Abstract
Sucrose synthase (Sus; EC 2.4.1.13) is a key enzyme of sucrose metabolism in plant cells, providing carbon for respiration and for the synthesis of cell wall polymers and starch. Since Sus is important for plant cell growth, insights into its structure, localization, and features are useful for defining the relationships between nutrients, growth, and cell morphogenesis. We used the pollen tube of tobacco (Nicotiana tabacum) as a cell model to characterize the main features of Sus with regard to cell growth and cell wall synthesis. Apart from its role during sexual reproduction, the pollen tube is a typical tip-growing cell, and the proper construction of its cell wall is essential for correct shaping and direction of growth. The outer cell wall layer of pollen tubes consists of pectins, but the inner layer is composed of cellulose and callose; both polymers require metabolic precursors in the form of UDP-glucose, which is synthesized by Sus. We identified an 88-kD polypeptide in the soluble, plasma membrane and Golgi fraction of pollen tubes. The protein was also found in association with the cell wall. After purification, the protein showed an enzyme activity similar to that of maize (Zea mays) Sus. Distribution of Sus was affected by brefeldin A and depended on the nutrition status of the pollen tube, because an absence of metabolic sugars in the growth medium caused Sus to distribute differently during tube elongation. Analysis by bidimensional electrophoresis indicated that Sus exists as two isoforms, one of which is phosphorylated and more abundant in the cytoplasm and cell wall and the other of which is not phosphorylated and is specific to the plasma membrane. Results indicate that the protein has a role in the construction of the extracellular matrix and thus in the morphogenesis of pollen tubes.
Sucrose synthase (Sus; EC 2.4.1.13) is a key enzyme of Suc metabolism and catalyzes the reversible transformation of Suc into Fru and UDP-Glc. Although its common name suggests synthetic activity, Suc in phloem seems to be produced mainly by other enzyme complexes, such as Suc-P synthase and Suc-P hydrolase. The main activity of Sus, therefore, is restricted to tissues that metabolize Suc (Baroja-Fernandez et al., 2003; Konishi et al., 2004), and monitoring of its activity provides useful information about the transport and consumption of carbohydrates during the development of plant cells and tissues (Wittich and Willemse, 1999). Because of its critical function, Sus is localized in different cell structures, such as the cytoskeleton (Winter et al., 1998), cell membranes (Matic et al., 2004), and the tonoplast (Etxeberria and Gonzalez, 2003). The protein is present in two distinct forms. The soluble form of Sus is generally involved in the respiration process. The second form of Sus is associated with plasma membranes or cell walls and is generally involved in the synthesis of cell wall components, providing metabolites (usually UDP-Glc) for callose synthase and cellulose synthase (Amor et al., 1995). The association of Sus with cell membranes may be regulated by phosphorylation/dephosphorylation processes (Anguenot et al., 2006). This model is supported by evidence obtained with cotton fibers (Ruan et al., 1997), in which mutants deficient in fiber synthesis showed altered levels of Sus (Ruan and Chourey, 1998) and suppression of its gene activity inhibited fiber growth (Ruan et al., 2003). The enzyme has also been localized in association with thickenings of the secondary cell wall (Salnikov et al., 2003). Another example is Zinnia elegans, in which Sus is present at higher concentrations close to the plasma membrane and microtubules, just below the secondary wall thickenings (Salnikov et al., 2001). In maize (Zea mays), three distinct Sus genes have been identified. Two (Sh1 and Sus1) coded for two isoenzymes (SUS-SH1 and SUS1) that have a critical role in the synthesis of cellulose. The third gene (Sus2) encoded a protein necessary for the synthesis of starch precursors (Chourey et al., 1998). In roots of Triticum aestivum under hypoxic conditions, the enzyme activity of Sus increased considerably in the apical and central regions of roots and overlapped the deposition pattern of cellulose (Albrecht and Mustroph, 2003).
The pollen tube is a fundamental cell during sexual reproduction in higher plants and is characterized by fast apical growth that requires much energy. Pectins, cellulose, and callose are the main components of cell walls and are produced continuously to sustain pollen tube growth. During its development in the anther, pollen accumulates large quantities of carbohydrates, which constitute a large part of its dry weight (Pacini et al., 2006) and provide the sugars needed for pollen germination and growth. Sugars in the pistil (or in pollen germination media) are necessary to maintain pollen tube growth (Labarca and Loewus, 1972) and are hypothetically taken up inside the pollen tube by specific transporters (Ylstra et al., 1998). Subsequent catalysis of sugars produces ATP, which is required for growth, as confirmed by the high density of mitochondria with cristae in the subapical region and in the first segment of the base domain (Lovy-Wheeler et al., 2006). Distribution of mitochondria coincides with high levels of NADH (Cardenas et al., 2006), suggesting that pollen tubes derive most of their energy requirements from respiration.
The anisotropic development of pollen tubes is reflected in the nonuniform distribution of the cell wall components (Geitmann and Steer, 2006). The walls of the tube apex can be defined as primary cell walls, while the walls of mature pollen tubes are of the secondary type. Therefore, the temporal events occurring in somatic cells have been changed into a spatial model adapted to the pollen tube. The apical region of pollen tubes is characterized by a pectin layer (Li et al., 1995) that extends for the entire length of the tube and forms the outer layer of the cell wall. In some cases, such as in Arabidopsis (Arabidopsis thaliana; Derksen et al., 2002), this pectin layer is progressively substituted by the secondary layer, which consists mainly of callose. This secondary layer starts approximately 30 μm from the apex in tobacco (Nicotiana tabacum; Ferguson et al., 1998) and may increase in thickness, as in Petunia (Herrero and Dickinson, 1980), or remain constant, as in Solanum (Parre and Geitmann, 2005). Callose is also the main component of callose plugs, which form regularly in the mature region and allow cytoplasm to concentrate in the tube apex (Cresti and VanWent, 1976). Callose is produced by the enzyme callose synthase (Brownfield et al., 2007), which is localized in the plasma membrane (Ferguson et al., 1998). Cellulose occurs in lower quantities than callose (Schlupmann et al., 1994) and is generally localized in the inner layer of the cell wall (Ferguson et al., 1998). Since cellulose is a crystalline component of the cell wall, its orientation is potentially important for the architecture of the cell wall (Ferguson et al., 1998; Derksen et al., 1999). Although scarcely produced, the cellulose layer may be important in vivo (Lennon and Lord, 2000). Application of inhibitors of cellulose synthesis perturbs tube growth, suggesting that cellulose is necessary for the regular growth of pollen tubes (Anderson et al., 2002). The putative cellulose-synthesizing enzyme of pollen tubes has not been identified or characterized.
