probability of expressing regret following tubal sterilization was 12.7% (95% CI, 11.2–14.3). Several patient characteristics have been determined to be predictors of regret: young age at the time of sterilization, sterilization postpartum, divorce and/or remarriage subsequent to sterilization, being poor (eg, Medicaid patients), and being of Hispanic origin. Reprinted from Fertility and Sterility, Volume 74, Schmidt JE et al, “Requesting information about and obtaining reversal after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization,” pp. 892–898, Copyright 2000, with permission from Elsevier.
Some studies report that tubal ligation increases a woman’s subsequent risk of needing a hysterectomy.39–41 Indeed, the CREST study found a 5-year cumulative probability of hysterectomy of 8.4% (95% CI, 2.4–9.0) compared with 1.8% of nonsterilized women.42 Other cohort studies have found no overall increased risk of needing a hysterectomy following sterilization, but rather conclude that women who choose tubal sterilization for contraception may more likely select a surgical solution for menstrual disorders.43
In general, female sterilization is protective against ectopic pregnancy because few pregnancies occur in sterilized women. However, if pregnancy does occur, it is more likely to be an ectopic pregnancy following tubal sterilization. The CREST study demonstrated that the 10-year probability of ectopic pregnancy for all tubal sterilization methods studied was 7.3 ectopic pregnancies per 1000 procedures (95% CI, 5.0–9.6), or one third of all post-tubal sterilization pregnancies. Risk differed by occlusion method employed and age of patient at time of procedure, with bipolar coagulation and age under 30 years associated with the highest risk.44
Controversy exists regarding whether female sterilization results in menstrual changes, such as dysmenorrhea, menorrhagia, and metrorrhagia, coined post-tubal ligation syndrome. The best studies, including CREST data, suggest that when controlling for confounding variables, no significant change in menstrual cycle occurs following tubal sterilization.45 Women who have had transcervical sterilization can undergo endometrial ablation with thermal balloon ablation according to a study that proves feasibility and safety.46 There are no data on the feasibility or safety of using other ablation techniques, including other thermal methods, cryoablation, or laser ablation, following transcervical sterilization. Techniques using microwave or radio frequencies should be avoided once the transcervical sterilization coils are in place due to the risk of heat transmission by the coils and subsequent damage to the surrounding tissues.
Many couples are concerned regarding the impact of sterilization on “sexual interest” (libido). A CREST study determined that 80% of the 4576 women studied reported no change in sexual interest or sexual pleasure following tubal sterilization. Of the remaining women who noted a change, the majority reported positive sexual effects in interest and pleasure.47 Similarly, in a much smaller trial, vasectomy was not associated with adverse effects on male libido, erectile function, or sexual satisfaction, despite widespread myths.48
Despite extensive study, no other long-term health complications have been found with sterilization. Female sterilization has been found not to result in increased incidence of breast cancer,49 endometrial cancer,50 or decreased bone density.51 Multiple studies have demonstrated that vasectomy has no effect on the risk of prostate and testicular cancer52–54 or cardiovascular disease.55,56
Special Considerations
Ensuring Informed Consent
In order to ensure that a patient is fully informed, he or she must be made aware that temporary contraceptives are also available, that sterilization is a surgical procedure that carries surgical risks, that there are some potential health benefits to sterilization, and that, if successful, the procedure will prevent the patient from having any more children. The patient, and the patient’s partner, if involved in the consent process, should consider the sterilization procedure as permanent and something that cannot be reversed. They should also understand that they can change their minds anytime before the procedure is performed. Furthermore, because several patient characteristics have been identified that increase the risk of future regret following sterilization, patients who are young, postpartum, in the midst of relationship or financial crisis, or have low parity should undergo particularly extensive counseling.
