Abstract
Mature plant cell walls lose their ability to expand and become unresponsive to expansin. This phenomenon is believed to be due to cross-linking of hemicellulose, pectin, or phenolic groups in the wall. By screening various hydrolytic enzymes, we found that pretreatment of nongrowing, heat-inactivated, basal cucumber (Cucumis sativus) hypocotyls with pectin lyase (Pel1) from Aspergillus japonicus could restore reconstituted exogenous expansin-induced extension in mature cell walls in vitro. Recombinant pectate lyase A (PelA) and polygalacturonase (PG) from Aspergillus spp. exhibited similar capacity to Pel1. Pel1, PelA, and PG also enhanced the reconstituted expansin-induced extension of the apical (elongating) segments of cucumber hypocotyls. However, the effective concentrations of PelA and PG for enhancing the reconstituted expansin-induced extension were greater in the apical segments than in the basal segments, whereas Pel1 behaved in the opposite manner. These data are consistent with distribution of more methyl-esterified pectin in cell walls of the apical segments and less esterified pectin in the basal segments. Associated with the degree of esterification of pectin, more calcium was found in cell walls of basal segments compared to apical segments. Pretreatment of the calcium chelator EGTA could also restore mature cell walls' susceptibility to expansin by removing calcium from mature cell walls. Because recombinant pectinases do not hydrolyze other wall polysaccharides, and endoglucanase, xylanase, and protease cannot restore the mature wall's extensibility, we can conclude that the pectin network, especially calcium-pectate bridges, may be the primary factor that determines cucumber hypocotyl mature cell walls' unresponsiveness to expansin.
The primary cell wall in the plant consists of a complex network of cellulose microfibrils that are embedded in an extensively hydrated matrix of hemicelluloses and pectins with small quantities of structural proteins intercalated into the matrix (Cosgrove, 2000). During growth, the cell wall must selectively loosen the network to accommodate volumetric expansion of the cell and withstand the expansive forces generated by cell turgor pressure. The loosening of cell walls in plants is related to: expansin (McQueen-Mason and Cosgrove, 1995) being able to independently induce the acid-growth of the cell wall (Cleland, 1983); numerous enzymes and proteins in the cell walls being able to alter cell wall structure (Fry, 1995; Yuan et al., 2001; Okamoto-Nakazato, 2002; Van Sandt et al., 2007); and an increased cell wall susceptibility due to the action of expansin (Cosgrove, 1999).
As cells mature, cell walls lose the ability to expand (Van Volkenburgh et al., 1985; Cosgrove, 1989). Growth cessation after cell maturation is generally irreversible and is typically accompanied by cell wall tightening (Kutschera, 1996). It has been reported that expansin activity was lacking in a nongrowing region of cucumber (Cucumis sativus) hypocotyls, but the extensibility of mature cell walls could not be restored by addition of exogenous expansin (McQueen-Mason et al., 1992). In maize (Zea mays) roots, cell walls of a mature region (nonelongating) adjacent to the elongation zone were not able to extend despite a greater amount of extractable expansin protein in the walls compared to the elongating region (Wu et al., 1996a). Thus, it was proposed that modification of cell walls, such as wall polymer cross-linking in nongrowing walls, is one of the major causes of unresponsiveness to expansin (Wu et al., 1996a; Cosgrove et al., 1997).
Various modifications to cell wall structure, related to tightening during maturation, have been proposed. These include the changes in the hemicellulose networks that occur during wall maturation. Pauly et al. (2001) reported an increase in the total amount of xyloglucan and a decrease in its enzyme accessibility as the tissue matured in peas (Pisum sativum). The arabinoxylan in maize coleoptiles also showed a decrease in branchedness as the coleoptiles matured, and the mixed-link β-d-glucans disappeared with growth cessation in the coleoptiles (Carpita, 1984). Following growth cessation of barley (Hordeum vulgare) coleoptiles, the (1→3, 1→4)-β-d-glucan contents also rapidly decreased, and the ratio of substituted to unsubstituted 4-linked xylosyl units decreased from about 4:1 to 1:1 (Gibeaut et al., 2005).
Deesterification of methyl-esterification pectin may also be associated with growth cessation in both grasses and dicotyledons and may contribute to wall tightening by strengthening pectin-calcium networks (Yamaoka and Chiba, 1983; Yamaoka et al., 1983; Goldberg, 1984; Ezaki et al., 2005). There is more unesterified pectin in the cell walls of nonelongating regions than in elongating regions (Fenwick et al., 1997; Fujino and Toh, 1998; Liberman et al., 1999). Pectin deesterification is generally believed to be catalyzed by pectin methylesterase (PME; Pelloux et al., 2007). PME was highly expressed in the elongated zones of flax hypocotyls (Al-Qsous et al., 2004). Expression of PME from Aspergillus aculeatus in Arabidopsis (Arabidopsis thaliana) resulted in a reduction in elongation of the hypocotyls (Derbyshire et al., 2007). Expression of fungal PME in tobacco (Nicotiana tabacum) led to a change in cell wall metabolism and a dwarfed phenotype (Hasunuma et al., 2004). Application of an exogenous PME induced thickening of the apical cell wall and inhibited pollen tube growth (Bosch et al., 2005). Silencing of the tobacco pollen PME NtPPME1 resulted in retarded in vivo pollen tube growth (Bosch and Hepler, 2006).
Cross-linking of phenolic groups between cell wall polymers, such as structural proteins and pectin, also coincides with wall maturation (Goldberg et al., 1986; Tan et al., 1991). Peroxidases are believed to be involved in cross-linking between phenolic compounds in cell walls (Fry, 1988). During strawberry (Fragaria ananassa) callus development, the highest acidic isoperoxidase activity was observed when the callus ceased to grow (Arnaldos et al., 2002). Peroxidase activity associated with cell walls increased from the subapical region (the fast elongating region) toward the basal region of Pinus pinaster hypocotyls (Sanchez et al., 1996). Similarly, peroxidase activity increased in the leaf of Lolium temulentum when expansion of the leaf ceased (Bacon et al., 1997). In addition, hydrogen peroxide, a substrate for peroxidase, has been shown to increase cell wall inextensibility in vitro in maize coleoptiles, and its action has been demonstrated to be suppressed by peroxidase inhibitors (Schopfer, 1996).
Cosgrove (1998) suggested that cell wall matrix cross-linking might increase the size of structural units that are passively dragged along as the wall creeps, and that the increase translated into greater resistance to creep. Given sufficient cross-linking, the wall will become unresponsive to expansin (Cosgrove, 1998). Because many structural changes in the wall may occur simultaneously as the cell wall matures, the molecular mechanism of wall tightening might be complex.
To understand what kind of modification in mature cell walls plays a key role in causing unresponsiveness to expansin, we screened for agents that were capable of restoring exogenous expansin-induced wall extension in the nonelongating regions (the basal regions) of cucumber hypocotyls. Herein we report that fungal pectinases possess such a capability. Together with other data in this study, we provide strong evidence to support that modification of the pectin network, forming calcium-pectate bridges, is a primary factor in determining cucumber hypocotyl mature cell walls' unresponsiveness to expansin.