Pollen tubes convert most of the energy stored in internal and external carbohydrates to generate intracytoplasmic movement and to construct the cell wall matrix. Intracellular movement promotes the progressive accumulation of organelles and molecules in pollen tubes. Proper construction of cell walls is fundamental for morphogenesis and directional growth. In an attempt to associate carbohydrate metabolism with the process of cell wall synthesis, we tested the hypothesis that Sus has a critical role in this relationship. Since Sus plays different roles in carbon metabolism and is a critical intersection for directing carbon to different sinks (Ruan et al., 1997), we used pollen tubes as a cell model for investigating the role of Sus in the flow of energy necessary for cell wall construction and cell growth. As a first step, we characterized Sus immunologically and biochemically in tobacco pollen tubes. We then examined how Sus was distributed in relation to cell wall construction and pollen tube growth.
RESULTS
Control of Immunological Cross-Reactivity
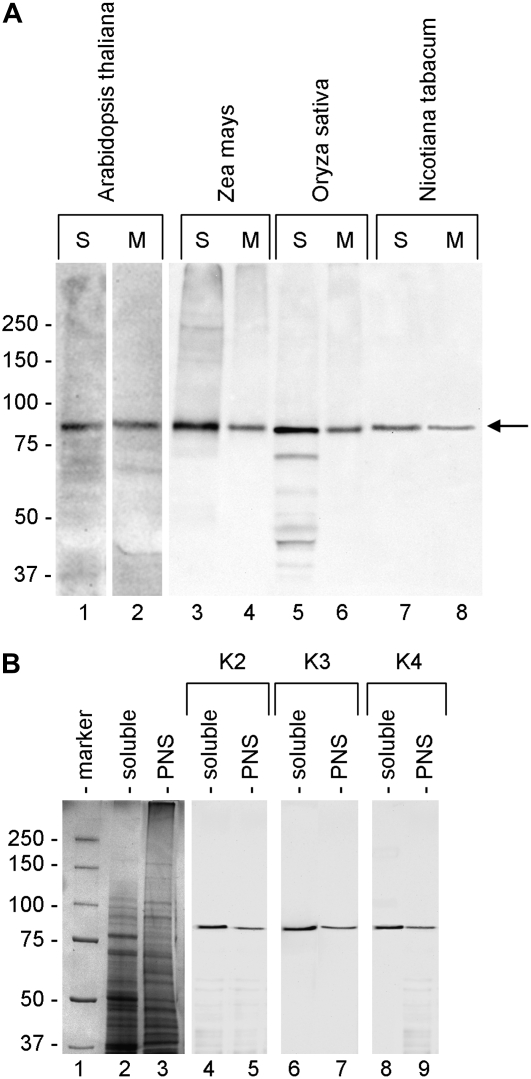
The cross-reactivity of anti-Sus was initially tested against different protein extracts. As the three antibodies (K2, K3, and K4) had almost the same pattern of cross-reactivity, Figure 1A only shows the cross-reactivity of K2. The antibody was tested on the soluble (S) and the membrane protein (M) fractions of whole flowering plants of Arabidopsis (lanes 1 and 2) and of leaves of maize (lanes 3 and 4), rice (Oryza sativa; lanes 5 and 6), and tobacco (lanes 7 and 8). In all cases, the antibody cross-reacted with an approximately 90-kD polypeptide in the soluble and membrane fractions.
Figure 1.
Immunoreactivity of anti-Sus antibodies against extracts from different plants. A, Sus antibody K2 was probed against soluble (S) and membrane (M) protein extracts from whole flowering plants of Arabidopsis (lanes 1 and 2) and from leaves of maize (lanes 3 and 4), rice (lanes 5 and 6), and tobacco (lanes 7 and 8). In all cases, the antibody cross-reacted with a single polypeptide having a molecular mass of 85 to 90 kD (arrow). The polypeptide was found in the soluble and insoluble protein pools. The same amount of protein (20 μg) was loaded in all lanes. The cross-reactivity of K3 and K4 antibodies was nearly identical to that of K2. Markers of molecular mass are indicated at left. B, Cross-reactivity of K2 (lanes 4 and 5), K3 (lanes 6 and 7), and K4 (lanes 8 and 9) antibodies against soluble and PNS proteins from tobacco pollen tubes (gel lanes 2 and 3, respectively). The three antibodies cross-reacted with a single band at 88 kD in all samples. Molecular mass markers are in lane 1. The same amount of protein (20 μg) was loaded in each lane.
The three antibodies were then assessed on the soluble and postnuclear supernatant (PNS) proteins of tobacco pollen tubes (Fig. 1B). Lanes 2 and 3 show the electrophoretic profiles of the two samples, while lanes 4 to 9 show the immunoblots with antibodies K2, K3, and K4. In all cases, we observed strong specific cross-reactivity against an 88-kD polypeptide.