Given the elective and permanent nature of sterilization, there are several special legal policies that should be considered during the consent process. In all states, there are special consent forms for all women undergoing publicly funded sterilization. A few states have special consents for all women seeking sterilization. Medicaid-funded sterilizations require a 30-day waiting period between consent and the procedure (except in the special circumstances of premature delivery or emergency abdominal surgery in the setting of prior consent) and that the patient be at least 21 years old and mentally competent.57 There are no such restrictions for male sterilization. Arbitrary denial of patient sterilization by health care professionals has been ruled in US courts to violate a man’s or woman’s basic rights. No official laws or policies dictate the sterilization of patients who are mentally challenged.
Reversal of Female and Male Sterilization
Even with comprehensive counseling and sound patient resolve for permanent contraception, changes in life happen that result in many couples considering reversal of the sterilization procedure. According to the CREST study, within 14 years of tubal sterilization 14.3% (95% CI, 12.4–16.3) of sterilized women request information regarding reversal.58 Within 5 years of vasectomy 1.4% of men and 2.0% of their wives request reversal.38 Patients seeking sterilization reversal should be informed that reversal requires major surgery and its accompanying risks, may not restore fertility, and is expensive and rarely covered by insurance. Some patients may not be candidates due to such factors as age or original sterilization technique. Transcervical sterilization is not reversible and these patients require in vitro fertilization (IVF) to become pregnant. Although the literature is scant, it appears that microinsert presence in the bilateral tubes does not diminish IVF success rates. In fact, several infertility clinics are reporting the use of transcervical sterilization in the setting of infertility with hydrosalpinx as a possible method to improve IVF success.59,60 Pregnancy rates following tubal ligation reversal are variable with subsequent live birth rates ranging from 25% to 87%.5 Vasectomy reversal success is also highly variable because, as with female tubal reanastomosis, pregnancy rates depend on the procedure performed as well as the length of time since the procedure was performed and the age of the female partner.29,61 This is further complicated by the finding that 60% of men develop antisperm antibodies, which further decreases the likelihood of pregnancy. Therefore, pregnancy rates following vasectomy reversal are reported to range from 7% to 89.7%.61
Cost
With over 60 million Americans periodically without health insurance in a given year62 and only 72% of employer-based health plans covering all contraceptive methods,63 cost is an important factor for many couples when choosing their contraceptive method. Sterilization carries a high upfront cost. However, given the length of effectiveness of this method, it can become very cost-effective depending on the age at which it is performed.64,65
The outpatient, in-office nature of vasectomy and transcervical female sterilization gives these methods very favorable cost profiles over time as compared with other methods. In a cost analysis of all contraceptive options available in 1995, Trussell and colleagues found vasectomy to be one of the most cost-effective methods of contraception at 5 years of use.64 In a more recent study of female sterilization techniques, Levie and Chudnoff demonstrated significant cost savings with transcervical female sterilization when compared with laparoscopic tubal ligation as long as the transcervical procedure was performed in the office setting, despite the relatively high cost of the device.66 Unless performed at the time of cesarean delivery, minilaparotomy and laparoscopic tubal sterilization are much more expensive than either vasectomy or in-office transcervical sterilization.
Conclusions
For men and women who no longer desire fertility, sterilization is a safe and highly effective option. Future regret is an important consideration that must be taken into account before any permanent sterilization procedure is performed, but with appropriate patient selection and counseling this problem is minimized. Male and female sterilization are performed both comfortably and cost effectively in the doctors’ offices rather than operating rooms, making sterilization a more convenient choice for permanent birth control.
Main Points.
Methods of tubal occlusion include electrosurgical methods using unipolar or bipolar electrocoagulation or mechanical methods such as the Hulka-Clemens spring clip, the Filshie hinged clip, or the Falope or Yoon silastic ring/band.
Transcervical tubal occlusion is a nonincisional method of sterilization, in which a metal microinsert is placed under hysteroscopic guidance into the interstitial portion of each fallopian tube.
Tubal ligation is a highly effective method of sterilization, although effectiveness varies by the ligation method employed and by patient age, race, and ethnicity.
Tubal ligation has demonstrated protection against the development of ovarian cancer and a reduced risk of the development of pelvic inflammatory disease.
