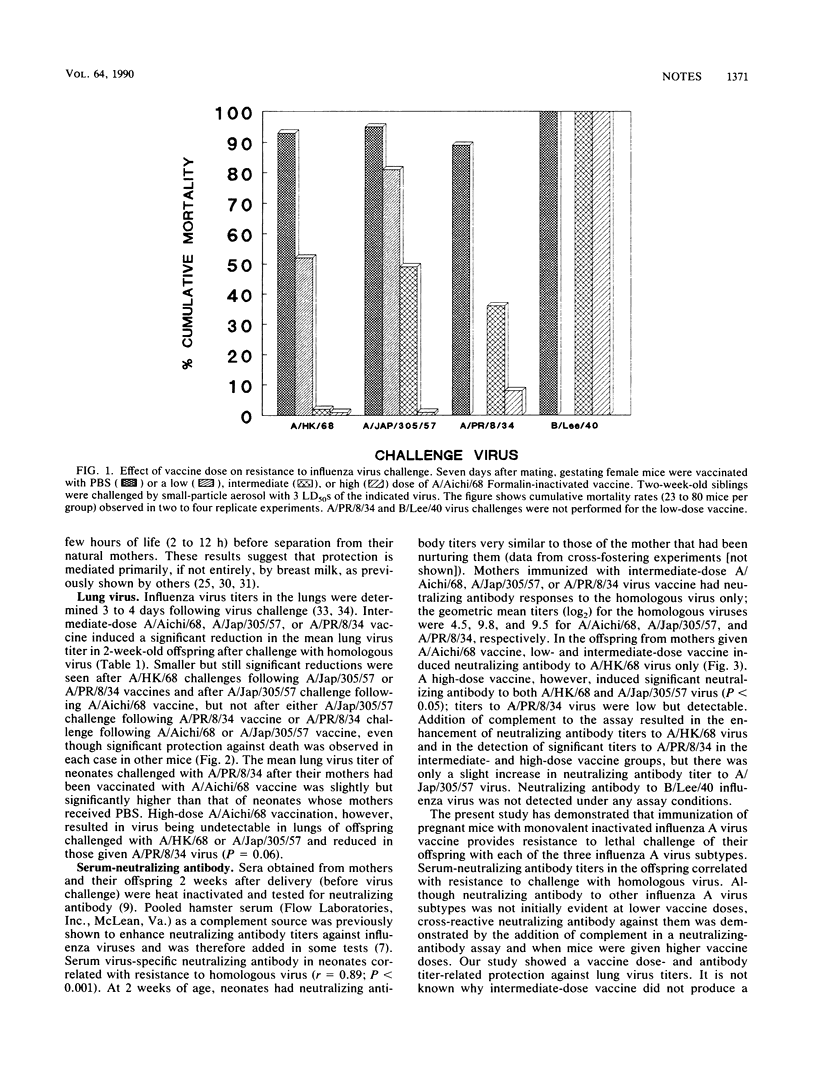
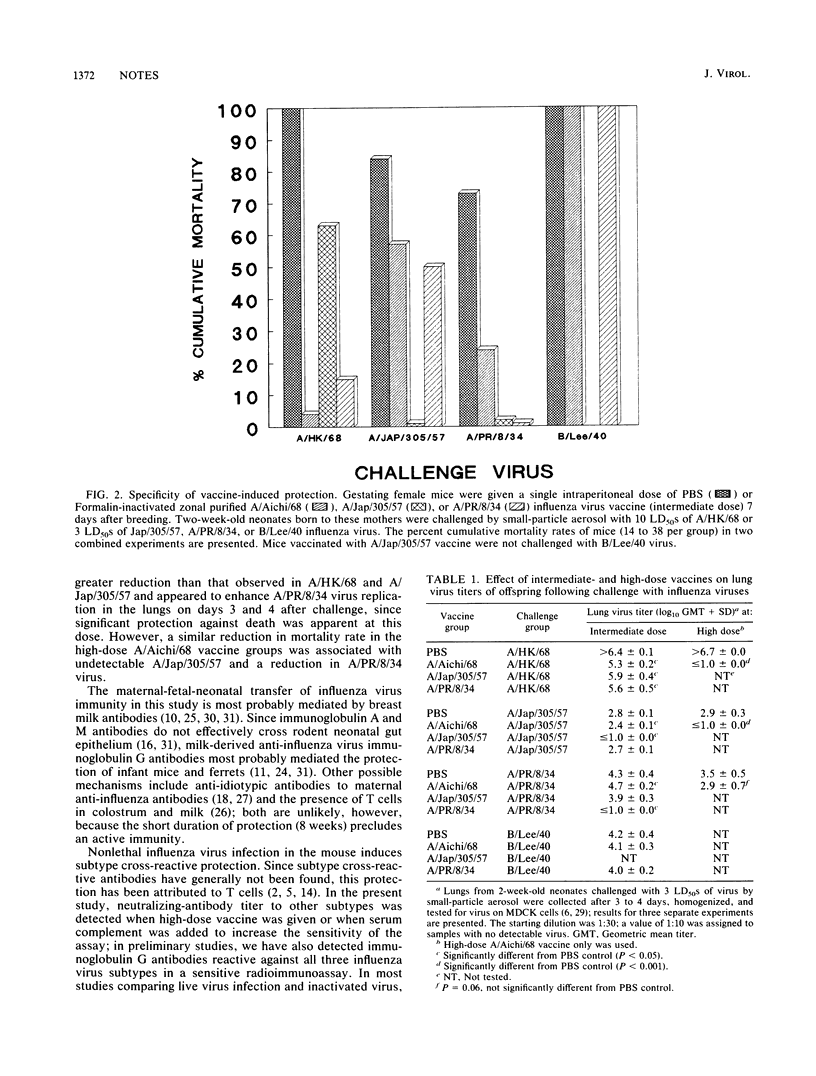
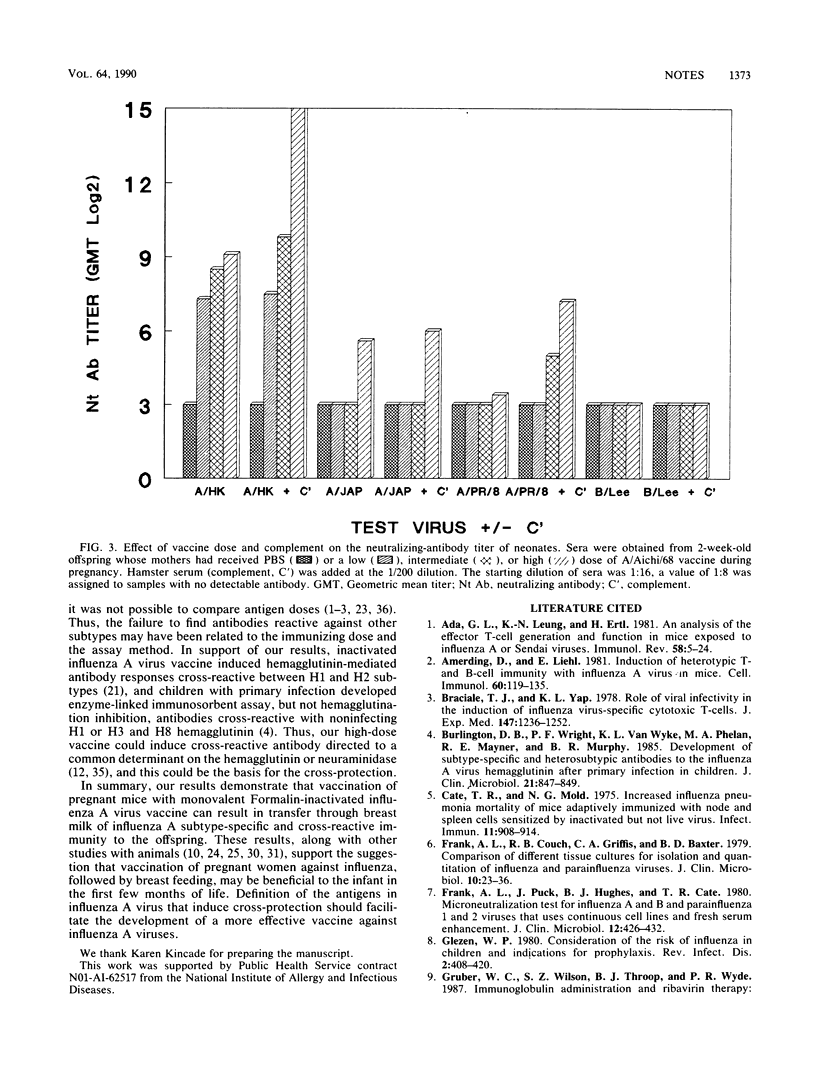
Abstract
A single intraperitoneal injection of pregnant mice with a monovalent Formalin-inactivated influenza A virus vaccine protected their offspring against a lethal challenge dose of the same influenza A virus H3N2, H2N2, and H1N1 subtypes, as well as against challenge with the other two subtypes. Degree of protection was vaccine dose related. Cross-fostering of neonates indicated that protection was conferred by breast milk antibodies. Serum virus-specific neutralizing antibodies in the mothers and neonates correlated with resistance to vaccine virus, but were detected against other subtypes only in a complement enhancement test or when high doses of vaccine were given.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Armerding D., Liehl E. Induction of homotypic and heterotypic T- and B-cell immunity with influenza A virus in mice. Cell Immunol. 1981 May 1;60(1):119–135. doi: 10.1016/0008-8749(81)90253-7. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Yap K. L. Role of viral infectivity in the induction of influenza virus-specific cytotoxic T cells. J Exp Med. 1978 Apr 1;147(4):1236–1252. doi: 10.1084/jem.147.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlington D. B., Wright P. F., van Wyke K. L., Phelan M. A., Mayner R. E., Murphy B. R. Development of subtype-specific and heterosubtypic antibodies to the influenza A virus hemagglutinin after primary infection in children. J Clin Microbiol. 1985 May;21(5):847–849. doi: 10.1128/jcm.21.5.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Mold N. G. Increased influenza pneumonia mortality of mice adoptively immunized with node and spleen cells sensitized by inactivated but not live virus. Infect Immun. 1975 May;11(5):908–914. doi: 10.1128/iai.11.5.908-914.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. L., Couch R. B., Griffis C. A., Baxter B. D. Comparison of different tissue cultures for isolation and quantitation of influenza and parainfluenza viruses. J Clin Microbiol. 1979 Jul;10(1):32–36. doi: 10.1128/jcm.10.1.32-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. L., Puck J., Hughes B. J., Cate T. R. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol. 1980 Sep;12(3):426–432. doi: 10.1128/jcm.12.3.426-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P. Considerations of the risk of influenza in children and indications for prophylaxis. Rev Infect Dis. 1980 May-Jun;2(3):408–420. doi: 10.1093/clinids/2.3.408. [DOI] [PubMed] [Google Scholar]
- Husseini R. H., Sweet C., Overton H., Smith H. Role of maternal immunity in the protection of newborn ferrets against infection with a virulent influenza virus. Immunology. 1984 Jul;52(3):389–394. [PMC free article] [PubMed] [Google Scholar]
- Jakeman K. J., Smith H., Sweet C. Mechanism of immunity to influenza: maternal and passive neonatal protection following immunization of adult ferrets with a live vaccinia-influenza virus haemagglutinin recombinant but not with recombinants containing other influenza virus proteins. J Gen Virol. 1989 Jun;70(Pt 6):1523–1531. doi: 10.1099/0022-1317-70-6-1523. [DOI] [PubMed] [Google Scholar]
- Jones P. D., Ada G. L. Influenza virus-specific antibody-secreting cells in the murine lung during primary influenza virus infection. J Virol. 1986 Nov;60(2):614–619. doi: 10.1128/jvi.60.2.614-619.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Cells mediating delayed-type hypersensitivity in the lungs of mice infected with an influenza A virus. Scand J Immunol. 1980;12(5):393–400. doi: 10.1111/j.1365-3083.1980.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Leung K., Ashman R. B., Ertl H. C., Ada G. L. Selective suppression of the cytotoxic T cell response to influenza virus in mice. Eur J Immunol. 1980 Nov;10(11):803–810. doi: 10.1002/eji.1830101102. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M. Inhibition of pathogenic effect of effector T cells by specific suppressor T cells during influenza virus infection in mice. Nature. 1983 Aug 11;304(5926):541–543. doi: 10.1038/304541a0. [DOI] [PubMed] [Google Scholar]