RESULTS AND DISCUSSION
Screening of Hydrolytic Enzymes with an Ability to Restore Susceptibility of Mature Cell Walls to Expansin
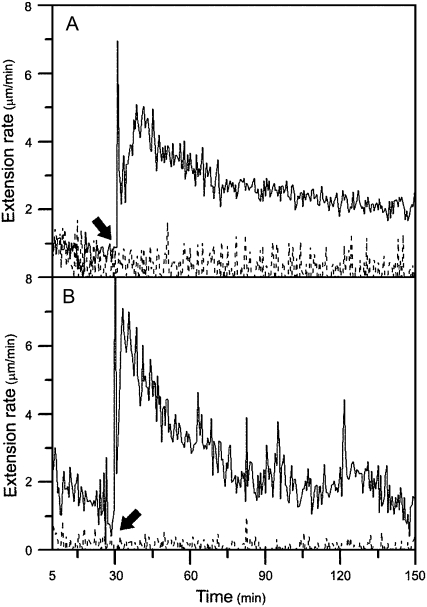
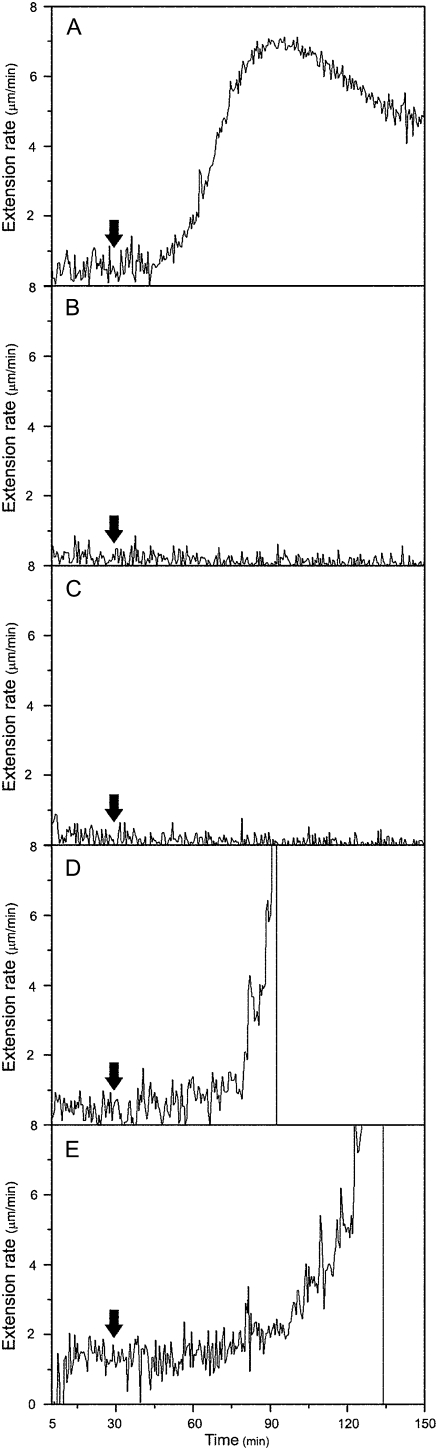
Mature cell walls in the basal regions (nonelongating) of cucumber hypocotyls usually do not respond to exogenous expansin (Fig. 1A). Because various cross-links related to wall maturation may have caused this unresponsiveness, we reasoned that removal of such cross-links, such as by hydrolytic enzymes, may restore reconstituted exogenous expansin-induced extension in mature cell walls. Thus, we established a bioassay using an extensometer to screen various hydrolytic enzymes that can cleave various components in mature cell walls. We first tested protein fractions isolated from plants. The basal segments were pretreated with plant extracts and then measured for their extension by expansin with an extensometer. The attempts were unsuccessful due to low hydrolytic enzyme activities in the extracts. We then examined several commercial proteases, cellulases, and pectinases from fungi for their abilities to restore mature walls' extensibility. One of the pectinase preparations from Aspergillus japonicus, pectolyase, was able to restore the mature segments' susceptibility to expansin in vitro; that is, mature walls pretreated with the fungal pectolyase could be extended in the presence of exogenous expansin in vitro (Fig. 1B).
Figure 1.
Mature cell walls pretreated with pectolyase showed a reconstituted expansin-induced extension under constant load using the extensometer in vitro. A, Growing apical 1-cm segments or nongrowing basal 1-cm segments of cucumber hypocotyls were frozen, thawed, abraded, pressed, and heated with water for 100 s in a microwave oven, and then stretched under tension in the bathing buffer (50 mm sodium acetate, pH 4.5) in an extensometer for 30 min, after which (indicated by an arrow) the bathing buffer was replaced with 0.1 mL of bathing buffer containing 2 mg mL−1 of crude expansin. The growing apical cell walls showed a reconstituted expansin-induced extension (solid line; rate = 4.36 ± 0.39 μm min−1), whereas the nongrowing mature basal walls did not respond to exogenous expansin (dashed line). B, The nongrowing basal segments were frozen, thawed, abraded, pressed, and heated with water for 100 s in a microwave oven, and then pretreated with 0.25 mg mL−1 pectolyase in bathing buffer for 10 min at 25°C, washed with bathing buffer three times, and, finally, suspended under tension in the bathing buffer in an extensometer for 30 min, after which (indicated by an arrow) the bathing buffer was replaced with 0.1 mL of bathing buffer containing 2 mg mL−1 of crude expansin (solid line; rate = 3.48 ± 0.25 μm min−1) or bathing buffer alone (dashed line; no extension). The curve is representative of 10 samples, and the rate is the mean ± se from three independent experiments.
The Active Component in Pectolyase Preparation Is a Pectin Lyase
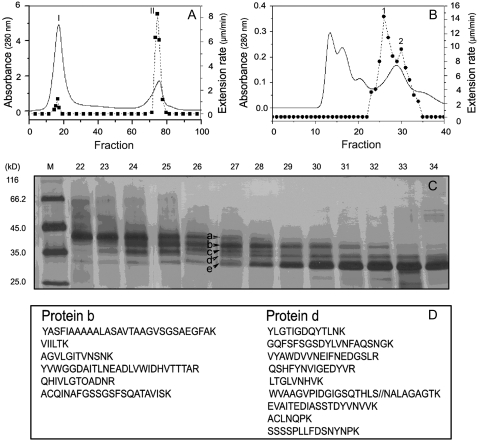
Commercial pectolyase is a mixture of proteins with multihydrolase activities and lyase activities, such as endoglucanase, xyloglucanase, xylanase, protease, and pectinase (data not shown). To identify the protein responsible for restoring cell wall extension, we fractionated pectolyase by CM-Sepharose ion-exchange chromatography and found a single active peak (peak II in Fig. 2A). This peak II fraction was further separated using Bio-Gel P-60 gel filtration into four fractions (Fig. 2B), one of which possessed two overlapping active peaks (peaks 1 and peak 2 in Fig. 2B) and contained at least five major bands of proteins (a, b, c, d, and e) on SDS-PAGE (Fig. 2C). Further attempts to purify the active component were not successful because the protein content of each active peak was too low to be sufficiently resolved. As seen in Figure 2C, proteins a and e were not associated with the activity because the maximum amount of protein a and e appeared in tubes 22 and 34, respectively, in which the restoring activity was extremely low. Thus, we focused on identifying the remaining three protein bands (b, c, and d) by liquid chromatography-electron spectroscopic imaging-tandem mass spectroscopy (LC-ESI-MS/MS). The amino acid sequencing of partial fragments from these three proteins indicated that protein b matched pectin lyase (Pel1; National Center for Biotechnology Information [NCBI] protein accession no. BAB82467) from Aspergillus oryzae, protein d matched XYNA-ASPAC endo-1,4-β-xylanase (NCBI protein accession no. BAA25847) from A. japonicus (Fig. 2D), and protein c was a mixture of protein b and protein d (data not shown).
Figure 2.
Fraction and identification of the active component in pectolyase that can restore reconstituted expansin-induced extension in mature cell walls. A, Fractionations of pectolyase from a CM-Sepharose column showed a single peak (peak II) of activity (solid line, the absorbance; dashed line, the restoring activity). B, Fractionations of peak II in A from Bio-Gel P-60 gel-permeation chromatography showed two overlapping peaks (1 and 2) of activity (solid line, the absorbance; dashed line, the restoring activity). C, Silver-stained SDS-PAGE of proteins in collecting tubes around peaks 1 and 2, in which five protein bands were, respectively, marked as a, b, c, d, and e. D, Peptide sequences of proteins b and d identified by LC-ESI-MS/MS. Protein bands b and d were excised from the gel and digested with trypsin. The digested peptides were subjected to LC-ESI-MS/MS analysis using a Finnigan LCQ Deca XP Plus LC/MS/MS. Identification was obtained by comparing the experimental data with the NCBI nonredundant protein database and was validated after considering at least four peptide sequences per protein (Bruneel et al., 2005).
To determine which of the two proteins contributed to the restoration activity, we constructed expression vectors with Pel1 complementary DNA (cDNA; GenBank accession no. EF452419) from A. oryzae (Kitamoto et al., 2001) and xylanase III cDNA from Trichoderm reesei (Xu et al., 1989), which has a 62% identity to XYNA-ASPAC endo-1,4-β-xylanase in amino acid sequence, and produced recombinant proteins in Escherichia coli. Results showed that pretreatment with recombinant Pel1 alone could restore the mature cell walls' susceptibility to expansin (Fig. 3A), whereas recombinant xylanase III could not (data not shown). Therefore, the active component in pectolyase was identified as Pel1.