Transcervical sterilization is an office-based sterilization under local anesthesia, thereby removing the risks of both invasive laparoscopic incisions and general anesthesia.
Patient regret following sterilization is the most common long-term complication of sterilization with rates reported from 0.9% to 26% for female sterilization and less than 5% for male sterilization.
In order to ensure that a patient is fully informed, he or she must be made aware that temporary contraceptives are also available, that sterilization is a surgical procedure that carries surgical risks, that there are some potential health benefits to sterilization, and that, if successful, the procedure will prevent the patient from having any more children.
The outpatient, in-office nature of vasectomy and transcervical sterilization gives these 2 methods favorable cost profiles over time as compared with other contraceptive methods.
References
- 1.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 & 2001. Perspect Sex Reprod Health. 2006;38:90–96. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 2.Halpern V, Grimes DA, Lopez L, Gallo MF. Strategies to improve adherence and acceptability of hormonal methods for contraception. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD004317.pub2. CD004317. DOI: 10.1002/14651858.CD004317.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Hatcher RA, Trussell J, Stewart F, et al. Contraceptive Techonology. 18th ed. Vol. 549. New York: Ardent Media; 2004. [Google Scholar]
- 4.Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004;350:1–35. [PubMed] [Google Scholar]
- 5.Westhoff C, Davis A. Tubal sterilization: focus on the U.S. experience. Fertil Steril. 2000;73:913–922. doi: 10.1016/s0015-0282(00)00481-7. [DOI] [PubMed] [Google Scholar]
- 6.MacKay AP, Kieke BA , Jr, Koonin LM, et al. Tubal sterilization in the United States, 1994–1996. Fam Plan Perspect. 2001;33:161–165. [PubMed] [Google Scholar]
- 7.Peterson HB, Xia Z, Hughes JM, et al. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol. 1996;174:1161–1170. doi: 10.1016/s0002-9378(96)70658-0. [DOI] [PubMed] [Google Scholar]
- 8.Essure [package insert] San Carlos, CA: Conceptus Incorporated; 2002. [Google Scholar]
- 9.Quinones RG, Alavarado AD, Ley EC. Tubal electrocoagulation under hysteroscopic control. Am J Obstet Gynecol. 1975;121:1111–1113. doi: 10.1016/s0002-9378(16)33599-2. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JM, Carignan CS, Cher D, et al. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59–67. doi: 10.1016/s0029-7844(03)00373-9. [DOI] [PubMed] [Google Scholar]
- 11.Valle RF, Carignan CS, Wright TC, et al. Tissue response to the STOP microcoil transcervical permanent contraceptive device: results from a prehysterectomy study. Fertil Steril. 2001;76:974–980. doi: 10.1016/s0015-0282(01)02858-8. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration (FDA), authors FDA approves new female sterilization device. FDA Talk Paper. 2002;T02-41:1–2. [Google Scholar]
- 13.Mino M, Arjona JE, Cordon J, et al. Success rate and patient satisfaction with the Essure™ sterilisation in an outpatient setting: a prospective study of 857 women. BJOG. 2007;114:763–766. doi: 10.1111/j.1471-0528.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 14.Veersema S, Vleugels MPH, Timmermans A, et al. Follow-up of successful bilateral placement of Essure microinserts with ultrasound. Fertil Steril. 2005;84:1733–1736. doi: 10.1016/j.fertnstert.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO), authors Female Sterilization: A Guide to Provision in Services. Geneva, Switzerland: WHO; 1992. [Google Scholar]
