Abstract
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States, and persistent HPV infection is strongly associated with risk of cervical cancer and genital warts. The recently approved quadrivalent HPV vaccine targets the HPV strains responsible for approximately 70% of cervical cancers and 90% of genital warts. It is also effective in reducing the incidence of HPV-related conditions, especially when given prior to exposure to HPV. The vaccine is recommended for all girls aged 11 to 12 with catch-up vaccination for women up to age 26, and most insurance plans cover the vaccine. A second bivalent HPV vaccine is currently pending approval by the US Food and Drug Administration (FDA). HPV vaccination reduces the incidence of HPV-related cancers and precancerous lesions in the United States and abroad, though decisions regarding implementation of vaccination remain.
Key words: Human papillomavirus, Cervical cancer, Genital warts, Sexually transmitted disease, Vaccination
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States with approximately 80% of women having acquired an infection by the age of 50.1 Although most HPV infections clear, persistent HPV infection is strongly associated with risk of cervical cancer and genital warts. The recently approved quadrivalent (types 6, 11, 16, and 18) HPV vaccine targets the HPV strains responsible for approximately 70% of cervical cancers and 90% of genital warts. It is also effective in reducing the incidence of HPV-related conditions, including cervical intraepithelial neoplasia (CIN) grades I, II, and III; adenocarcinoma in situ (AIS); vulvar and vaginal neoplasia; and genital warts, especially when given prior to exposure to HPV. The vaccine is recommended for all girls aged 11 to 12 with catch-up vaccination for women aged up to 26, and most insurance plans cover the vaccine. A second bivalent HPV vaccine is currently pending approval by the US Food and Drug Administration (FDA). HPV vaccination reduces the incidence of HPV-related cancers and precancerous lesions in the United States and abroad, though decisions regarding implementation of vaccination remain.
HPV Infection
The human papillomavirus is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces. More than 100 different types of the virus exist, including approximately 30 to 40 strains that infect the human genital tract. Of these, there are oncogenic or high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, and 58) that are associated with cervical, vulvar, vaginal, and anal cancers, and non-oncogenic or low-risk types (6, 11, 40, 42, 43, 44, and 54) that are associated with genital warts.2 HPV 16 is the most oncogenic, accounting for almost half of all cervical cancers, and HPV 16 and 18 together account for approximately 70% of cervical cancers.3 HPV 6 and 11 are the most common strains associated with genital warts and are responsible for approximately 90% of these lesions.
High-risk strains of HPV are now well established as the causative agents responsible for cervical dysplasia and cervical cancer.4 The American Cancer Society estimated 11,150 new cases of invasive cervical cancer in the United States in 2007 and about 3670 cervical cancer deaths that same year.5 Although cervical cancer as a cause of death in the United States has drastically declined over the last 50 years due to Papanicolaou testing, it is still the second leading cause of cancer-related death in women worldwide. There are 510,000 women diagnosed with invasive cervical cancer per year worldwide and 288,000 deaths, with approximately 80% of these cases in the developing world.6 In addition to cervical cancer, HPV has also been implicated as etiology for other less common genital cancers, including vulvar, vaginal, and anal carcinomas.7,8
Low-risk strains of HPV are responsible for anogenital condyloma or genital warts. Although not a life-threatening condition, genital warts are a major cause of morbidity as well as psychosocial distress and embarrassment for many patients. Approximately 1 million new cases of genital warts are diagnosed in the United States each year, of which 70% are estimated to persist beyond 4 months.9 Several treatment options are available for persistent genital warts; however none are uniformly successful. Recurrence rates vary tremendously, from 5% to 65% depending on treatment modality.10
The HPV virus is transmitted through direct skin-to-skin contact. Although infection is most often spread through penetrative vaginal or anal intercourse, other types of sexual contact can transmit HPV, and infection has been reported in self-reported “virgins.”11,12 Most HPV infections are acquired within the first years of sexual activity, as demonstrated by a study of 603 college students, in which it was found that approximately 40% of HPV infections are acquired within 2 years of the first sexual experience (Figure 1).11 The risk of infection is proportionately related to number of sexual partners, which has been demonstrated in several studies.11,13,14 Condoms can decrease the risk of transmission of HPV; however, the extent of this protection has not been fully elucidated.1 It should be noted that women having sexual contact with men who use condoms are still at risk of acquiring an infection, as condoms are not 100% protective. Given that a large proportion of infections are without noticeable symptoms, most individuals are unaware that they have HPV, and can unknowingly transmit the virus to others.