Isolation of Sus from Tobacco Pollen Tubes
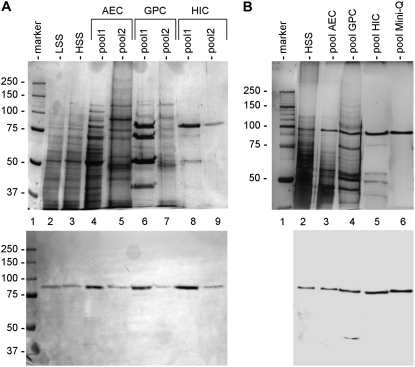
To demonstrate that the cross-reacting 88-kD polypeptide had the typical biochemical properties of Sus, we first isolated the protein from tobacco pollen tubes. As a control, Sus was also isolated from maize kernels (Fig. 2). Maize Sus (Fig. 2A) was isolated through sequential chromatography on anion exchange chromatography (AEC), gel permeation chromatography (GPC), and hydrophobic interaction chromatography (HIC) columns. After fractionation by AEC, the K2 antibody identified two distinct pools, eluting at 10% and 50% elution buffer, respectively (approximately 100 and 500 mm KCl). The two pools, designated pool1-AEC and pool2-AEC (lanes 4 and 5 in the top panel), were processed separately by GPC. The resulting pools (pool1-GPC and pool2-GPC) eluted with an apparent molecular mass of 400 kD (lanes 6 and 7). The two protein pools were subsequently fractionated by HIC, which produced two final pools (pool1-HIC and pool2-HIC, lanes 8 and 9, respectively), both eluting at approximately 250 mm (NH4)2SO4. The purity of the two samples was estimated at approximately 85% and 90%, respectively, using Quantity One software. The bottom panel shows the corresponding immunoblot with K2 antibody on the fractions in the top panel.
Figure 2.
Purification of Sus from maize kernels and tobacco pollen tubes. A, Sus was isolated from a high-speed supernatant (HSS) of maize kernels using a combination of AEC, GPC, and HIC. The gel in the top panel illustrates the main protein pools obtained during the isolation of maize Sus, whereas the bottom panel shows the corresponding blot with the K2 antibody. The first purification step (AEC) yielded two cross-reacting bands, which were collected in two distinct pools and processed separately. The final samples (lanes 8 and 9) contained the two cross-reactive bands at 86 kD. The same amount of protein was loaded in each lane (20 μg), except for lanes 8 (10 μg) and 9 (2 μg). Molecular mass markers are in lane 1. LSS, Low-speed supernatant. B, Gel (top) representing the main purification steps of Sus from pollen tubes. The 88-kD cross-reacting band was isolated from a high-speed supernatant through chromatography with AEC, GPC, HIC, and Mini-Q columns. The position of the Sus band was evaluated using the K2 antibody (bottom). Ten micrograms of proteins was loaded in each lane, except for lane 6 (5 μg). Molecular mass markers are in lane 1.
The protocol used for maize kernels was adapted for Sus isolation from pollen tubes (Fig. 2B). In this case, we detected only a single cross-reacting protein. The top panel shows gel analysis of the main fractions. The fraction pool after AEC (pool-AEC, lane 3) eluted around 150 mm KCl and was further fractionated by GPC. Pool-GPC (lane 4) eluted at approximately 200 kD and was further fractionated by HIC, which yielded pool-HIC (lane 5). Unlike for the isolation of maize Sus, we performed an additional purification step by AEC on a Mini-Q column. The final pool (pool-Mini-Q) contained a single band at 88 kD, the purity of which was more than 95%. The bottom panel shows the corresponding immunoblot with K2 antibody on the fractions shown in the top panel.
Enzyme Activity of Pollen Sus
The enzyme activity of Sus from pollen tubes was analyzed in the cleavage and synthesis pathways by quantifying Suc and Fru (Table I). In the test, maize Sus was assayed to validate experimental conditions and compare enzyme properties. In the case of Suc cleavage, we found that no products were obtained in the absence of pollen Sus or when denatured enzyme was used. When active pollen Sus was used, Suc was degraded to almost 50% of its molar concentration. In contrast, the two forms of maize Sus were more active in the cleavage pathway. When pollen Sus was assayed in the synthesis direction, it only converted one-third of the initial Fru into Suc. The two forms of maize Sus catalyzed the conversion of greater amounts of Fru into Suc.
Table I.
Enzyme assays of Sus activity from maize kernels and tobacco pollen tubes
The enzyme activity of Sus was evaluated in the synthesis and degradation pathways by measuring concentrations of Suc and Fru. Sugar values are indicated as μmol ± sd and were calculated by integrating the corresponding peaks of the chromatograms. Results are averages of three independent experiments. In the degradation pathway, Suc was almost entirely recovered and no Fru was released in the absence or presence of denatured pollen enzyme. In the presence of the two maize Sus forms (I and II), Suc was degraded to a corresponding amount of Fru. The reaction was evident but weaker in the presence of pollen Sus. In the synthesis pathway, no Suc was detected in the absence or presence of denatured pollen enzyme. The two maize Sus forms (I and II) yielded a considerable amount of Suc; pollen Sus yielded a smaller amount of Suc.