- 16.Zipper J, Kessel E. Quinacrine sterilization: a retrospective. Int J Obstet Gynecol. 2003;83:S7–S11. doi: 10.1016/S0020-7292(03)90084-1. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V, Kaza RM, Singh I, et al. An evaluation of the no-scalpel vasectomy technique. BJU Int. 1999;83:283–284. doi: 10.1046/j.1464-410x.1999.00934.x. [DOI] [PubMed] [Google Scholar]
- 18.Dassow P, Bennett JM. Vasectomy: an update. Am Fam Physician. 2006;74:2069–2074. [PubMed] [Google Scholar]
- 19.Barone MA, Nazerali H, Coretes M, et al. A prospective study of time and number of ejaculations to azoospermia after vasectomy by ligation and excision. J Urol. 2003;174:29–36. doi: 10.1097/01.ju.0000075505.08215.28. [DOI] [PubMed] [Google Scholar]
- 20.Essure [package insert] Vol. 3. Mountain View, CA: Conceptus Incorporated; 2007. [Google Scholar]
- 21.Ory EM, Hines RS, Cleland WH, Rehberg JF. Pregnancy after microinsert sterilization with tubal occlusion confirmed by hysterosalpingogram. Obstet Gynecol. 2008;111:508–510. doi: 10.1097/01.AOG.0000296487.36158.41. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson DJ, Costello C, Trussell J, et al. The risk of pregnancy after vasectomy. Obstet Gynecol. 2004;103:848–850. doi: 10.1097/01.AOG.0000123246.11511.e4. [DOI] [PubMed] [Google Scholar]
- 23.Irwin KL, Weiss NS, Lee NC, et al. Tubal sterilization, hysterectomy, and the subsequent occurrence of epithelial ovarian cancer. Am J Epidemiol. 1991;134:362–369. doi: 10.1093/oxfordjournals.aje.a116098. [DOI] [PubMed] [Google Scholar]
- 24.Rosenblatt KA, Thomas DB The World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives, authors. Reduced risk of ovarian cancer in women with a tubal ligation or hysterectomy. Cancer Epidemiol Biomarkers Prev. 1996;5:933–935. [PubMed] [Google Scholar]
- 25.Hankinson SE, Hunter DJ, Colditz GA, et al. Tubal ligation, hysterectomy, and risk of ovarian cancer: a prospective study. JAMA. 1993;270:2813–2818. [PubMed] [Google Scholar]
- 26.Narod SA, Sun P, Ghadirian P, et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet. 2001;357:1467–1470. doi: 10.1016/s0140-6736(00)04642-0. [DOI] [PubMed] [Google Scholar]
- 27.Edelman DA. Pelvic inflammatory disease and contraceptive practice. Adv Contracept. 1986;2:141–144. doi: 10.1007/BF01849223. [DOI] [PubMed] [Google Scholar]
- 28.Abbuhl SB, Muskin EB, Shofer FS. Pelvic inflammatory disease in patients with bilateral tubal ligation. Am J Emerg Med. 1997;15:271–274. doi: 10.1016/s0735-6757(97)90012-7. [DOI] [PubMed] [Google Scholar]
- 29.Hendrix NW, Chauhan SP, Morrison JC. Sterilization and its consequences. Obstet Gynecol Surv. 1999;54:766–777. doi: 10.1097/00006254-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Jamieson DJ, Hillis SD, Duerr A, et al. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96:997–1002. doi: 10.1016/s0029-7844(00)01082-6. [DOI] [PubMed] [Google Scholar]
- 31.Leslie TA, Illing RO, Cranston DW, Guillebaud J. The incidence of chronic scrotal pain after vasectomy: a prospective audit. Br J Urol Int. 2007;100:1330–1333. doi: 10.1111/j.1464-410X.2007.07128.x. [DOI] [PubMed] [Google Scholar]
- 32.Hillis SD, Marchbanks PA, Tylor LR, et al. Poststerilization regret: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 1999;93:889–895. doi: 10.1016/s0029-7844(98)00539-0. [DOI] [PubMed] [Google Scholar]
- 33.Shain RN, et al. Psychosocial consequences of vasectomy in developed and developing countries. In: Zatuchni GI, et al., editors. Male Contraception: Advances and Future Prospects. Philadelphia: Harper & Row; 1968. pp. 34–53. [Google Scholar]