Figure 1.
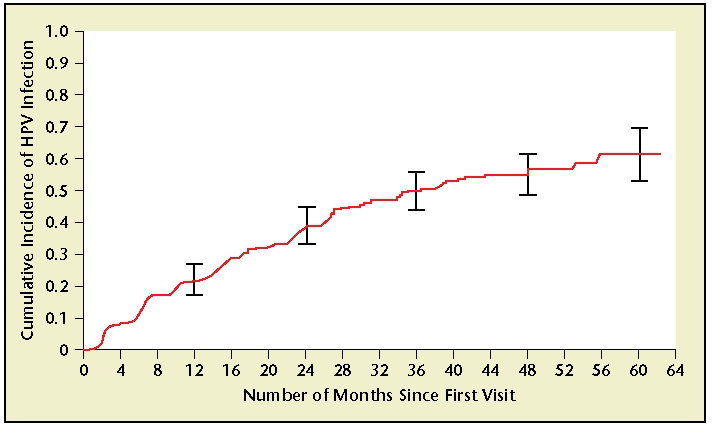
Cumulative incidence of human papillomavirus (HPV) infection from time of first sexual intercourse (n = 94) among women in Washington State, 1990–2000. Vertical bars, 95% confidence intervals at 12, 24, 36, 48, and 60 months. Reprinted with permission from Winer RL et al., “Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students,” American Journal of Epidemiology, 2003, Volume 157, Number 3, pp. 218–226, by permission of Oxford University Press.
Studies on the natural history of HPV infections have shown that in young women, the vast majority of HPV infections are transient. A study of college-age women showed that approximately 70% of women with HPV infections became HPV negative within 1 year and as many as 91% of them became HPV negative within 2 years, with a median duration of infection of 8 months.13 Certain HPV types, such as HPV 16, are associated with increased rates of persistence; however, in the previously mentioned study, the 24-month clearance of HPV 16 was 72%. Thus, the majority of these infections clear. Other factors associated with persistent HPV infection include age higher than 30, parity, infection with multiple HPV subtypes, immunosuppression, smoking, and oral contraceptive use.15
The risk of HPV infections that do not clear is persistence and progression of cervical epithelial abnormalities. Early HPV infections may be manifest by mild changes in the cervical epithelium, which can be detected by Papanicolaou testing. Cytologic changes in the squamous epithelium are termed squamous intraepithelial lesions (SILs) and can be characterized as low grade or high grade. When diagnosed by histology, HPV-associated changes are termed cervical intraepithelial neoplasia and are graded from 1 to 3, depending on the depth of abnormal cells. CIN 1 includes mild dysplasia and condyloma (anogenital warts) and includes lesions in which only one third of the depth of the epithelium is abnormal. CIN 2 or moderate dysplasia includes lesions with abnormal proliferation of up to two thirds of the epithelium, and in CIN 3, which includes severe dysplasia and carcinoma in situ (CIS), the entire epithelium is abnormal.16 Similar grading exists for vulvar intraepithelial neoplasia (VIN 1-3) and vaginal intraepithelial neoplasia (VAIN 1-3).
Cervical intraepithelial neoplasia caused by HPV can clear without treatment, but the rate of clearance varies according to the severity of the lesion; high-grade lesions have a greater rate of progression to cervical cancer (Figure 2). Sixty percent of CIN 1 lesions are likely to regress spontaneously, 10% are likely to progress to CIN 3, and only 1% is likely to progress to invasive cancer. For CIN 3, these numbers are quite different: approximately one third of these lesions are likely to regress, and the rate of progression to cervical cancer is greater than 12% if left untreated.17
Figure 2.
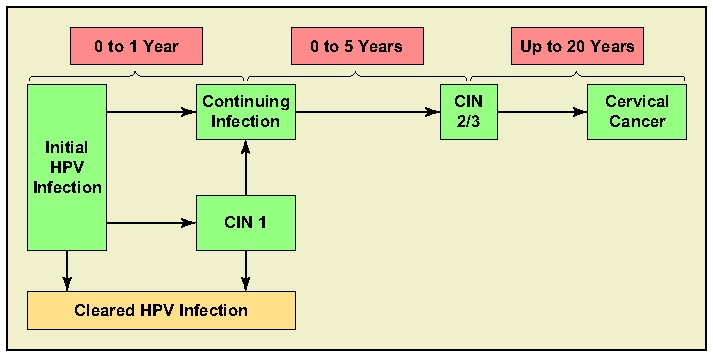
Human papillomavirus (HPV) clearance versus progression. CIN, cervical intraepithelial neoplasia.