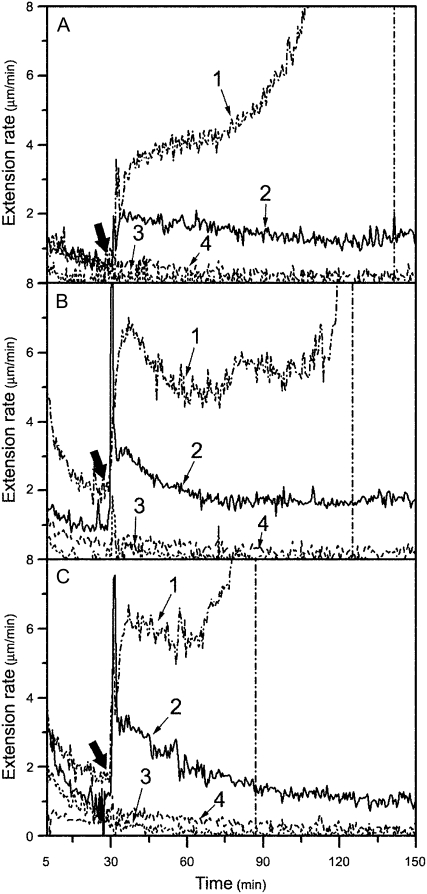
Figure 3.
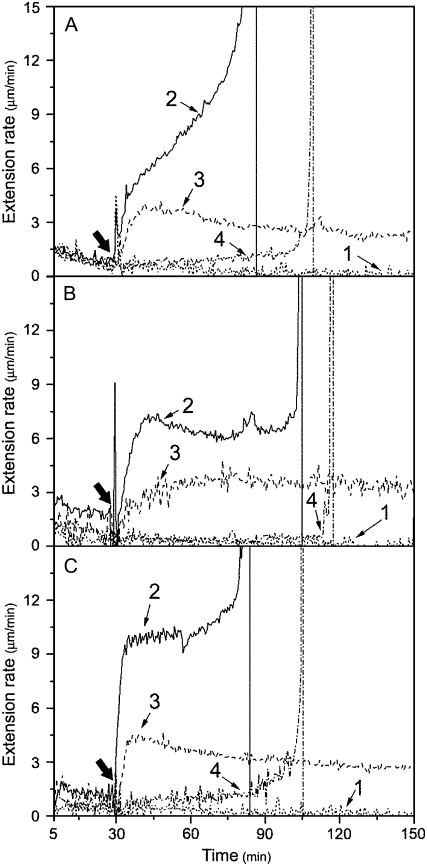
Effects of pretreatment with recombinant Pel1, PelA, and PG on mature cucumber hypocotyl walls' susceptibility to expansin. A, Nongrowing basal segments were frozen, thawed, abraded, pressed, and heated with water for 100 s in a microwave oven. These were then pretreated with 33 units mL−1 (line 1, representative; line 4, mean) or 22 units mL−1 (line 2, mean) of Pel1 in 50 mm sodium acetate buffer (buffer B, pH 5.0), or with cell extraction (line 3, mean) in buffer B (pH 5.0) from E. coli containing empty pET-28a (+) expression plasmid for 30 min at 30°C, washed with buffer B (pH 5.0) three times, and, finally, were stretched under tension in bathing buffer in an extensometer for 30 min, after which the bathing buffer was replaced with 0.1 mL of bathing buffer containing either 2 mg mL−1 of crude expansin or bathing buffer alone (only line 4 for minus expansin control). B, Nongrowing basal segments were pretreated with 1.50 units mL−1 (line 1, representative; line 4, mean) or 1.15 units mL−1 (line 2, mean) of PelA, or cell extraction (line 3, mean) in buffer B (pH 5.5); others for extension assay as described in A. C, The nongrowing basal segments were pretreated with 0.08 units mL−1 (line 1, representative; line 4, mean) or 0.06 units mL−1 (line 2, mean) of PG, or cell extraction (line 3, mean) in buffer B (pH 5.0); others for extension assay as described in A. Curves are the mean (mean) or representative (representative, based on its breakage) of three independent experiments. Thick arrows indicate when the bathing buffer was replaced.
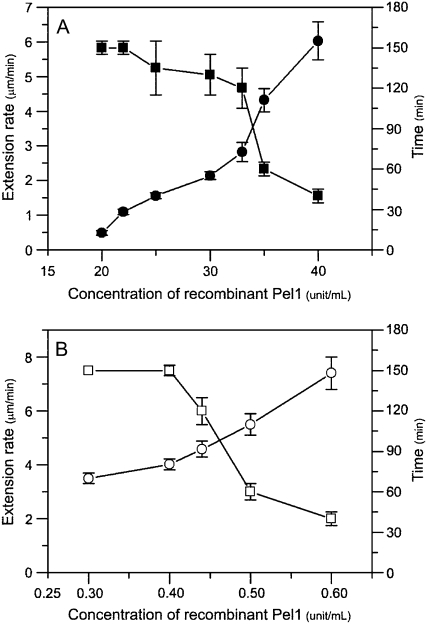
Pel1's restoring ability was concentration dependent (Fig. 4). With an increased concentration of Pel1 in pretreatment of mature basal cell walls, the reconstituted expansin-induced extension rate also increased, but the sustained time of wall extension decreased and the extending wall materials ultimately broke (Fig. 4A). For example, the reconstituted expansin-induced extension rate of basal segments pretreated with 22 units mL−1 of Pel1 was 1.09 ± 0.16 μm min−1 and wall extension continued for more than 150 min; by contrast, pretreatment with 33 units mL−1 of Pel1 increased the extension rate to 2.82 ± 0.28 μm min−1, but wall extension was only sustained for approximately 120 min before breaking. This disruption was due to the action of Pel1 retained in the mature walls after pretreatment, because pretreated walls could not be heated again to inactivate the Pel1. Additional heating damaged the hypocotyls, resulting in soft basal segments that were unable to be clamped to the extensometer for detection.
Figure 4.
Concentration dependence of the effect of recombinant expressed Pel1 on the extensibility of cucumber hypocotyls. A, Reconstituted expansin-induced extension rates (•) and sustained times (▪) of pretreated basal segments of cucumber hypocotyls varied with Pel1 concentrations in buffer B, pH 5.0. Details are described in Figure 3A. B, The reconstituted expansin-induced extension rates (○) and sustained times (□) of the apical segments varied with Pel1 concentrations in replacing bathing buffer containing 1 mg mL−1 of expansin plus different concentrations of Pel1. Details are described in Figure 1A. Data are means ± se from three independent experiments.
The substrate specificity analysis confirmed that recombinant Pel1 more efficiently cleaved 90% methyl-esterification pectin, but less efficiently cleaved 60% methyl-esterification pectin, and could not cleave 30% esterified and unesterified pectin at pH 5.0, 40°C (Table I). On the other hand, recombinant xylanase III from Trichoderma reesei QM9414 only hydrolyzed xylan and arabinoxylan among the polysaccharides detected in the cell walls (Table I), suggesting that the recombinant xylanase was active but lacked restoration capability.
Table I.