- Masurel N., de Bruijne J. I., Beuningh H. A., Schouten H. J. Haemagglutination-inhibition antibodies against influenza A and influenza B in maternal and neonatal sera. J Hyg (Lond) 1978 Feb;80(1):13–19. doi: 10.1017/s0022172400053353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T., Liu Y. C., Schulman J. L., Bona C. A. Shared idiotopes among monoclonal antibodies specific for A/PR/8/34 (H1N1) and X-31(H3N2) influenza viruses. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1809–1812. doi: 10.1073/pnas.81.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Henderson F. W., Clyde W. A., Jr, Collier A. M., Denny F. W. Pneumonia: an eleven-year study in a pediatric practice. Am J Epidemiol. 1981 Jan;113(1):12–21. doi: 10.1093/oxfordjournals.aje.a113061. [DOI] [PubMed] [Google Scholar]
- Murray D. L., Imagawa D. T., Okada D. M., St Geme J. W., Jr Antibody response to monovalent A/New Jersey/8/76 influenza vaccine in pregnant women. J Clin Microbiol. 1979 Aug;10(2):184–187. doi: 10.1128/jcm.10.2.184-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble G. R., Kaye H. S., Kendal A. P., Dowdle W. R. Age-related heterologous antibody responses to influenza virus vaccination. J Infect Dis. 1977 Dec;136 (Suppl):S686–S692. doi: 10.1093/infdis/136.supplement_3.s686. [DOI] [PubMed] [Google Scholar]
- Puck J. M., Glezen W. P., Frank A. L., Six H. R. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis. 1980 Dec;142(6):844–849. doi: 10.1093/infdis/142.6.844. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Schulman J. L. Cellular immune responses of mice to influenza virus vaccines. J Immunol. 1980 Nov;125(5):2182–2188. [PubMed] [Google Scholar]
- Reuman P. D., Kris R. M., Ayoub E. M., Small P. A., Jr Maternal-infant transfer of influenza-specific immunity not detectable by haemagglutination inhibition. Microbios. 1988;54(220-221):135–147. [PubMed] [Google Scholar]
- Reuman P. D., Paganini C. M., Ayoub E. M., Small P. A., Jr Maternal-infant transfer of influenza-specific immunity in the mouse. J Immunol. 1983 Feb;130(2):932–936. [PubMed] [Google Scholar]
- Ruben F. L., Holzman I. R., Fireman P. Responses of lymphocytes from human colostrum or milk to influenza antigens. Am J Obstet Gynecol. 1982 Jul 1;143(5):518–522. doi: 10.1016/0002-9378(82)90540-3. [DOI] [PubMed] [Google Scholar]