Just as the acquisition of HPV is highest among young women, the prevalence of abnormal cervical cytology is also highest in younger women (Figure 3). In a study that looked at Papanicolaou test results of more than 80,000 women ages 10 to over 70, the rate of SIL was highest in the subgroup aged 10 to 19.18 Although the majority of these lesions will clear spontaneously, the risk of progression of cervical lesions is increased with earlier age of sexual activity. This was demonstrated in a case-controlled study of 206 women with CIN and 327 women with invasive cancer in which the relative risk for CIN and invasive cervical cancer increased with decreasing age at first intercourse. For example, the risk of invasive cervical cancer was 5 times higher among women who reported first intercourse before the age of 18 as compared to those who were 22 years or older.19 Thus, younger age at first intercourse is not only related to increasing rates of cervical abnormalities in young women, but a higher risk of progression to cervical cancer.
Figure 3.
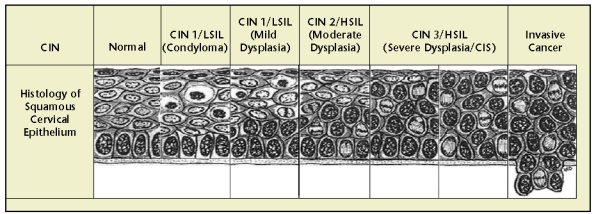
Histology of squamous intraepithelial lesions. CIN, cervical intraepithelial neoplasia; CIS, carcinoma in situ; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade intraepithelial lesion. Reprinted with permission from Bonnez W.16
HPV Vaccine
In June 2006, the FDA approved the first vaccine against HPV. Gardasil® (Merck & Co., Inc., Whitehouse Station, NJ) is a prophylactic quadrivalent vaccine against HPV types 6, 11, 16, and 18 made from noninfectious viruslike particles (VLPs) and it is given as a series of 3 injections over a 6-month period (at 0, 2, and 6 months). The vaccine targets the 4 HPV types that together cause 70% of cervical cancer, AIS, CIN 3, VIN 2/3, and VAIN 2/3 cases; 50% of CIN 2 cases; 35% to 50% of all CIN 1, VIN 1, and VAIN 1 cases; and 90% of genital warts.
The efficacy of Gardasil was demonstrated in 4 large, randomized, phase II and III studies that enrolled a total of 20,541 women aged 16 to 26. The primary endpoints measured in these trials were precancerous lesions (CIN) grade 2/3 and AIS. These endpoints were chosen given their relatively greater prevalence and known status as immediate precursors to invasive cervical cancer. Women were enrolled regardless of baseline HPV status, and 73% were HPV negative to all 4 subtypes at enrollment. The remaining 27% had evidence of infection with at least 1 of the HPV subtypes, but only 7% had evidence of exposure to more than 1 HPV type. Thus, 93% of participants were negative for at least 3 of the 4 HPV subtypes. The primary goal was to study the effect of the prophylactic vaccine on HPV-naive women, and thus the primary study population was women who were HPV naive to the relevant HPV strain, who remained HPV negative until 1 month after the vaccination period, received all 3 vaccine doses, and had no protocol violations (per-protocol efficacy treatment group). Secondary analysis looked at the efficacy of the vaccine in all subjects enrolled on day 1 regardless of HPV status who received at least 1 dose of the vaccine. This secondary analysis approximated the impact of giving the vaccine to the general population of American young women.