Substrate specificity of five recombinant enzymes against cell wall polysaccharides
Means ± se of three replicates.
| Cell Wall Polysaccharide | Enzyme Activity
|
||||
|---|---|---|---|---|---|
| Pel1 | PelA | PG | XynIII | Cel12A | |
| units mg−1 protein | |||||
| Pectin esterified ∼90/100 | 65.2 ± 1.2 | 0 | 0 | 0 | 0 |
| Pectin esterified ∼60/100 | 1.9 ± 0.2 | 0.43 ± 0.02 | 8.0 ± 0.7 | 0 | 0 |
| Pectin esterified ∼30/100 | 0 | 4.1 ± 0.1 | 153 ± 1 | 0. | 0 |
| Poly-GalA | 0. | 6.1 ± 0.2 | 204 ± 4 | 0 | 0 |
| Rhamnogalacturonan | 0 | 0.89 ± 0.03 | 4.1 ± 0.2 | 0 | 0 |
| Cellulose carboxymethyl sodium salt | 0 | 0 | 0 | 0 | 5.3 ± 0.7 |
| Xyloglucan | 0 | 0 | 0 | 0 | 8.3 ± 0.5 |
| 1-3,1-4-β-Glucan | 0 | 0 | 0 | 0 | 13.0 ± 0.9 |
| Xylan | 0 | 0 | 0 | 850 ± 2 | 0 |
| Arabinoxylan | 0 | 0 | 0 | 318 ± 2 | 0 |
Effects of Other Fungal Pectinases on Mature Cell Walls' Susceptibility to Expansin
Young, growing cell walls are characterized by a high level of highly methylesterified galacturonan and a low level of acidic pectins, whereas older, mature walls contain richer unesterified acidic pectins and more poorly methylesterified galacturonan (Liberman et al., 1999). We, therefore, reasoned that other fungal pectinases specific for unesterified pectins should have similar capabilities to or be even stronger than Pel1. For this reason, we cloned and expressed an α-1,4-endo-poly-GalA lyase, which is known as pectate lyase A (PelA; EC 4.2.2.2, GenBank accession no. EF452421). This acts on an unesterified region of homogalacturonan pectin, from Aspergillus nidulans (Ho et al., 1995) and an endo-polygalacturonase (PG; EC 3.2.1.15, GenBank accession no. EF452420), which catalyzes the hydrolytic cleavage of glycosidic α-1,4 linkages in polygalacturonide chains of pectin (Kitamoto et al., 1993), from A. oryzae.
As shown in Table I, recombinant PelA and PG expressed in E. coli mainly degraded unesterified acidic pectin rather than esterified (90%) pectin as well as other wall polysaccharides. As predicted, pretreatment with a low concentration of PelA (1.15 units mL−1) or PG (0.06 units mL−1) led to a significant reconstituted expansin-induced extension of basal segments of cucumber hypocotyls, reaching 2.16 ± 0.62 (Fig. 3B) and 1.87 ± 0.83 μm min−1 (Fig. 3C), respectively. These rates are much higher compared to that from Pel1 pretreatment on the basis of per-unit enzymes. Similarly, reconstituted expansin-induced extension rates of pretreated basal segments were even higher with increasing concentrations of PelA or PG, but the segments broke after a short period of expansin-induced extension (data not shown). For example, when the pretreatment concentration was increased to 1.50 units mL−1 for PelA (Fig. 3B) or 0.08 units mL−1 for PG (Fig. 3C), reconstituted expansin-induced extension rates of the basal segments reached 4.30 ± 0.72 μm min−1 (PelA) or 5.45 ± 0.77 μm min−1 (PG), respectively. However, these segments only extended for about 60 to 100 min after addition of expansin solution before breaking.
The PelA from A. nidulans used in this study was more similar to pectate lyases from tomatoes (Solanum lycopersicum) than that from other fungi or bacteria (Ho et al., 1995; Zhao et al., 2007b). Wing et al. (1990) first reported that plant pectate lyases were expressed at maximal levels in mature tomato flowers, anthers, and pollen. Later, genes encoding PGs and pectate lyases were also reported in pollen (Brown and Crouch, 1990; Wing et al., 1990; Niogret et al., 1991; Allen and Lonsdale, 1993). Pectinases were thought to break the pectin network in pollen and loosen the pollen cell wall to enable pollen emergence and tube growth (Taniguchi et al., 1995; Wu et al., 1996b; Marín-Rodríguez et al., 2002). Recently, Parre and Geitmann (2005) observed that exogenous pectinases at moderate concentrations stimulated pollen germination and tube growth of Solanum chacoense. Apparently, pollen germination follows a reverse process of maturation during which inextensible mature walls are restored to extensible cell walls by pectinases.
A Synergistic Effect of Fungal Pectinases with Expansin on the Apical Segment Extension
The recombinant Pel1, PelA, or PG alone could not induce the extension of growing walls in the reconstitution assay, but they acted synergistically with expansin to enhance wall extension (Fig. 5), which was consistent with effects of crude fungal pectinases reported by Cosgrove and Durachko (1994). Expansin plus Pel1, PelA, or PG increased the reconstituted expansin-induced extension rate of the apical segments of cucumber hypocotyls by 37.1%, 45.4%, or 169.1%, respectively, as compared to expansin alone. Because of continuous hydrolysis and β-elimination of pectinases during measurements, the hypocotyls eventually broke after a prolonged time period. Similarly, with an increase in Pel1 concentration, the Pel1 synergism became stronger (Fig. 4B). However, the higher concentration of Pel1 often led to breakage of extending tissues during long-term wall extension measurement.
Figure 5.
Enhancement of reconstituted expansin-induced extension of growing cell wall by recombinant Pel1, PelA, and PG. A, Enhancement of Pel1. Reconstituted expansin-induced extension of apical segments of cucumber hypocotyls following replacement of bathing buffer alone (line 1), or containing 1 mg mL−1 of expansin plus 0.44 units mL−1 of Pel1 (line 2, representative), or containing 1 mg mL−1 of expansin (line 3, mean), or containing 0.44 units mL−1 of Pel1 (line 4, representative). B, Enhancement of PelA. Reconstituted expansin-induced extension of apical segments of cucumber hypocotyls following replacement of bathing buffer alone (line 1, mean), or containing 1 mg mL−1 of expansin plus 6.10 units mL−1 of PelA (line 2, representative), or containing 1 mg mL−1 of expansin (line 3, mean), or containing 6.10 units mL−1 of PelA (line 4, representative). C, Enhancement of PG. The reconstituted expansin-induced extension of apical segments of cucumber hypocotyls following replacement of bathing buffer alone (line 1, mean) or containing 1 mg mL−1 of expansin plus 1.0 units mL−1 of PG (line 2, representative), or containing 1 mg mL−1 of expansin (line 3, mean), or containing 1.0 units mL−1 of PG (line 4, representative). Details are described in Figure 1A. Curves are the mean (mean) or representative (representative, based on its breakage) of three independent experiments. Thick arrows indicate when bathing buffer was replaced.
Notably, a synergistic effect with expansin on the apical segment extension only occurred at higher concentrations of PelA (6.10 units mL−1; Fig. 5B) or PG (1.00 units mL−1; Fig. 5C), whereas 1.15 units mL−1 of PelA or 0.06 units mL−1 of PG, which could restore the extensibility of the basal segments of the hypocotyls to expansin (see Fig. 3, B and C), had no effect on the reconstituted expansin-induced extension of the apical segments. In contrast, the effective concentration of Pel1 for synergism with expansin action in the apical segments was much lower than that for restoring activity in the basal segments (0.44 units mL−1 for the apical segment versus 22 units mL−1 for the basal segment, comparing Fig. 5A with Fig. 3A). Because PelA and PG are more effective on less esterified pectins (Table I), a stronger capacity for PelA and PG in restoration of mature walls' susceptibility to expansin may very well indicate a less degree of esterification of pectin in mature cell walls (making PelA and PG more effective) compared to the growing cell walls. Similarly, Pel1 favored highly esterified pectin substrates (Table I) and a stronger effect of Pel1 on reconstituted expansin-induced extension in young cell walls indicated a higher degree of esterification of pectin in growing cell walls compared to mature cell walls.
Restoration of Mature Cell Walls' Susceptibility to Expansin by EGTA
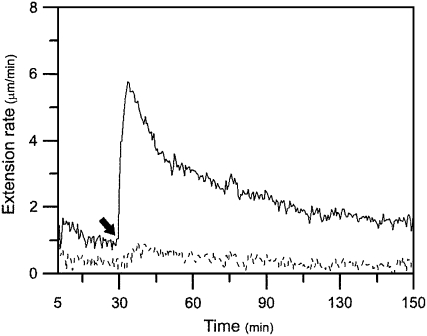
Calcium bridges are an important cross-link in the pectin network in cell walls. Some researchers have reported that calcium ions inhibited extension of growing cell walls and the calcium chelator, such as EGTA, could promote extension of growing cell walls (Cleland, 1977; Rayle, 1988; Virk and Cleland, 1990; Ezaki et al., 2005; Proseus and Boyer, 2006). To test the role of calcium-pectate bridges in mature cell wall's unresponsiveness to expansin, we used the calcium chelating agent EGTA to pretreat the nongrowing basal segments of cucumber hypocotyls to remove calcium ions before assaying the walls' extension. Results showed that pretreating the basal segments with 100 mm EGTA conferred much stronger reconstituted exogenous expansin-induced extension in mature walls but did not cause tissue breakage during long-term wall extension measurement in most cases (Fig. 6), which was very different from pretreatment with high concentrations of pectinases (compare Fig. 6 with Fig. 3).