- 34.Wilcox LS, Zeger SL, Chu SY, et al. Risk factors for regret after tubal sterilization: 5 years of follow-up in a prospective study. Fertil Steril. 1991;55:927–933. [PubMed] [Google Scholar]
- 35.Chi I, Gates D, Thapa S. Performing tubal sterilizations during women’ s postpartum hospitalization: a review of the United States and international experiences. Obstet Gynecol Surv. 1992;47:71–79. doi: 10.1097/00006254-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Howard G. Who asks for vasectomy reversal and why? BMJ. 1982;285:490–492. doi: 10.1136/bmj.285.6340.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke L, Gregson S. Who has a vasectomy reversal? J Biosco Sci. 1986;18:253–259. doi: 10.1017/s0021932000016229. [DOI] [PubMed] [Google Scholar]
- 38.Jamieson DJ, Kaufman SC, Costello C, et al. A comparison of women’ s regret after vasectomy versus tubal sterilization. Obstet Gynecol. 2002;99:1073–1079. doi: 10.1016/s0029-7844(02)01981-6. [DOI] [PubMed] [Google Scholar]
- 39.Cohen MM. Long-term risk of hysterectomy after tubal sterilization. Am J Epidemiol. 1987;125:410. doi: 10.1093/oxfordjournals.aje.a114547. [DOI] [PubMed] [Google Scholar]
- 40.Goldhaber MK, Armstrong MA, Golditch IM, et al. Long-term risk of hysterectomy among 80,007 sterilized and comparison women at Kaiser Permanente. Am J Epidemiol. 1993;138:508. doi: 10.1093/oxfordjournals.aje.a116885. [DOI] [PubMed] [Google Scholar]
- 41.Stergachis A, Shy KK, Grothaus LC, et al. Tubal sterilization and the long-term risk of hysterectomy. JAMA. 1990;264:2893–2898. [PubMed] [Google Scholar]
- 42.Hillis SD, Marchbanks PA, Taylor LR, et al. Higher hysterectomy risk for sterilized than nonsterilized women: findings from the U.S. Collaborative Review of Sterilization. Obstet Gynecol. 1998;91:241–246. doi: 10.1016/s0029-7844(97)00648-0. [DOI] [PubMed] [Google Scholar]
- 43.Shy KK, Sterganchis A, Grothaus LG, et al. Tubal sterilization and risk of subsequent hospital admission for menstrual disorders. Am J Obstet Gynecol. 1992;166:1698–1705. doi: 10.1016/0002-9378(92)91559-s. [DOI] [PubMed] [Google Scholar]
- 44.Peterson HB, Xia Z, Hughes JM, et al. The risk of ectopic pregnancy after tubal sterilization. N Engl J Med. 1997;336:762–767. doi: 10.1056/NEJM199703133361104. [DOI] [PubMed] [Google Scholar]
- 45.Peterson HB, Jeng G, Folger SG, et al. The risk of menstrual abnormalities after tubal sterilization. N Engl J Med. 2000;343:1681–1687. doi: 10.1056/NEJM200012073432303. [DOI] [PubMed] [Google Scholar]
- 46.Valle R, Valdez J, Wright R, et al. Concomitant Essure tubal sterilization and Termachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152–158. doi: 10.1016/j.fertnstert.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 47.Costello C, Hillis SD, Marchbanks PA, et al. The effect of interval tubal sterilization on sexual interest and pleasure. Obstet Gynecol. 2002;100:511–517. doi: 10.1016/s0029-7844(02)02042-2. [DOI] [PubMed] [Google Scholar]
- 48.Bertero E, Hallak J, Gromatzky C, et al. Assessment of sexual function in patients undergoing vasectomy using the international index of erectile function. Int Braz J Urol. 2005;31:452–458. doi: 10.1590/s1677-55382005000500006. [DOI] [PubMed] [Google Scholar]
- 49.Irwin KL, Lee NC, Peterson HB, et al. Hysterectomy, tubal sterilization, and the risk of breast cancer. Am J Epidemiol. 1998;127:1192. doi: 10.1093/oxfordjournals.aje.a114912. [DOI] [PubMed] [Google Scholar]