In a combined analysis of the data from all 4 clinical trials, the efficacy of the quadrivalent vaccine in the perprotocol group was 99% against HPV 16/18-related CIN 2/3 or AIS. Specifically the efficacy was 100% against CIN 2, 98% against CIN 3, and 100% against AIS. By HPV type, the vaccine prevented 99% of HPV 16-related lesions and 100% of HPV 18-related lesions.20 Protection against genital warts caused by HPV types 6, 11, 16, and 18 was likewise high, with the vaccine preventing 99% of these infections. These efficacy results reflect the tremendous impact of vaccinating girls and adolescents before sexual activity and prior to exposure to HPV.21
The analysis of the intent-to-treat populations in the Gardasil trials also demonstrated a significant reduction in CIN 2/3 and AIS lesions, even in women with prior exposure to HPV, in those who deviated from the protocol, or in those who did not complete the full vaccine series. In this group, the quadrivalent vaccine showed an efficacy of 44% for HPV 16/18-related CIN 2/3 or AIS. When broken down by lesions, it prevented 50% of CIN 2, 39% of CIN 3, and 54% of AIS lesions. By HPV type, 42% of HPV 16-related lesions were prevented, and 81% of HPV 18-related lesions.20 These data show that although the benefit of the vaccine is clearly highest when given to an HPV-naive population, there is still a significant reduction in HPV-related precancerous lesions when given to the general population regardless of HPV status. There was no evidence that the vaccine had any impact on the natural history of previously acquired HPV infections.20
Data from 3 of the trials of the quadrivalent vaccine have been analyzed to look at vaginal and vulvar lesions, and show similar rates of efficacy as for cervical lesions. In the per-protocol HPV population, the vaccine was 100% effective at preventing HPV 16/18-related VIN 2/3 and VAIN 2/3 over a 3-year follow-up period. In the intention-to-treat population, the vaccine was 71% effective against HPV 16/18-related VIN 2/3 and VAIN 2/3 and 49% effective against these lesions regardless of HPV type (Table 1). These data show that the quadrivalent vaccine is effective at preventing vulvar and vaginal lesions associated with HPV 16 and 18, and over time may contribute to decreased incidence of vaginal and vulvar invasive cancers.
Table 1.
Primary Analysis of Efficacy Against Human Papillomavirus Type 16 and 18—Related Cervical Intraepithelial Neoplasia Grade 2/3 and Adenocarcinoma In Situ
| Vaccine (N = 10,291) |
Placebo (N = 10,292) |
||||||
|---|---|---|---|---|---|---|---|
| n | Cases | Rate* | n | Cases | Rate* | Efficacy (95% CI) | |
| Per-protocol susceptible population† | |||||||
| HPV 16/18-related CIN 2/3 or AIS | 8579 | 1 | < .1 | 8550 | 85 | .4 | 99% (93–100) |
| By lesion type | |||||||
| CIN 2 | 8579 | 0 | 0 | 8550 | 56 | .3 | 100% (93–100) |
| CIN 3 | 8579 | 1 | < .1 | 8550 | 51 | .2 | 98% (89–100) |
| AIS | 8579 | 0 | 0 | 8550 | 7 | < .1 | 100% (31–100) |
| By HPV type | |||||||
| HPV 16-related | 7455 | 1 | < .1 | 7265 | 73 | .4 | 99% (92–100) |
| HPV 18-related | 7450 | 0 | 0 | 7381 | 18 | .1 | 100% (78–100) |
| Unrestricted susceptible population‡ | |||||||
| HPV 16/18-related CIN 2/3 or AIS | 9729 | 3 | < .1 | 9737 | 121 | .4 | 98% (93–100) |
| By lesion type | |||||||
| CIN 2 | 9729 | 1 | < .01 | 9737 | 77 | .3 | 99% (93–100) |
| CIN 3 | 9729 | 2 | < .01 | 9737 | 75 | .3 | 97% (90–100) |
| AIS | 9729 | 0 | 0 | 9737 | 10 | < .1 | 100% (55–100) |
| By HPV type | |||||||
| HPV 16-related | 8502 | 3 | < .1 | 8497 | 103 | .4 | 97% (91–99) |
| HPV 18-related | 8383 | 0 | 0 | 8410 | 25 | .1 | 100% (84–100) |
| ITT populations§ | |||||||
| HPV 16/18-related CIN 2/3 or AIS | 10,291 | 142 | .5 | 10,292 | 255 | .9 | 44% (31–55) |
| By lesion type | |||||||
| CIN 2 | 10,291 | 82 | .3 | 10,292 | 163 | .5 | 50% (34–62) |
| CIN 3 | 10,291 | 99 | .3 | 10,292 | 162 | .5 | 39% (21–53) |
| AIS | 10,291 | 6 | < .1 | 10,292 | 13 | < .1 | 54% (−30–86) |
| By HPV type | |||||||
| HPV 16-related | 10,291 | 134 | .5 | 10,292 | 232 | .8 | 42% (28–54) |
| HPV 18-related | 10,291 | 8 | < .1 | 10,292 | 42 | .2 | 81% (59–92) |
Each woman counted only once in each applicable row.
Cases per 100 person-years at risk.
Women with at least 1 follow-up visit post-dose 3: 8492 vaccine vs 8462 placebo for analysis of HPV 16/18 endpoints; 7401 vs 7203 for analysis of HPV 16 endpoints; 7381 vs 7314 for analysis of HPV 18 endpoints.