Figure 6.
Effect of EGTA on mature cucumber hypocotyl cell walls' susceptibility to expansin. Nongrowing basal segments were frozen, thawed, abraded, pressed, and heated with water for 100 s in a microwave oven, and were pretreated with either 100 mm EGTA (pH 8.3) or distilled H2O (pH 8.3) for 30 min at 25°C, washed with bathing buffer three times, and, finally, were hung under tension in the bathing buffer in an extensometer for 30 min, after which (indicated by an arrow) the bathing buffer was replaced with 0.1 mL of bathing buffer containing 2 mg mL−1 of crude expansin. The basal segments that had been pretreated with EGTA could extend after adding expansin at 4.02 ± 0.73 μm min−1 (solid line), whereas the basal segments that had been pretreated with distilled H2O (pH 8.3) did not extend after adding expansin (dashed line). Curves are the mean of four independent experiments. Data are means ± se from four independent experiments.
Because the middle lamella between cells consists of calcium-pectate bridges, one could suspect that when EGTA pretreatment breaks most (if not all) of the calcium-pectate bridges in mature walls, cell separation and segment breakage would occur under a constant load in an extensometer. However, this phenomenon was not observed in most cases, implying that other cross-links must also be present in the polysaccharide network to support cell wall structural integrity. Recently, some reports indicate cross-links between pectin molecules(Vincken et al., 2003), pectin and xyloglucan (Thompson and Fry, 2000; Brett et al., 2005; Cumming et al., 2005; Popper and Fry, 2005), as well as pectin and cellulose (Zykwinska et al., 2005) in type I primary plant cell walls. These cross-links must provide extra cohesion between cell walls in addition to calcium-pectate bridges.
Distribution of Pectin and Calcium Ions along the Growth Gradient of Cucumber Hypocotyls
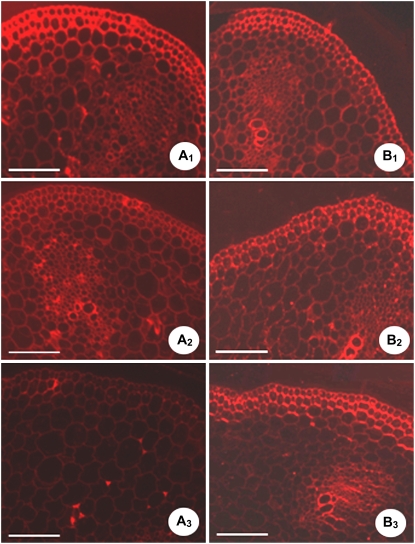
The distribution of highly methylated pectins along the growth gradient was explored with JIM7 antibodies. The highest, the intermediate, and the weakest fluorescence labeling was observed, respectively, in the apical (Fig. 7A1), the middle (Fig. 7A2), and the basal (Fig. 7A3) part of the hypocotyls. This is consistent with the growth gradient along cucumber hypocotyls, as reported in other species (Goldberg et al., 1986; McCann et al., 1993; Liberman et al., 1999). The higher degree of pectin methyl-esterification is considered to be required for normal cell elongation in Arabidopsis hypocotyls (Derbyshire et al., 2007). Unfortunately, a significant amount of difference in unesterified pectin existed between the apical and basal cell walls and was not detected by JIM5 antibodies, which often fail to label the carboxyl groups of unesterified pectin engaged in salt linkages with calcium ions (Fig. 7, B1–B3; Jauneau et al., 1998; Liberman et al., 1999).
Figure 7.
JIM7 (A1–A3) and JIM5 (B1–B3) labeling is shown along the growth gradient of cucumber hypocotyls. Cross sections of cucumber hypocotyls were made at: A1 and B1, the apical part of the hypocotyls (0.5 cm below the hook); A2 and B2, the middle part of the hypocotyls (4 cm below the hook); A3 and B3, the basal part of the hypocotyls (8 cm below the hook). Results were from three independent experiments; each experiment included three cucumber hypocotyls and nine transverse sections from each region of each hypocotyl. Bars = 400 μm.
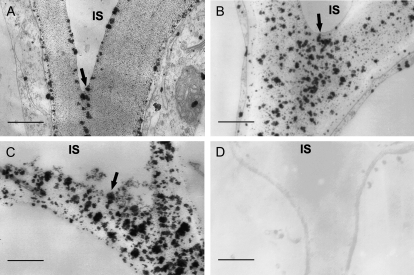
Because unesterified pectins usually bind calcium to form calcium-pectate bridges in the cell walls, the calcium localization in the cucumber hypocotyls was determined using the pyroantimonate precipitation procedure; a polar distribution of calcium precipitates along the hypocotyls was clearly observed, with the basal portion showing higher levels of calcium (Fig. 8). In the apical region of the hypocotyls, there were less calcium precipitate particles in the walls, which were scattered onto two sides of the walls close to the protoplasm membrane and a narrowed center region of lamellae between the cells (Fig. 8A). In the middle region, calcium precipitates were intense, coarse, and unevenly distributed throughout the cell walls, especially in the lamellar region between cells (Fig. 8B). In the basal region, calcium precipitate particles in the walls and the lamellar region became denser (Fig. 8C). An increase in Ca2+ in the mature cell walls suggested an increase in acidic unesterified pectin and an intensified formation of the calcium-pectate bridge in the pectin network in mature walls.
Figure 8.
Calcium localization in cell walls along the growth gradient of cucumber hypocotyls. A to C, Transverse sections were made in the region of 0.5 cm (A), 4 cm (B), and 8 cm (C) below the hook and assayed with the potassium pyroantimonate method for calcium localization. The particles of calcium precipitation in cell walls of the cell junctions between epidermis and subepidermis were displayed. Particles of calcium precipitation disappeared in the walls of basal segments that had been pretreated with 100 mm EGTA. D, Details are described in Fig. 6. The experiment was repeated three times with the same results. Three cucumber hypocotyls and nine transverse sections from each region of each hypocotyl were examined in each experiment. IS, intercellular space. Bars = 0.5 μm.
In Figure 6, we have demonstrated that pretreatment with 100 mm EGTA could restore reconstituted expansin-induced extension in mature hypocotyl cell walls. Here we further proved that in the mature basal segment of the hypocotyls that were pretreated with 100 mm EGTA, calcium-pyroantimonate precipitate particles disappeared completely (Fig. 8D). Therefore, we can conclude that calcium cross-bridging of nonmethylesterfied pectin chains may be the primary factor that determines cucumber hypocotyl mature cell walls' unresponsiveness to expansin.
Notably, pretreatment with pectinases or EGTA could also restore reconstituted expansin-induced extension in mature mung bean (Vigna radiata) hypocotyls but could not make the mature walls of wheat (Triticum aestivum) coleoptiles susceptible to β-expansin (data not shown). Because graminaceous monocot cell walls (type II) contain much less pectin (Carpita and Gibeaut, 1993), other cross-links may be involved in wall tightening during maturation.