| Enzyme | Degradation
|
Synthesis
|
||
|---|---|---|---|---|
| Suc | Fru | Suc | Fru | |
| No enzyme | 205.3 ± 4.2 | 2.0 ± 1.8 | 3.2 ± 1.1 | 215.2 ± 8.8 |
| Denatured enzyme | 214.0 ± 10.6 | 2.78 ± 0.8 | 2.9 ± 1.5 | 198.7 ± 3.2 |
| Pollen Sus | 87.6 ± 6.6 | 83.2 ± 6.1 | 73.0 ± 4.4 | 133.2 ± 3.6 |
| Maize Sus (I) | 73.0 ± 7.6 | 122.1 ± 4.2 | 108.0 ± 4.1 | 116.5 ± 2.6 |
| Maize Sus (II) | 29.2 ± 3.2 | 160.9 ± 5.6 | 164.8 ± 5.9 | 83.2 ± 5.1 |
Subcellular Distribution of Pollen Sus
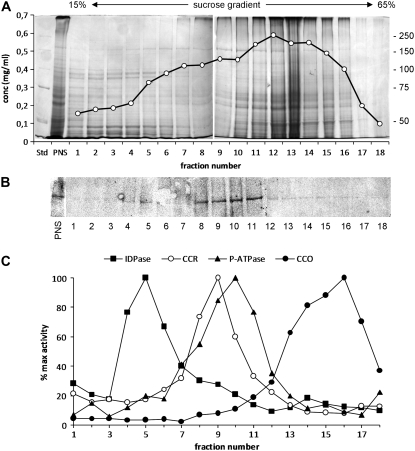
The immunoblot analysis of Figure 1 revealed a cross-reacting band associated with the membrane fraction of pollen tubes. The subcellular distribution of Sus was consequently investigated by fractionating the pool of pollen tube organelles along a 15% to 65% linear Suc gradient and by probing each fraction with Sus antibodies. The 20 fractions showed different protein concentrations, with the highest protein contents usually recovered around fractions 12 to 13 (42% Suc; Fig. 3A). Immunoblot analysis revealed that the 88-kD polypeptide elutes in fractions 8 to 11, corresponding to 34% to 42% Suc concentration (Fig. 3B), and a weaker signal was also found in fractions 4 to 6 (24%–28% Suc). These fractions were also analyzed for specific organelle markers (Fig. 3C). IDPase activity (a marker of Golgi apparatus and vesicles) was mostly detected in fractions 4 to 6, strictly corresponding to the smaller peak of the 88-kD polypeptide. A marker of rough endoplasmic reticulum (cytochrome c reductase) was found mainly in fractions 8 to 10, whereas vanadate-sensitive ATPase activity (a marker of plasma membrane) was detected in fractions 8 to 11, almost overlapping the second major peak of Sus. The cytochrome c oxidase activity (a marker of mitochondria) peaked around fractions 14 to 17.
Figure 3.
Distribution of Sus in organelle fractions of tobacco pollen tubes. The distribution of Sus was investigated by Suc gradient fractionation of PNS. A, Representative SDS-PAGE of fractions obtained after separating PNS with 15% to 65% linear Suc gradients. Molecular mass markers are at right. The gel is superimposed on the protein concentration profile, expressed as mg/mL. B, Immunoblot of the same fractions with the Sus antibody. The protein was found in fractions 8 to 11 and to some extent in fractions 4 to 6. C, Analysis of organelle markers in all fractions. The activity of each enzyme marker is reported as the percentage of maximum enzyme activity. Markers were IDPase activity (for Golgi apparatus and vesicles), cytochrome c reductase (CCR; for rough endoplasmic reticulum), P-ATPase activity (for plasma membrane), and cytochrome c oxidase activity (CCO; for mitochondria). Three different membrane fractionations were used to calculate the enzyme activity of organelle markers.
Detection of Sus in Plasma Membrane and Cell Wall
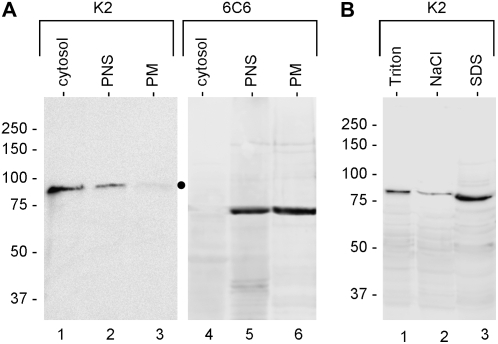
Sus is reported to be associated with the plasma membrane of plant cells. We investigated the association of pollen Sus with the plasma membrane by isolating a plasma membrane fraction by the two-aqueous-phase partitioning technique (Fig. 4A). The Sus antibodies failed to detect any signal (apart from a faint band; dot) in purified plasma membrane (lane 3), whereas the antibodies identified the 88-kD polypeptide in the soluble (lane 1) and PNS (lane 2) samples. As a control, the same fractions were probed with the 6C6 antibody (which cross-reacts with a 77-kD plasma membrane protein of pollen tubes); the antibody recognized the protein in the plasma membrane fraction (lane 6) as well as in the PNS fraction (lane 5) but not among soluble proteins (lane 4).
Figure 4.
Analysis of purified plasma membrane and cell wall proteins from pollen tubes. A, The absence/presence of Sus in the plasma membrane of pollen tubes was evaluated by immunoblot analysis of plasma membrane purified by the two-aqueous-phase partitioning method. Lanes 1 and 4, cytosolic proteins; lanes 2 and 5, PNS; lanes 3 and 6, plasma membrane fraction (PM). Samples were probed with the K2 antibody, revealing 88-kD Sus in the soluble and PNS fractions but not (or very weakly) in the plasma membrane (lane 3, dot). As a control, samples were also probed with the 6C6 antibody, which cross-reacted with a plasma membrane-associated polypeptide (lanes 5 and 6). Equivalent protein amounts were loaded in each lane. Markers of molecular mass are at left. B, Localization of Sus in the cell wall of pollen tubes. Putative Sus was associated with cell walls of pollen tubes. The K2 antibody labeled Sus in Triton-extracted proteins (lane 1), a low signal was found in NaCl-extracted proteins (lane 2), while the polypeptide was mostly recovered in SDS-extracted proteins (lane 3). Equal protein amounts were loaded in each lane. Markers of molecular mass are at left.