Women with at least 1 follow-up visit post—dose 1: 9348 vaccine vs 9406 for analysis of HPV 16/18 endpoints; 8165 vs 8200 for analysis of HPV 16 endpoints; 8151 vs 8209 for analysis of HPV 18 endpoints.
Women with at least 1 dose and had at least 1 follow-up visit post—dose 1: 9841 vaccine and 9904 placebo. 95% CI, 95% confidence interval; AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; N, number randomized to group; n = number in specified population.
Reprinted from The Lancet, Volume 369, Ault KA et al, “Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomized clinical trials,” pp. 1861–1868, Copyright 2007, with permission from Elsevier.
In addition to the efficacy of the quadrivalent vaccine against the HPV subtypes that it is formulated for recent analysis of the data from the phase III trials demonstrated that the vaccine also offers cross-protection against other viral subtypes that are not in the vaccine. This finding is not surprising because the HPV family of viruses shares many proteins. Among the study population of HPV-naive women, the efficacy of the quadrivalent vaccine against CIN 1 to 3 or AIS due to 10 oncogenic nonvaccine HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) was 27%, and efficacy against CIN 2/3 and AIS against the same 10 HPV strains was 38%.22 These data are the first to show a substantial reduction in cervical lesions caused by 10 nonvaccine HPV types that together cause about 20% of cervical cancers worldwide. This cross-reactivity may provide additional protection to young women who are vaccinated with the quadrivalent vaccine.
Currently, the duration of protection of the quadrivalent vaccine has been demonstrated for up to 5 years, but its duration beyond that is not yet known. Immunogenicity studies have shown that the antibody levels peak after the third dose, fall by 1 log over the next 18 months, and then level off.23 Antibody levels are maintained at or above the level seen with natural infection for the approximately 5 years of follow-up analysis currently available, and sustained efficacy of the quadrivalent vaccine against CIN and persistent infection has been demonstrated in follow-up analysis for the same duration of time.24 Whereas preliminary studies do show an increase to antibody titers after a challenge dose given 5 years after the initial vaccination, it is currently unknown if a booster dose will be necessary. Follow-up studies are currently ongoing to determine the duration of protection for at least 14 years after vaccination (Table 2).
Table 2.
Characteristics of 2 Candidate Human Papillomavirus Vaccines and Trial Populations
| Quadrivalent Vaccine | Bivalent Vaccine | |
|---|---|---|
| Characteristic | Merck [Gardasil] | GlaxoSmithKline [Cervarix] |
| Virus-like particles [VLPs] of genotypes | 6, 11, 16, 18 | 16, 18 |
| Substrate | Yeast [Saccharomyces cerevisiae] | Baculovirus expression system |
| Adjuvant | Proprietary aluminium hydroxyphosphate | Proprietary aluminium hydroxide |
| sulfate (225 µg; Merck aluminium adjuvant) | (500 µg) plus 50 µg 3-deacylated | |
| monophosphoryl lipid A (GSK AS04 | ||
| adjuvant) | ||
| Schedule used in trials: 3 intramuscular | 2 months between doses 1 and 2; | 1 month between doses 1 and 2; |
| doses of 0.5 mL with intervals of: | 6 months between doses 1 and 3 | 6 months between doses 1 and 3 |
| Countries/regions included in | Brazil (34%); Europe (21%); | Brazil and North America (over 50% |
| phase II trials | United States (45%) | of women were from Brazil) |
| Countries/regions included in | North America (25%); Latin America | North America (12%); Latin America |
| phase III trials | (27%); Europe (44%): Asia-Pacific (4%) | (34%); Europe (30%); Asia-Pacific (25%) |
| Adolescent safety/immunogenicity | Boys and girls 9–15 years | Girls 10–14 years |
| bridging trials | Boys 10–18 years | |
| Other trials in progress or due to start | Efficacy, immunogenicity bridging | Efficacy, immunogenicity bridging |
| and safety studies in women 25–45 years; | and safety studies in women > 26 | |
| studies of administration at the same time | years; studies of administration at the | |
| as other vaccines; safety and immunogenicity | same time as other vaccines; safety | |
| in HIV-infected persons and other | and immunogenicity in African | |
| immunocompromised groups; efficacy | populations, including HIV-infected | |
| study in men | women |
HIV, human immunodeficiency virus.
Reprinted from Cutts FT et al,21 with permission of the World Health Organization.