Effects of Pretreatment of Other Wall Hydrolytic Enzymes on the Extensibility of Mature Cell Walls
In several current cell wall models, the cellulosic framework is interconnected by hemicellulosic polysaccharides to form a cellulose/hemicellulose network that provides structural integrity and strength, and physically regulates wall extension as the cell grows (Carpita and Gibeaut, 1993; Cosgrove, 2005). Xyloglucan is the major hemicellulosic component in dicotyledonous and nongraminaceous monocotyledonous plants. Cel12A, an endoglucanase, could hydrolyze xyloglucan and other hemicelluloses rather than pectin (Yuan et al., 2001; also see Table I). As previously reported by Yuan et al. (2001), a low concentration (0.014 units mL−1) of Cel12A could induce a delayed extension of heat-inactivated apical segments of cucumber hypocotyls (Fig. 9A). However, a higher concentration (0.68 units mL−1) of Cel12A did not induce extension of basal segments by itself (Fig. 9B), nor did it make the mature basal segment susceptible to expansin via pretreatment (Fig. 9C). Interestingly, a low concentration (0.014 units mL−1) of Cel12A could result in delayed breakage of basal segments pretreated with pectinases (Fig. 9D) or EGTA (Fig. 9E). It is unlikely that the pretreatment concentration of Cel12A was too low to function effectively in mature walls. Therefore, the xyloglucan network is not a crucial cross-link involved in wall maturation. Furthermore, rather than delaying extension, Cel12A induced breakage of mature walls pretreated with pectinase or EGTA, implying that pectin plays an important, cross-linking role in retaining the structural integrity of walls. In pretreated mature walls, pretreatment with pectinases or EGTA destroyed the pectin network, which made wall framework retained only by xyloglucan so that basal segments of the hypocotyls must break at a weak point with the hydrolysis of xyloglucan by Cel12A during the bioassays. By contrast, in the unpretreated, young, growing walls, pectin cross-linking was kept intact so it could protect the hypocotyls from breaking when the xyloglucan network was hydrolyzed by Cel12A during a delayed, long-term extension. Apparently, the pectin network does not simply fill in the spaces between cellulose/hemicellulose networks. The pectin matrix may tether cellulose fibrils together, as suggested by Zykwinska et al. (2005).
Figure 9.
Action of endo-xyloglucanase Cel12A on mature and young cell walls. A, Cel12A alone could induce delayed extension of heat-inactivated cell walls of apical segments with a rate of 4.68 ± 1.07 μm min−1, when the replacing bathing buffer contained 0.014 units mL−1 Cel12A. B, Cel12A could not induce extension of basal segments when the replacing bathing buffer contained 0.68 units mL−1 Cel12A. C, Pretreatment of basal segments with Cel12A (0.68 units mL−1 Cel12A in bathing buffer for 30 min at 30°C) could not restore reconstituted expansin-induced extension in mature walls. D and E, Cel12A could cause breakage of basal segments that had been pretreated with 1.15 units mL−1 of PelA (D is as described in Fig. 3B) or 100 mm of EGTA (E is as described in Fig. 6) rather than extension. The curves in A, B, and C are the mean (mean) of three independent experiments and the curves in D and E are the representatives of three independent experiments based on their breakage. Arrows indicate when the bathing buffer was replaced.
In addition, we pretreated the mature basal segments of cucumber hypocotyls with pronase, peptin, or trypsin and found no apparent effect of these proteases on mature wall extensibility (data not shown).
MATERIALS AND METHODS
Chemicals
Crude microbial pectolyase (P-3026), pepsin (P 7000), and trypsin (T 4799) were obtained from Sigma, and pronase (537088) was purchased from Calbiochem. JIM5 and JIM7 were from the Paul Knox Cell Wall Lab. Anti-rat IgG TRITC conjugate (T 4280), EGTA (E 8145), pectin esterified approximately 90% (P 9561), pectin esterified approximately 60% (P 9436), pectin esterified approximately 30% (P 9311), poly-GalA (P 1879), cellulose carboxymethyl sodium salt (C 5678), xylan (X-0627), and 1-3,1-4-β-glucan (G 5011) were purchased from Sigma. Rhamnogalacturonan (P-RHAM1), xyloglucan (P-XYGLN), and arabinoxylan (P-RAXY) were obtained from Megazyme.
Plant Materials
Cucumber (Cucumis sativus ‘Burpee Pickler’) seeds were sown on gauze that was soaked with distilled water and placed in 45 × 35 × 12 cm flats with lids. Seedlings were grown for 4 d at 30°C in the dark until they reached 8.5 cm in length. The hypocotyls were quickly excised, frozen at −20°C, and used for experiments within 5 d.
Extension Measurements
Reconstituted exogenous expansin-induced cell wall extension of apical or basal segments (1-cm long) of cucumber hypocotyls was measured with a constant load extensometer, as described by McQueen-Mason et al. (1992).
To detect the agents that can restore expansin-induced extension of mature cell walls, frozen basal segments of the hypocotyls were thawed, abraded with carborundum, pressed between two glass slides, heated for 100 s with water in a microwave oven, and incubated in Eppendorf tubes with 0.5 mL of the bathing buffer (50 mm sodium acetate, pH 4.5) containing different pectinases, other hydrolytic enzymes, or EGTA under experimental conditions as indicated in “Results and Discussion.” They were then washed with bathing buffer three times and secured between two clamps for reconstituted wall extension measurement by expansins using the extensometer. The tissue segments stretched under tension in the bathing buffer for around 30 min, after which the bathing buffer was replaced with 0.1 mL of replacing buffer (bathing buffer containing expansin or other proteins). The cell wall extension rate is defined as changes of wall length per min calculated by subtracting the stable wall extension rate before the replacement from the stable maximum wall extension rate after replacement (McQueen-Mason et al., 1992). All extension measurements reported here were repeated at least three times, and the numbers of total samples were ≥10.
Extraction of α-Expansin
Cucumber hypocotyls were grown as described above, and crude α-expansin fraction was extracted from the hypocotyls, as described by McQueen-Mason et al. (1992), and stored at −80°C until use.
Pel1 Purification and Identification
Two-hundred milligrams of pectolyase dissolved in buffer A (20 mm sodium acetate, pH 4.5) were loaded onto a CM-Sepharose column (20 × 1 cm, 4°C; Sigma) preequilibrated with buffer A. After washing with buffer A, proteins were first eluted with 42 mL of 0.1 m NaCl in buffer A and then with 182 mL of a linear gradient of 0.1 to 0.5 m NaCl in buffer A at 0.4 mL min−1. The active fraction (peak II) eluted from the column was concentrated on an Ultrafree-CL 5,000 spin column (Millipore) and loaded onto a Bio-Gel P-60 gel filtration chromatography column (100 × 1 cm, 4°C; Bio-Rad) preequilibrated with 20 mm NaCl in buffer A. Proteins were eluted with 90 mL of 20 mm NaCl in buffer A at 0.1 mL min−1. Fractions were tested for their ability to restore the expansin-induced extension in mature walls.
The partially purified active fractions from the Bio-Gel P-60 gel filtration were separated by SDS-PAGE, and proteins in the SDS-PAGE were identified by LC-ESI-MS/MS (Finnigan LCQ Deca XP Plus LC/MS/MS) at the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Science.
Cloning and Expression of Pectinases and Hemicellulases
Cloning and expression of Pel1 (Zhao et al., 2007a), PelA (Zhao et al., 2007b), PG (Zhang et al., 2007), Cel12A (Yuan et al., 2001), and xylanase III (Lu et al., 2004) were performed according to our previous reports.
Enzyme Activity Assay
To assay Pel1 enzyme activity, 0.5 mL of the reaction mixture containing 50 mm sodium acetate buffer (buffer B, pH 5.0), 0.1% pectin or other wall polysaccharides, and an appropriate amount of Pel1 was incubated at 40°C for 10 min. Conditions similar to Pel1 activity assays were employed to assay PelA enzyme activity, except that the reaction mixture contained a buffer B with pH 5.5, 1 mm calcium chloride, and an appropriate amount of PelA; additionally, the incubation temperature was 35°C (Macmillan and Vaughn, 1963). To assay PG enzyme activity, conditions similar to the Pel1 activity assay were employed, except that the reaction mixture contained an appropriate amount of PG, and the incubation temperature was 30°C. To determine the unsaturated oligosaccharide concentration, the reaction was terminated by adding 1 mL of 0.02 n HCl, and was measured at 235 nm (Kitamoto et al., 2001). To determine the concentration of the reducing sugars, the reaction was terminated by adding 0.5 mL dinitrosalicylic acid reagent and measured for the A520 (Miller, 1959).
To assay xylanase III enzyme activity, 0.2 mL of the reaction mixture containing buffer B (pH 5.0), 10 mg mL−1 xylan, or arabinoxylan, or other wall polysaccharides, and an approximate amount of xylanase III was incubated at 37°C for 60 min (Lu et al., 2004). To assay Cel12A enzyme activity, 0.2 mL of the reaction mixture containing buffer B (pH 4.5), 10 mg mL−1 cellulose carboxymethyl sodium salt, xyloglucan, or 1-3,1-4-β-glucan, or other wall polysaccharides, and an approximate amount of Cel12A was incubated at 37°C for 30 min (Yuan et al., 2001). To determine the concentration of the reducing sugars, the reaction mixture was mixed with 1 mL of 100 mm borate buffer (pH 9.0) and 200 μL 1% 2-cyanoacetamide, heated at 98°C for 10 min, and measured for A276 (Gross et al., 1982).