We also evaluated the association of Sus with the pool of cell wall proteins from tobacco pollen tubes (Fig. 4B). Proteins were extracted from the cell wall by differential extraction with Triton X-100, NaCl, and SDS. The 88-kD polypeptide was found in the cell wall protein pool after extraction with Triton X-100 (lane 1). A clear signal was also detected in NaCl-extracted proteins (lane 2), and the strongest signal was found in the SDS-extracted protein pool (lane 3).
Isoform Composition of Pollen Sus
The previous results indicated that pollen Sus is associated with plasma membrane and Golgi compartments and with the cell wall. The enzyme assays indicated that the protein shares typical biochemical properties of the Sus family. To assess the variability of Sus isoforms with regard to the cellular compartments, we analyzed the total protein pool, the soluble fraction, PNS, and SDS-extracted cell wall proteins by bidimensional electrophoresis and immunoblot. The total protein extract (Fig. 5A) showed two cross-reacting spots at 88 kD with a pI of 5.5 to 6.0 (arrow and arrowhead). The blot was overloaded to detect all possible isoforms. The cytoplasmic fraction contained an abundant protein pool in which the K2 antibody again recognized the two protein spots (Fig. 5B). Cross-reactivity was almost identical in the PNS pool (Fig. 5C). When the SDS-extracted cell wall proteins were analyzed, the antibody cross-reacted with two protein spots that were identical in molecular mass and pI (Fig. 5D). The relative intensity of the left spot (the more acidic one; arrowhead) was similar in all cases and was always less than that of the basic spot on the right (arrow). Unlike the acidic spot, the basic spot showed a relative intensity 10 times greater in the soluble fraction, being relatively similar in the PNS and cell wall pools. Control experiments were done by mixing different samples. Immunoblot of those combinations always provided the same spot pattern, suggesting that spot position was identical in all samples.
Figure 5.
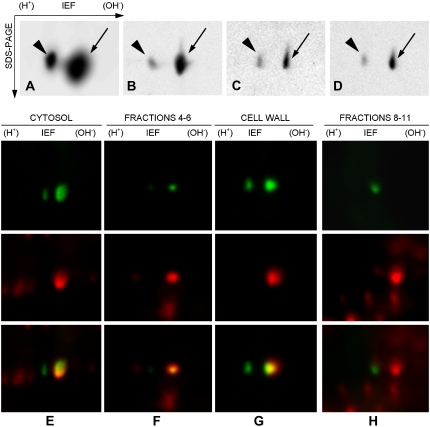
A to D, 2-D analysis of different pollen tube fractions: total proteins from tobacco pollen tubes (A), soluble protein pool (B), PNS (C), and SDS-extracted proteins from the cell wall (D). In all cases, the K2 antibody cross-reacted with two protein spots at 88 kD showing a pI of 5.5 to 6.0. In total protein extracts, the more acidic spot (arrowhead) was smaller than the basic spot (arrow) by a factor of three to four. This blot was overloaded to identify all possible cross-reacting spots. In all cases, the acidic spot had lower intensity than the basic spot. The intensity of the acidic spot did not change substantially in the different subcellular fractions. On the contrary, the basic spot was more intense in the soluble pool (approximately 10-fold). Only the blot segment containing the cross-reacting spots is reported. IEF, isoelectric focusing. E to H, Double western blot after 2-D electrophoresis of cytosolic fraction (E), Golgi vesicle-enriched sample (F; fractions 4–6 from Suc gradients), cell wall protein fraction (G), and plasma membrane-enriched fraction (H; fractions 8–11 from Suc gradients) with anti-Sus (green; top panels) and anti-phospho-Ser (red; middle panels). The bottom panels show the overlay.
To investigate the phosphorylation status of Sus in different pollen tube fractions, we performed double two-dimensional (2-D) immunoblot analyses using a monoclonal anti-phospho-Ser antibody in combination with the polyclonal anti-Sus. Simultaneous visualization of the two immunological reactions was achieved using the DyeChrome Double Western Blot Stain Kit (see Supplemental Fig. S1 for analysis on one-dimensional (1-D) gels and phosphoprotein standards). When tested on cytosolic proteins (Fig. 5E), two spots were detected by anti-Sus (top panel) but only one spot was also labeled by anti-phospho-Ser (middle panel), as shown by the overlap of the two pseudocolored images (bottom panel). The vesicle sample (Fig. 5F, corresponding to fractions 4–6 in Fig. 3) also showed a couple of spots when labeled by anti-Sus (top); again, only one spot was also labeled by anti-phospho-Ser (middle), as confirmed by the overlap (bottom). The SDS-extracted cell wall proteins (Fig. 5G) showed a similar pattern, as only one of the two spots identified by anti-Sus (top) was also labeled by anti-phospho-Ser (middle, with overlap in the bottom panel). The membrane sample from fractions 8 to 11 of Figure 3 showed a dissimilar pattern (Fig. 5H); anti-Sus labeled a single spot (top) that was not labeled by anti-phospho-Ser (middle), as confirmed by overlapping pseudocolored images (bottom). The big red dot in Figure 8H (middle and bottom panels) is a phosphorylated plasma membrane protein that was not recognized by anti-Sus (as shown in the top panel); this means that the protein spot was not Sus but an unrelated protein that migrates very close to Sus.
Figure 8.