Although the efficacy of the quadrivalent vaccine has not been tested in individuals younger than 16, there are immunogenicity data from HPV trials to show that the immunologic responses among 9- to 15-year-old girls at 1 month post-dose 3 were not inferior to anti-HPV responses in 16- to 26-year-old adolescents and young adults.25 Thus, given equal if not greater immunogenic responses among 9- to 15-year-old girls, the efficacy of the quadrivalent vaccine in this age group is inferred.
A second HPV vaccine, the bivalent Cervarix™ (GlaxoSmithKline, Philadelphia, PA), is currently pending FDA approval. The bivalent vaccine is an L1 VLP vaccine against HPV 16 and 18, which is also given as a series of three injections (0, 1, and 6 months). Phase III trials of the bivalent vaccine enrolled 18,644 women aged 15 to 25, of whom 9258 received the vaccine. As in the trials of the quadrivalent vaccine, primary analysis was done in women who were HPV negative at enrollment and who completed the vaccination series, and CIN 2+ lesions (CIN 2, CIN 3, AIS, and invasive cancer) were used as the primary endpoints. Combined efficacy for the bivalent vaccine was 90.4% against HPV 16 and 18-associated CIN 2+ lesions, with 93.3% efficacy against HPV 16-related lesions and 83.3% efficacy against HPV 18-related lesions. Additional analysis of the 23 women with CIN 2+ lesions revealed that 14 of them had at least 1 other HPV type in the lesion. In 3 of these cases, the HPV 16 or 18 detected was thought unlikely to be the cause of the cervical abnormality due to evidence of preceding infection with other oncogenic subtypes. Thus, the authors concluded that if these cases were excluded, the efficacy of the vaccine would be 100%. The bivalent vaccine was also 89.2% effective against CIN 1 lesions.26
As with the quadrivalent vaccine, the bivalent vaccine demonstrates evidence of some cross-reactivity against other HPV types. Six-month protection against persistent infection was seen with HPV 45 (59.9% efficacy) and HPV 31 (36.1% efficacy), and 12-month protection against 12 combined non-16 or 18 HPV types was 27.1%.26 The bivalent vaccine is only targeted at HPV 16 and 18 and is thus not designed to offer protection against genital warts.
Approval of Cervarix was initially expected at the end of 2007, but the FDA delayed its approval and requested additional information from the manufacturer. There is uncertainty whether the vaccine will be available in the United States in 2008. The bivalent vaccine is currently available in Europe and was recently approved in Australia.
Administering the Vaccine
The efficacy of HPV vaccination is greatest when given to HPV-naive women. Given the high correlation between HPV infection and onset of sexual contact (digital, oral, anal, or vaginal), the ideal time to give the vaccine is prior to initiation of sexual activity. Data from the 2003 Youth Risk Behavior Survey revealed that 7.4% of youths report sexual intercourse before age 13, and by the end of high school (grade 12) more than 60% of adolescents have had intercourse, with 20.3% having had more than 4 lifetime partners.27
When the FDA approved Gardasil in June 2006, the vaccine was approved for use in girls and women aged 9 to 26. The Advisory Committee on Immunization Practices (ACIP) recommended that the vaccine should be administered to girls aged 11 to 12 as part of the routine vaccination schedule, and that it could be administered to girls as young as 9 years old. It should also be offered to girls and women aged 13 to 26 who have not yet completed the vaccination series.28 The American College of Obstetricians and Gynecologists, the Society for Adolescent Medicine, and the American Academy of Family Physicians support similar recommendations. As gynecologists, it is our responsibility to vaccinate all women between 9 and 26 who have not yet completed the vaccination series.