- Sigal N. H., Chan M., Reale M. A., Moran T., Beilin Y., Schulman J. L., Bona C. The human and murine influenza-specific B cell repertoires share a common idiotope. J Immunol. 1987 Sep 15;139(6):1985–1990. [PubMed] [Google Scholar]
- Sumaya C. V., Gibbs R. S. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. J Infect Dis. 1979 Aug;140(2):141–146. doi: 10.1093/infdis/140.2.141. [DOI] [PubMed] [Google Scholar]
- Sun C. S., Wilson S. Z., Wyde P. R. Limited efficacy of aerosolized recombinant alpha interferon against virulent influenza A/HK infection in mice. Proc Soc Exp Biol Med. 1986 Feb;181(2):298–304. doi: 10.3181/00379727-181-42257. [DOI] [PubMed] [Google Scholar]
- Sweet C., Bird R. A., Jakeman K., Coates D. M., Smith H. Production of passive immunity in neonatal ferrets following maternal vaccination with killed influenza A virus vaccines. Immunology. 1987 Jan;60(1):83–89. [PMC free article] [PubMed] [Google Scholar]
- Sweet C., Jakeman K. J., Smith H. Role of milk-derived IgG in passive maternal protection of neonatal ferrets against influenza. J Gen Virol. 1987 Oct;68(Pt 10):2681–2686. doi: 10.1099/0022-1317-68-10-2681. [DOI] [PubMed] [Google Scholar]
- Wilson S. Z., Knight V., Wyde P. R., Drake S., Couch R. B. Amantadine and ribavirin aerosol treatment of influenza A and B infection in mice. Antimicrob Agents Chemother. 1980 Apr;17(4):642–648. doi: 10.1128/aac.17.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Wilson M. R., Cate T. R. Interferon production by leukocytes infiltrating the lungs of mice during primary influenza virus infection. Infect Immun. 1982 Dec;38(3):1249–1255. doi: 10.1128/iai.38.3.1249-1255.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R., Nelson D. L. Specificity of in vitro anti-influenza virus antibody production by human lymphocytes: analysis of original antigenic sin by limiting dilution cultures. J Immunol. 1984 Feb;132(2):928–935. [PubMed] [Google Scholar]
- Zweerink H. J., Askonas B. A., Millican D., Courtneidge S. A., Skehel J. J. Cytotoxic T cells to type A influenza virus; viral hemagglutinin induces A-strain specificity while infected cells confer cross-reactive cytotoxicity. Eur J Immunol. 1977 Sep;7(9):630–635. doi: 10.1002/eji.1830070910. [DOI] [PubMed] [Google Scholar]