A1 to A3, Distribution of Sus in pollen tubes treated with brefeldin A. A1 shows a control pollen tube. After treatment with brefeldin A for 20 min (A2), faint staining is evident at the cell border (arrow); arrowheads indicate circular structures near the border. After 30 min of treatment (A3), indefinite structures were visible in the base domain (arrows). Bar = 10 μm for all images. B1, Immunogold labeling of Sus in brefeldin A-treated pollen tubes. Staining was found mainly in multivesicular structures located in subcortical regions. Bar = 360 nm. B2 and B3, Staining of brefeldin A-treated pollen tubes also showed low levels of Sus in the cell wall (CW). Bar = 360 nm. C, Localization of Sus in tobacco pollen tubes grown in medium containing glycerol. Staining in the apical domain was weak, and labeling in the cortical region (arrow) was not as intense as with Suc medium. Bar = 10 μm. D, Staining of Sus in tobacco pollen tubes grown in PEG-based medium. Staining was again weak and punctate at the cell border (arrow); pollen tubes also showed diffuse cytoplasmic staining. Bar = 15 μm. E, Elongation rates of pollen tubes grown in Suc- and glycerol-based media. After 90 and 120 min of growth, pollen tubes in glycerol medium grew irregularly and often burst (very high sd); at 180 min, pollen tubes in glycerol medium were not counted. Error bars indicate sd.
In control experiments, samples were pretreated with 20 units of alkaline phosphatase, which removed almost all signals detected by anti-phospho-Ser. The two Sus spots were not labeled after staining with monoclonal anti-phospho-Thr and anti-phospho-Tyr (data not shown).
Intracellular Localization of Pollen Sus
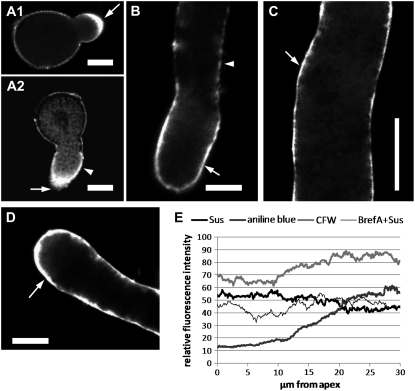
The distribution of Sus in tobacco pollen tubes was analyzed by immunofluorescence microscopy with K2, K3, and K4 antibodies. Results were extremely similar for all three antibodies. In germinating pollen grains (Fig. 6A1), labeling was localized mainly in the tube apex (arrow). A confocal analysis in the central focal plane of similar tubes confirmed that labeling was not only cortical but also detected in the tip domain (Fig. 6A2, arrow); in this specific pollen tube, labeling started to accumulate at the cell periphery (arrowhead). A pollen tube at the start of germination is shown in Supplemental Figure S2A. After elongation (tube length, 50–100 μm), the protein was distributed along the cell border in the apical domain (Fig. 6B, arrow); the signal also appeared and increased progressively in the basal domain (arrowhead). At 100 to 150 μm of pollen tube length, the signal at the tube border was stronger and extended toward the pollen grain (Fig. 6C, arrow). The staining pattern along the cell border was continuous (neither spotted nor clustered), and the pollen tube cytoplasm always showed weak diffuse staining. After 3 h of growth (Fig. 6D; length, approximately 200 μm), peripheral apical staining was still intense and remained constant even after longer elongation times. Fluorescence intensity was measured along the curvature of the pollen tube from the extreme tip down to the cell border in 200-μm-long pollen tubes (n = 20; Fig. 6E, thick black line); anti-Sus labeling was greater in the first 20 μm of the tubes, decreasing toward the base. As a control, preimmune serum provided no staining (data not shown).
Figure 6.
Localization of Sus in tobacco pollen tubes by immunofluorescence microscopy. A1, Distribution of Sus in germinating pollen tubes; labeling was found at the tube apex (arrow). A2, Confocal view of a pollen tube showing Sus in the apex (arrow) and in the cell border (arrowhead). B, In short pollen tubes (50–100 μm), labeling was localized mainly in the cortex of the apical domain (arrow) but also accumulated at the base of pollen tubes (arrowhead). C, As the tubes grew (100–150 μm), the protein distributed progressively along the cell border (arrow). D, Three hours after germination (approximately 200 μm), staining was still more intense in the cortical region of the apex (arrow). Preimmune serum did not cause staining. Bars = 10 μm. E, Graphic representation of the fluorescence intensity of Sus along the cell border before (thick black line) and after (thin black line) treatment with brefeldin A (BrefA). Pollen tubes were also stained for callose with Aniline Blue (dark gray thick line) and for cellulose with Calcofluor White (CFW; light gray thick line). Results are averages of 20 200-μm-long pollen tubes. Fluorescence intensity was determined with ImageJ software. Values represent relative fluorescence only and cannot be used to compare different stain intensities.
Measurement of anti-Sus labeling in 200-μm-long tubes after brefeldin A treatment (Fig. 6E, thin black line; see next paragraph for details) showed loss of high-intensity staining in the apical domain. The two major cell wall polymers (callose and cellulose) were also measured in similar tubes after labeling with Aniline Blue and Calcofluor White, respectively. Callose (Fig. 6E, thick dark gray line) showed a lower intensity at the tip and increased progressively 10 to 20 μm from the tube apex, reaching a maximum of approximately 30 μm. Cellulose (thick light gray line) was detected in the apical domain and showed a similar pattern to callose except for a lower rate of increase.