The HPV vaccine is generally well tolerated by patients. In clinical trials, the most common side effects were injection site pain, swelling, and erythema. These were seen at higher rates among patients receiving the active vaccine compared with those receiving placebo; however, these side effects were rated as mild or moderate in intensity by 94.3% of participants. There was overall no difference in the rates of serious side effects between vaccine and placebo recipients. Very few patients (0.1%) withdrew from the trials due to adverse reactions. The only absolute contraindication to Gardasil is hypersensitivity to the active substances or to any of the inactive ingredients in the vaccine, and individuals who develop symptoms suggestive of hypersensitivity after receiving a dose of the vaccine should not receive further doses. The vaccine is not recommended for use in pregnant women, and caution should be used in women who are breastfeeding. Effectiveness does not appear to be altered by use of oral contraceptives or concomitant administration of the hepatitis B vaccine, although other vaccines have not been studied. The immunologic response may be decreased in patients receiving immunosuppressive therapies.29
Access and Law
Private insurance carriers tend to follow ACIP guidelines, and this has proven to be the case with the HPV quadrivalent vaccine as well. As of September 29, 2007, health plans covering about 98% of privately insured individuals in the United States had decided at the national level to reimburse for the quadrivalent vaccine. Nevertheless, these decisions at the national level can still be affected by state and regional decisions and coverage may vary. Although most girls and women in the target age for HPV vaccination have private insurance, 1 in 10 (12%) girls aged 9 to 18 and 3 in 10 (29%) women aged 19 to 26 are uninsured.30
The Vaccines for Children (VCF) Program is a federally funded program that vaccinates children who are covered by Medicaid, or who are Medicaid-eligible, uninsured, American Indian or Alaskan Native. VCF has added the Gardasil vaccine to its coverage list. States that have a State Children’s Health Insurance Program separate from their Medicaid program are also required to cover ACIP-recommended vaccines, though the funding for this must come at the state level.30
For adults with Medicaid, vaccination coverage is optional and is decided on a state-by-state basis. There is currently no public funding available for uninsured adults for the HPV vaccine; however, Merck has established a vaccination assistance program for uninsured women whose income is below 200% of the poverty level, and on an individual basis additional exceptions can be made for patients with higher incomes.31
Since the ACIP recommendations to vaccinate all girls aged 11 to 12 were issued, there has been a tremendous amount of debate regarding whether to mandate the HPV vaccine as part of school vaccination programs. The debate includes considerations about the vaccine’s safety, cost, and moral objections to vaccinating girls against a sexually transmitted infection. School vaccination programs are regulated by the state and, in 2007, at least 24 states and the District of Columbia introduced legislation that would mandate HPV vaccine coverage, the majority of which are still under consideration. In February 2007, Texas was the first state to enact a mandate for vaccination by executive order of the governor; however, this mandate was later overturned by the state legislature. Virginia is the only other state that so far has also passed a bill mandating vaccination, which would go into effect October 2008, and a bill that would delay the requirement is currently under consideration.
Conclusion
The human papillomavirus is the most common sexually transmitted infection in the United States and is the cause of cervical cancer and genital warts. The quadrivalent HPV vaccine is 99% effective at preventing the high-grade cervical lesions caused by HPV types 16 and 18 that are precursors to cervical cancer, and is equally effective at preventing HPV 6 and 11-related genital warts when given to HPV-naive individuals. It can also reduce a significant number of infections when given to women with some prior HPV exposure. Efficacy has been shown for up to 5 years, and follow-up is ongoing. A second bivalent HPV vaccine has been developed that is effective against HPV 16 and 18, but is not yet available in the United States. The quadrivalent vaccine is currently recommended for girls aged 11 to 12, catch-up vaccination is recommended for women up to the age of 26, and most insurance plans cover the vaccine. Debate is currently ongoing with regard to whether the vaccine will become a part of the mandatory vaccination schedule.
Main Points.
High-risk strains of the human papillomavirus (HPV) are now well established as the causative agents responsible for cervical dysplasia. HPV 16 is the most oncogenic, accounting for almost half of all cervical cancers, and HPV 16 and 18 together account for approximately 70% of cervical cancers.
Most HPV infections are acquired within the first years of sexual activity, and the risk of infection is proportionally related to the number of sexual partners. However, the majority of HPV infections are transient in young women.
Cervical intraepithelial neoplasia (CIN) caused by HPV can clear without treatment, but the rate of clearance varies according to the severity of the lesion; high-grade lesions have a greater rate of progression to cervical cancer.
In a combined analysis of data from 4 clinical trials, the efficacy of the quadrivalent vaccine in the per-protocol group was 99% against HPV type 16/18-related CIN grade 2/3 or adenocarcinoma in situ.
Protection against genital warts caused by HPV types 6, 11, 16, or 18 was likewise high, with the vaccine preventing 99% of these infections.
The efficacy of HPV vaccination is greatest when given to HPV-naive women.
The Advisory Committee on Immunization Practices recommended that the vaccine should be administered to girls aged 11 to 12 as part of the routine vaccination schedule, and that it could be administered to girls as young as 9 years old. It should also be offered to girls and women aged 13 to 26 who have not yet completed the vaccination series. The American College of Obstetricians and Gynecologists, the Society for Adolescent Medicine, and the American Academy of Family Physicians support similar recommendations.