One enzyme unit for Pel1 or PelA was defined as the amount of protein that forms 1 μmol of 4, 5-unsaturated product in 1 min under assay conditions. The molar extinction coefficient for the unsaturated product at 235 nm was 4,600 m−1 cm−1 (Collmer et al., 1988).
One enzyme unit for PG, xylanase, or endoglucanase was defined as the amount of protein required to release a reducing sugar equivalent to 1 μmol of GalA, Xyl, or Glc per minute.
Immunocytochemical Localization of Pectins
Immunolabeling was performed as described by Lenartowska et al. (2001) with primary antibodies JIM5, which recognizes homopoly-GalA epitopes, or JIM7, which recognizes the epitopes of highly methyl-esterified regions of the pectins (Knox et al., 1990), and secondary antibodies (anti-rat IgG TRITC conjugate). The control was not incubated with the primary antibodies.
Calcium Localization
Samples were fixed according to the method from Mentré and Escaig (1988). Ultrathin sections were examined by transmission electron microscope (Hitachi 600-A-2). In controls, either potassium pyroantimonate was omitted or calcium was chelated by EGTA. Samples pretreated with EGTA in the restoring experiments were prepared as described above.
Sequences for Pel1 cDNA (from Aspergillus oryaze), PelA cDNA (from Aspergillus nidulans), and PG cDNA (from A. oryaze) can be found in the GenBank under accession numbers EF452419, EF452421, and EF452420, respectively.
Acknowledgments
We thank Dr. D.J. Cosgrove at Pennsylvania State University for helpful communication and discussion.
This work was supported by the National Natural Science Foundation of China (30170005) and the Jiangsu Province Natural Science Foundation (BK2001112, 2005104SBZB551).
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sheng Yuan (shengyuan@email.njnu.edu.cn).
References
- Al-Qsous S, Carpentier E, Klein-Eude D, Burel C, Mareck A, Dauchel H, Gomord V, Balange AP (2004) Identification and isolation of a pectin methylesterase isoform that could be involved in flax cell wall stiffening. Planta 219 369–378 [DOI] [PubMed] [Google Scholar]
- Allen RL, Lonsdale DM (1993) Molecular characterization of one of the maize polygalacturonase gene family members which are expressed during late pollen development. Plant J 3 261–271 [DOI] [PubMed] [Google Scholar]
- Arnaldos TL, Ferrer MA, García AAC, Muñoz R (2002) Changes in peroxidase activity and isoperoxidase pattern during strawberry (Fragaria × ananassa) callus development. J Plant Physiol 159 429–435 [Google Scholar]
- Bacon M, Thompson DS, Davies WJ (1997) Can cell wall peroxidase activity explain the leaf growth response of Lolium temulentum L during drought? J Exp Bot 48 2075–2085 [Google Scholar]
- Bosch M, Cheung AY, Hepler PK (2005) Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 138 1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hepler PK (2006) Silencing of the tobacco pollen pectin methylesterase NtPPME1 results in retarded in vivo pollen tube growth. Planta 223 736–745 [DOI] [PubMed] [Google Scholar]
- Brett C, Baydoun EA-H, Abdel-Massih RM (2005) Pectin-xyloglucan linkages in type I primary cell walls of plants. Plant Biosyst 139 54–59 [Google Scholar]
- Brown SM, Crouch ML (1990) Characterization of a gene family abundantly expressed in Oenothera organensis pollen that shows sequence similarity to polygalacturonase. Plant Cell 2 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneel A, Labas V, Mailloux A, Sharma S, Royer N, Vinh J, Pernet P, Vaubourdolle M, Baudin B (2005) Proteomics of human umbilical vein endothelial cells applied to etoposide-induced apoptosis. Proteomics 5 3876–3884 [DOI] [PubMed] [Google Scholar]
- Carpita NC (1984) Cell wall development in maize coleoptiles. Plant Physiol 76 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3 1–30 [DOI] [PubMed] [Google Scholar]
- Cleland RE (1977) Reevaluation of the effect of calcium ions on auxin-induced elongation. Plant Physiol 60 709–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE (1983) The capacity for acid-induced wall loosening as a factor in the control of Avena coleoptile cell elongation. J Exp Bot 34 676–680 [Google Scholar]
- Collmer A, Ried JL, Mount MS (1988) Assay methods for pectic enzymes. Methods Enzymol 161 329–335 [Google Scholar]
- Cosgrove DJ (1989) Characterization of long-term extension of isolated cell walls from growing cucumber hypocotyls. Planta 177 121–130 [PubMed] [Google Scholar]
- Cosgrove DJ (1998) Cell wall loosening by expansins. Plant Physiol 118 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ (1999) Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol 50 391–417 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407 321–326 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6 850–861 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Bedinger P, Durachko DM (1997) Group I allergens of grass pollen as cell wall-loosening agents. Proc Natl Acad Sci USA 94 6559–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Durachko DM (1994) Autolysis and extension of isolated walls from growing cucumber hypocotyls. J Exp Bot 45 1711–1719 [DOI] [PubMed] [Google Scholar]
- Cumming CM, Rizkallah HD, McKendrick KA, Abdel-Massih RM, Baydoun EA, Brett CT (2005) Biosynthesis and cell-wall deposition of a pectin-xyloglucan complex in pea. Planta 222 546–555 [DOI] [PubMed] [Google Scholar]
- Derbyshire P, McCann MC, Roberts K (2007) Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol 7 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki N, Kido N, Takahashi K, Katou K (2005) The role of wall Ca2+ in the regulation of wall extensibility during the acid-induced extension of soybean hypocotyl cell walls. Plant Cell Physiol 46 1831–1838 [DOI] [PubMed] [Google Scholar]
- Fenwick KM, Jarvis MC, Apperley DC (1997) Estimation of polymer rigidity in cell walls of growing and nongrowing celery collenchyma by solid-state nuclear magnetic resonance in vivo. Plant Physiol 115 587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC (1988) The Growing Plant Cell Wall: Chemical and Metabolic Analysis. Longman Scientific and Technical, New York
- Fry SC (1995) Polysaccharide-modifying enzymes in the plant cell wall. Annu Rev Plant Physiol Plant Mol Biol 46 497–520 [Google Scholar]
- Fujino T, Toh T (1998) Changes in pectin structure during epidermal cell elongation in pea (Pisum sativum) and its implications for wall architecture. Plant Cell Physiol 39 1315–1323 [Google Scholar]
- Gibeaut DM, Pauly M, Bacic A, Fincher GB (2005) Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 221 729–738 [DOI] [PubMed] [Google Scholar]
- Goldberg R (1984) Changes in the properties of cell wall pectin methylesterase along the Vigna radiata hypocotyl. Physiol Plant 61 58–63 [Google Scholar]
- Goldberg R, Morvan C, Roland JC (1986) Composition, properties and localization of pectins in young and mature cells of mung bean hypocotyls. Plant Cell Physiol 27 419–427 [Google Scholar]
- Gross M, Jacobs GH, Poulton JE (1982) A rapid and sensitive spectrophotometric assay for prunasin hydrolase activity employing purified mandelonitrile lyase. Anal Biochem 119 25–30 [DOI] [PubMed] [Google Scholar]
- Hasunuma T, Fukusaki E, Kobayashi A (2004) Expression of fungal pectin methylesterase in transgenic tobacco leads to alteration in cell wall metabolism and a dwarf phenotype. J Biotechnol 111 241–251 [DOI] [PubMed] [Google Scholar]
- Ho MC, Whitehead MP, Cleveland TE, Dean RA (1995) Sequence analysis of the Aspergillus nidulans pectate lyase pelA gene and evidence for binding of promoter regions to CREA, a regulator of carbon catabolite repression. Curr Genet 27 142–149 [DOI] [PubMed] [Google Scholar]