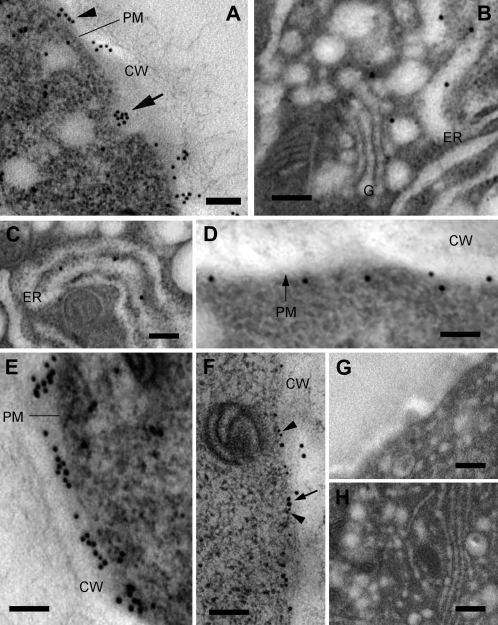
More information on the distribution of pollen Sus was obtained by immunoelectron microscopy. In this case, Sus was localized in the cytoplasm and more consistently between the cytoplasm and the cell wall, presumably in the plasma membrane (Fig. 7A, arrowhead), reminiscent of its pattern in cotton fiber (Ruan, 2007). Interestingly, the protein was also localized in secretory vesicle-like structures (arrow). The section of Figure 7A in the apical domain of pollen tubes shows a putative secretory vesicle (containing Sus) fusing with the plasma membrane. Staining of Sus was also found in the internal membrane system of the cell. Specifically, gold particles were found in association with vesicles close to Golgi bodies and with the endoplasmic reticulum (Fig. 7, B and C). Association of the enzyme with the plasma membrane is suggested by Figure 7D, where gold particles were detected along the cell border. However, Sus was also consistently found in the cell wall of pollen tubes (Fig. 7E). To confirm that Sus is associated with the plasma membrane of pollen tubes, we performed double labeling experiments with the 6C6 antibody (which cross-reacts with a plasma membrane-associated protein of 77 kD). The results indicated that the two proteins were codistributed at the interface between the cytoplasm and the cell wall (Fig. 7F), supporting evidence that Sus is associated with the plasma membrane. In controls with the preimmune serum, no staining of any structure was observed, either in the cortical (Fig. 7G) or in the cytoplasmic (Fig. 7H) domain of pollen tubes. Controls performed in the absence of primary antibodies were also negative (Supplemental Fig. S2B). Quantitative analysis of gold particles across the cell wall indicated that most Sus (approximately 70%) was situated in the cell wall region closer to the plasma membrane (first 20–40 nm), decreasing progressively beyond 40 nm. This suggests that Sus remains confined in the proximity of the plasma membrane after secretion, presumably in the inner callose-cellulose layer.
Figure 7.
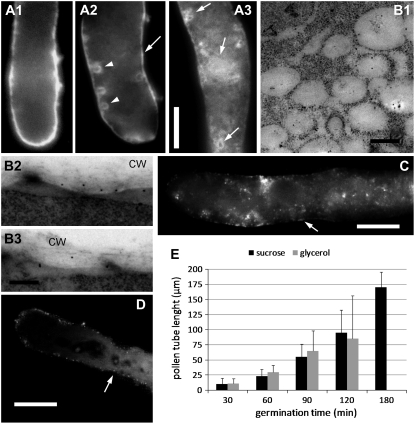
Distribution of Sus in tobacco pollen tubes visualized by immunoelectron microscopy. A, Section of pollen tubes in the apical domain. The arrowhead indicates gold particles deposited in the cell wall (CW); the arrow indicates particles in a secretory vesicle fusing with the plasma membrane (PM). Bar = 170 nm. B, Localization of Sus in association with Golgi bodies (G) and the endoplasmic reticulum (ER). Bar = 180 nm. C, Association of Sus with membranes of the endoplasmic reticulum. Bar = 180 nm. D, Sus was also associated with the plasma membrane of pollen tubes. Bar = 180 nm. E, Most Sus was associated with the pollen tube cell wall. Bar = 180 nm. F, Double immunogold labeling with anti-Sus (15-nm gold particle; arrow) and with 6C6 antibody (10-nm gold particle; arrowheads). The 6C6-reacting protein was found at the interface between the cytoplasm and the cell wall and corresponded to the distribution of Sus. Bar = 200 nm. G and H, Pollen tubes probed with the preimmune serum: cortical region (G) and cytoplasm (H). Bars = 300 nm.
When we assessed the distribution of Sus in pollen tubes treated with the protein transport inhibitor brefeldin A (Nebenfuhr et al., 2002), the control pollen tubes showed typical cortical staining (Fig. 8A1), whereas those treated with brefeldin A for 20 min (Fig. 8A2) showed a reduction in apical staining and a series of circular structures near the cell border (arrowheads). Typical cortical staining was partly maintained (arrow). In pollen tubes treated with brefeldin A for 30 min (Fig. 8A3), we observed the appearance of indefinite structures (possibly of membranous origin) in the cytoplasm (arrows). When analyzed by immunogold electron microscopy with anti-Sus antibody, those tubes showed that Sus was associated with large vesicular structures measuring approximately 350 nm (Fig. 8B1). Comparison of our results with the literature (Geitmann et al., 1996) suggested that the unidentified structures may arise from dictyosomes or from the fusion of secretory vesicles. At the same time, association of Sus with the cell wall was reduced drastically (Fig. 8, B2 and B3).
When we tested the effect of different osmotica on the distribution of Sus, the growth of pollen tubes germinating in medium containing 0.39 m glycerol or 0.1 m polyethylene glycol (PEG)-400 (O'Kelley, 1955; which are not metabolized by pollen tubes) was different from that observed in Suc-based medium. The pollen tube apex was scarcely labeled in either glycerol (Fig. 8C) or PEG medium (Fig. 8D; the tip was slightly brighter in PEG-grown pollen tubes but still less intense than in control tubes). This specific staining pattern was observed in short (approximately 50–100 μm) and longer (150–200 μm) pollen tubes. Labeling was weak and dotted in the cortical region (Fig. 8, C and D, arrows). Unlike the growth in Suc-based medium, the cytoplasm of pollen tubes was slightly labeled. Analysis of the germination rate of pollen tubes grown in Suc-based and glycerol-based media revealed that pollen tubes elongated in both media: growth proceeded constantly in Suc medium but reached a plateau at approximately 120 min in glycerol medium, when pollen tubes started to burst and growth ceased (Fig. 8E). Pollen germination in glycerol medium was apparently not delayed with respect to that in Suc medium, suggesting that the two osmotica did not influence the speed of pollen tube germination.