- 50.Castellsague X, Thompson WD, Dubrow R. Tubal sterilization and the risk of endometrial cancer. Int J Cancer. 1996;65:607–612. doi: 10.1002/(SICI)1097-0215(19960301)65:5<607::AID-IJC9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Fox KM, Cummings SR. Is tubal ligation a risk factor for low bone density and increased risk of fracture? Am J Obstet Gynecol. 1995;172:101–105. doi: 10.1016/0002-9378(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 52.Moller H, Knudsen LB, Lynge E. Risk of testicular cancer after vasectomy: cohort study of over 73,000 men. BMJ. 1994;309:295–299. doi: 10.1136/bmj.309.6950.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernal-Delgado E, Latour-Perez J, Pradas-Arnal F, et al. The association between vasectomy and prostate cancer: a systemic review of the literature. Fertil Steril. 1998;70:191–200. doi: 10.1016/s0015-0282(98)00142-3. [DOI] [PubMed] [Google Scholar]
- 54.Cox B, Sneyd MJ, Paul C, et al. Vasectomy and risk of prostate cancer. JAMA. 2002;287:3110–3115. doi: 10.1001/jama.287.23.3110. [DOI] [PubMed] [Google Scholar]
- 55.Nienhuis H, Goldacre M, Seagroatt V, et al. Incidence of disease after vasectomy: a record linkage retrospective cohort study. BMJ. 1992;304:743–746. doi: 10.1136/bmj.304.6829.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldacre MJ, Wotton CJ, Seagroatt V, et al. Cancer and cardiovascular disease after vasectomy: an epidemiological database study. Fertil Steril. 2005;84:1438–1443. doi: 10.1016/j.fertnstert.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 57.Coe v Bolton. No. C-87-785A (ND Ga Sept. 30, 1976)
- 58.Schmidt JE, Hillis SD, Marchbanks PA, et al. Requesting information about and obtaining reversal after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Fertil Steril. 2000;74:892–898. doi: 10.1016/s0015-0282(00)01558-2. [DOI] [PubMed] [Google Scholar]
- 59.Hitkari JA, Singh SS, Shapiro HM, et al. Essure treatment of hydrosalpinges. Fertil Steril. 2007;88:1663–1666. doi: 10.1016/j.fertnstert.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 60.Kerin JF, Cattanach S. Successful pregnancy outcome with the use of in vitro fertilization after Essure® hysteroscopic sterilization. Fertil Steril. 2007;87:1212. doi: 10.1016/j.fertnstert.2006.07.1549. e1–e4. [DOI] [PubMed] [Google Scholar]
- 61.The Practice Committee of the American Society for Reproductive Medicine, authors. Vasectomy reversal. Fertil Steril. 2006;86(suppl):S268–S271. doi: 10.1016/j.fertnstert.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 62.The Henry J. Kaiser Family Foundation, authors. The Uninsured: A Primer. Key Facts About Americans Without Health Insurance. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 2007. [Accessed January 26, 2008]. [Google Scholar]
- 63.The Kaiser Family Foundation and Health Research and Educational Trust, authors. Employer Health Benefits. 2004 Annual Survey. Menlo Park, CA: The Henry J. Kaiser Foundation; 2004. [Accessed January 26, 2008]. http://www.kff.org/insurance/7148/upload/2004-Employer-Health-Benefits-Survey-Full-Report.pdf. [Google Scholar]
- 64.Trussell J, Leveque JA, Koenig JD, et al. The economic value of contraception: a comparison of 15 methods. Am J Public Health. 1995;85:494–503. doi: 10.2105/ajph.85.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiou C-F, Trussell J, Reyes E, et al. Economic analysis of contraceptives for women. Contraception. 2003;68:3–10. doi: 10.1016/s0010-7824(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 66.Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Inv Gynecol. 2005;12:318–322. doi: 10.1016/j.jmig.2005.05.016. [DOI] [PubMed] [Google Scholar]