- Jauneau A, Roy S, Reis D, Vian B (1998) Probes and microscopical methods for the location of pectins in plant cells. Int J Plant Sci 159 1–13 [Google Scholar]
- Kitamoto N, Kimura T, Kito Y, Ohmiya K, Tsukagoshi N (1993) Structural features of a polygalacturonase gene cloned from Aspergillus oryzae KBN616. FEMS Microbiol Lett 111 37–41 [DOI] [PubMed] [Google Scholar]
- Kitamoto N, Yoshino-Yasuda S, Ohmiya K, Tsukagoshi N (2001) Sequence analysis and overexpression of a pectin lyase gene (pel1) from Aspergillus oryzae KBN616. Biosci Biotechnol Biochem 65 209–212 [DOI] [PubMed] [Google Scholar]
- Knox RB, Lintead PJ, King J, Cooper C (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181 512–552 [DOI] [PubMed] [Google Scholar]
- Kutschera U (1996) Cessation of cell elongation in rye coleoptiles is accompanied by a loss of cell-wall plasticity. J Exp Bot 47 1387–1394 [Google Scholar]
- Lenartowska M, Rodriguez-Garcia MI, Bednarska E (2001) Immunocytochemical localization of esterified and unesterified pectins in unpollinated and pollinated styles of Petunia hybrida Hort. Planta 213 182–191 [DOI] [PubMed] [Google Scholar]
- Liberman M, Mutaftschiev S, Jauneau A, Vian B, Catesson AM, Goldberg R (1999) Mung bean hypocotyls homogalacturonan: localization, organization and origin. Ann Bot (Lond) 84 225–233 [Google Scholar]
- Lu CM, Yuan S, Zhao QX (2004) Cloning and expression of xyn III from genomic DNA of Trichoderma reesei QM9414 by overlap-PCR. Sheng Wu Gong Cheng Xue Bao 20 764–769 [PubMed] [Google Scholar]
- Macmillan JD, Vaughn RD (1963) Purification and properties of a polygalacturonic acid-trans-eliminase produced by clostridium multifermentans. Biochemistry 3 564–572 [DOI] [PubMed] [Google Scholar]
- Marín-Rodríguez MC, Orchard J, Seymour GB (2002) Pectate lyase, cell wall degradation and fruit softening. J Exp Bot 53 2115–2119 [DOI] [PubMed] [Google Scholar]
- McCann MC, Stacey NJ, Wilson R, Roberts K (1993) Orientation of macromolecules in the walls of elongating carrot cells. J Cell Sci 106 1347–1356 [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ (1995) Expansin mode of action on cell walls. Analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol 107 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentré P, Escaig F (1988) Localization of cations by pyroantimonate. I. Influence of fixation on distribution of calcium and sodium. An approach by analytical ion microscopy. J Histochem Cytochem 36 49–54 [DOI] [PubMed] [Google Scholar]
- Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31 426–428 [Google Scholar]
- Niogret MF, Dubald M, Mandaron P, Mache R (1991) Characterization of pollen polygalacturonase encoded by several cDNA clones in maize. Plant Mol Biol 17 1155–1164 [DOI] [PubMed] [Google Scholar]
- Okamoto-Nakazato A (2002) A brief note on the study of yieldin, a wall-bound protein that regulates the yield threshold of the cell wall. J Plant Res 115 309–313 [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A (2005) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220 582–592 [DOI] [PubMed] [Google Scholar]
- Pauly M, Qin Q, Greene H, Albersheim P, Darvill A, York WS (2001) Changes in the structure of xyloglucan during cell elongation. Planta 212 842–850 [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12 267–277 [DOI] [PubMed] [Google Scholar]
- Popper ZA, Fry SC (2005) Widespread occurrence of a covalent linkage between xyloglucan and acidic polysaccharides in suspension-cultured angiosperm cells. Ann Bot (Lond) 96 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proseus TE, Boyer JS (2006) Calcium pectate chemistry controls growth rate of Chara corallina. J Exp Bot 57 3989–4002 [DOI] [PubMed] [Google Scholar]
- Rayle DL (1988) Calcium bridges are not load-bearing cell-wall bonds in Avena coleoptiles. Planta 178 92–95 [DOI] [PubMed] [Google Scholar]
- Sanchez M, Pena MJ, Revilla G, Zarra I (1996) Changes in dehydrodiferulic acids and peroxidase activity against ferulic acid associated with cell walls during growth of Pinus pinaster hypocotyl. Plant Physiol 111 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P (1996) Hydrogen peroxide-mediated cell-wall rigification in vitro in maize coleoptiles. Planta 199 43–49 [Google Scholar]
- Tan KS, Hoson T, Masuda Y, Kamisaka S (1991) Correlation between cell wall extensibility and the content of diferulic and ferulic acids in cell walls of Oryza sativa coleoptiles grown under water and in air. Physiol Plant 83 397–403 [Google Scholar]
- Taniguchi Y, Ono A, Sawatani M, Nanba M, Kohno K, Usui M, Kurimoto M, Matuhasi T (1995) Cry j I, a major allergen of Japanese cedar pollen, has pectate lyase enzyme activity. Allergy 50 90–93 [DOI] [PubMed] [Google Scholar]
- Thompson JE, Fry SC (2000) Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta 211 275–286 [DOI] [PubMed] [Google Scholar]
- Van Sandt VS, Suslov D, Verbelen JP, Vissenberg K (2007) Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot (Lond) 100 1467–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E, Schmidt MG, Cleland RE (1985) Loss of capacity for acid-induced wall loosening as the principal cause of the cessation of cell enlargement in light-grown bean leaves. Planta 163 500–505 [DOI] [PubMed] [Google Scholar]
- Vincken JP, Schols HA, Oomen RJ, McCann MC, Ulvskov P, Voragen AG, Visser RG (2003) If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol 132 1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk SS, Cleland RE (1990) The role of wall calcium in the extension of cell walls of soybean hypocotyls. Planta 182 559–564 [PubMed] [Google Scholar]
- Wing RA, Yamaguchi J, Larabell SK, Ursin VM, McCormick S (1990) Molecular and genetic characterization of two pollen-expressed genes that have sequence similarity to pectate lyases of the plant pathogen Erwinia. Plant Mol Biol 14 17–28 [DOI] [PubMed] [Google Scholar]
- Wu Y, Qiu X, Du S, Erickson L (1996. a) PO149, a new member of pollen pectate lyase-like gene family from alfalfa. Plant Mol Biol 32 1037–1042 [DOI] [PubMed] [Google Scholar]
- Wu Y, Sharp RE, Durachko DM, Cosgrove DJ (1996. b) Growth maintenance of the maize primary root at low water potentials involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiol 111 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Takakuwa N, Nogawa M, Okada H, Morikawa Y (1989) A third xylanase from Trichoderma reesei PC-3-7. Appl Microbiol Biotechnol 49 718–724 [Google Scholar]
- Yamaoka T, Chiba N (1983) Changes in the coagulating ability of pectin during growth of soybean hypocotyls. Plant Cell Physiol 24 1281–1290 [Google Scholar]
- Yamaoka T, Tsukada K, Takahashi H, Yamauchi N (1983) Purification of a cell wall-bound pectin-gelatinizing factor and examination of its identity with pectin methylesterase. J Plant Res 96 139–144 [Google Scholar]
- Yuan S, Wu Y, Cosgrove DJ (2001) A fungal endoglucanase with plant cell wall extension activity. Plant Physiol 127 324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YL, Zhao QX, Zhu H, Sun J, Han FM, Yuan S (2007) Expression of endopolygalacturonase A of Aspergillus oryzae in Escherichia coli. Sheng Wu Gong Cheng Xue Bao 23 101–105 [PubMed] [Google Scholar]
- Zhao QX, Yuan S, Zhang YL (2007. a) Expression of pectin lyase 1 from Aspergillus oryzae in Escherichia coli. Sheng Wu Gong Cheng Xue Bao 23 873–877 [PubMed] [Google Scholar]
- Zhao QX, Yuan S, Zhang YL, Zhu H, Dai CC, Yang F, Han FM (2007. b) Expression, purification and characterization of pectate lyase A from Aspergillus nidulans in Escherichia coli. World J Microbiol Biotechnol 23 1057–1064 [Google Scholar]
- Zykwinska AW, Ralet MC, Garnier CD, Thibault JF (2005) Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol 139 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]