Debate is currently ongoing with regard to whether the HPV vaccine will become part of the mandatory vaccination schedule.
References
- 1.Centers for Disease Control and Prevention, authors. Genital HPV Infection Fact Sheet. Rockville, MD: CDC National Prevention Information Network; 2004. [Google Scholar]
- 2.Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Clifford GM, Rana RK, Franceschi S, et al. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89:101–105. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch FX, Lorincz A, Muñoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society, authors. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 6.World Health Organization (WHO) Initiative for Vaccine Research, authors. Human Papilloma Infection and Cervical Cancer. Vol. 4. Geneva, Switzerland: WHO; 2008. [Accessed January 30, 2008]. http://www.who.int/vaccine_research/diseases/hpv/en/ [Google Scholar]
- 7.Hildesheim A, Han CL, Brinton LA, et al. Human papillomavirus type 16 and risk of preinvasive and invasive vulvar cancer: results from a seroepidemiological case-control study. Obstet Gynecol. 1997;90:748–754. doi: 10.1016/S0029-7844(97)00467-5. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society (ACS), authors Detailed Guide: Vaginal Cancer: What Are the Risk Factors for Vaginal Cancer? Atlanta, GA: ACS; 2006. [Accessed February 5, 2008]. http://www.cancer.org/docroot/CRI/content/CRI_2_4_2X_What_are_the_risk_factors_for_vaginal_cancer_55.asp?sitearea= [Google Scholar]
- 9.Lacey CJN. Therapy for genital human papillomavirus-related disease. J Clin Virol. 2005;32(suppl):S82–S90. doi: 10.1016/j.jcv.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician. 2004;70:2335–2342. [PubMed] [Google Scholar]
- 11.Winer RL, Lee S-K, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 12.Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidemiol Biomarkers Prev. 2001;10:101–106. [PubMed] [Google Scholar]
- 13.Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 14.Sellors JW, Karwalajtys TL, Kaczorowski J, et al. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168:421–425. [PMC free article] [PubMed] [Google Scholar]
- 15.Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis-role of parity, oral contraceptives and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–28. [PubMed] [Google Scholar]
- 16.Bonnez W. Papillomavirus. In: Richman DD, Whitley RJ, Hayden FJ, editors. Clinical Virology. 2nd ed. Washington, DC: American Society for Microbiology Press; 2002. pp. 557–596. [Google Scholar]
- 17.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12:186–192. [PubMed] [Google Scholar]
- 18.Mount SL, Papillo JL. A study of 10,296 pediatric and adolescent Papanicolaou smear diagnoses in northern New England. Pediatrics. 1999;103:539–545. doi: 10.1542/peds.103.3.539. [DOI] [PubMed] [Google Scholar]
- 19.La Vecchia C, Franceschi S, Decarli A, et al. Sexual factors, venereal diseases, and the risk of intraepithelial and invasive cervical neoplasia. Cancer. 1986;58:935–941. doi: 10.1002/1097-0142(19860815)58:4<935::aid-cncr2820580422>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Ault KA the Future II Study Group, authors. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomized clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 21.Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719–726. doi: 10.2471/BLT.06.038414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown D the Future Study Group, authors. HPV type 6/11/16/18 vaccine: first analysis of cross-protection against persistent infection, cervical intraepithelial neoplasia (CIN), and adenocarcinoma in situ (AIS) caused by oncogenic HPV types in addition to 16/18. Interscience Conference on Antimicrobial Agents and Chemotherapy; September 2007; Chicago, IL. [Google Scholar]
- 23.Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 24.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 25.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in male and female adolescents and young women. Pediatrics. 2006;118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 26.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomized controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 27.Grunbaum JA, Kann L, Kinchen S, et al. Youth risk behavior surveillance-United States, 2003. MMWR Surveill Summ. 2004;53:1–96. [PubMed] [Google Scholar]
- 28.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Vaccination Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 29.Merck & Co., Inc, authors. [Accessed February 6, 2008];Gardasil: prescribing information Web site. http://www.gardasil.com/prescribinginformation-about-gardasil.html.
- 30.The Henry J. Kaiser Family Foundation, authors. HPV Vaccine: Implementation and Financing Policy. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 2007. [Accessed February 6, 2008]. http://www.kff.org/womenshealth/upload/7602.pdf. [Google Scholar]
- 31.Merck & Co., Inc, authors. [Accessed March 5, 2008];Placing patients first by making medicines more affordable-who may qualify. http://www.merck.com/merckhelps/vaccines/qualify.html.