Quantitative Analysis of Sus in Different Cell Compartments
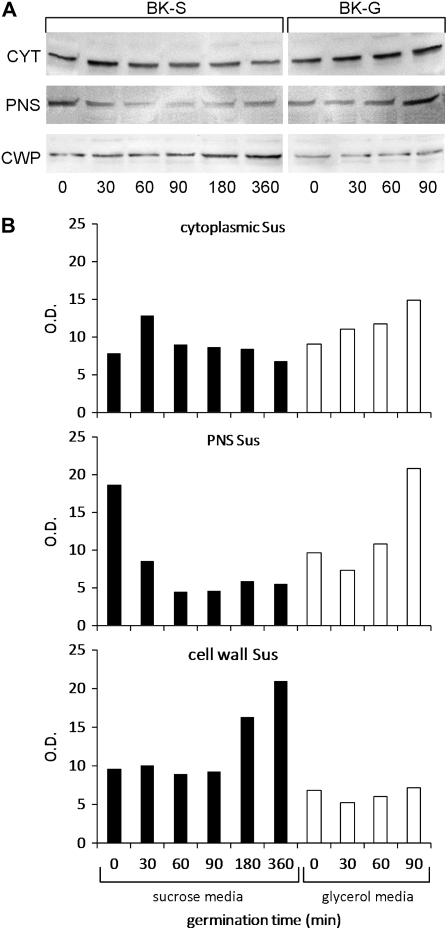
Since glycerol in the growth medium altered the distribution of Sus in pollen tubes, we assessed the difference by quantitative immunoblot analysis of proteins from different cell compartments: cytoplasmic, PNS, and SDS-extracted cell wall proteins (Fig. 9A). The assay was performed on pollen tubes germinated in glycerol- or Suc-based medium for different times (0, 30, 60, 90, 180, and 360 min). In the case of glycerol, the germination times were only 0, 30, 60, and 90 min because the tubes showed distorted growth after 90 min. When the immunoblot results were evaluated quantitatively (Fig. 9B), we found that the quantity of cytoplasmic Sus (top panel) showed a peak around 30 min in Suc-based medium (BK-S, black bars) and then decreased gradually during tube growth. When pollen tubes were grown in glycerol-based medium (BK-G, white bars), cytoplasmic Sus increased visibly with germination time. PNS-associated Sus (middle panel) was higher before germination in Suc-based medium, then remained almost steady at a lower level. On the contrary, in glycerol medium, the membrane-associated enzyme increased over 90 min. Cell wall-associated Sus (bottom panel) increased progressively with germination time in Suc medium, while in glycerol medium it remained at significantly lower levels. These results indicate that the concentration of cell wall-associated Sus increased progressively in Suc-based medium, while the amount of membrane-associated protein decreased correspondingly; in the case of glycerol-based medium, the quantity of cell wall-associated enzyme remained low while Sus appeared to accumulate in the membrane and soluble fractions.
Figure 9.
Analysis of the distribution of Sus in pollen tubes grown in medium containing Suc or glycerol. A, Immunoblot of cytoplasmic (CYT), PNS, and SDS-extracted cell wall proteins (CWP) from pollen tubes grown in Suc medium (BK-S) or glycerol medium (BK-G) for different times (0, 30, 60, 90, 180, and 360 min). The same quantity of protein was loaded in each lane. B, Quantitative analysis of immunoblots obtained in Suc (black bars) and glycerol (white bars) media showing relative concentrations of cytoplasmic Sus (top panel), PNS Sus (middle panel), and cell wall-associated Sus (bottom panel). In glycerol medium, only 0 to 90 min were considered because growth was abnormal after 90 min.
DISCUSSION
In this study, we attempted to characterize Sus in tobacco pollen tubes using different methodological approaches (biochemical, cytological, immunological, and proteomic). Tobacco pollen tubes were found to contain a Sus-like protein of 88 kD. The polypeptide shares several features with Sus of other species and is mainly associated with the pollen tube cell wall. Identification of Sus was based on cross-reactivity with antibodies against Sus of other species; however, the specific pattern of cross-reactivity coupled with enzyme assays indicated that the pollen protein belonged to the Sus family.
Pollen Tubes Contain a Protein Biochemically and Immunologically Related to Sus
The first approach to the characterization of Sus in tobacco pollen tubes was immunological. Analysis of leaf extracts from different plants confirmed the wide cross-reactivity of the antibodies K2, K3, and K4, which identified bands with similar molecular mass in flowering plants of Arabidopsis and in leaves of maize, rice, and tobacco. This finding is not surprising, because the amino acid sequence of Sus is highly conserved. A comparison among Sus from the four species indicated a similarity of approximately 70% to 80%. The immunological result was weaker in Arabidopsis; however, this finding is in agreement with the literature, because in some cases (like in rosette leaves of Arabidopsis) Sus is very low (Bieniawska et al., 2007). The molecular mass of the polypeptide subunit in pollen tubes was 88 kD, which is in agreement with the literature, where the molecular mass of Sus ranges from 80 kD in Daucus carota (Sebkova et al., 1995) to 94 kD in rice (Yen et al., 1994).
The native molecular mass of the protein was calculated by GPC on Sephacryl S-300 columns. Comparison with mass standards (β-amylase, 210 kD; bovine serum albumin, 66 kD; cytochrome c, 12 kD) indicated a molecular mass of 200 kD for pollen tube Sus, suggesting that the protein forms a dimer of two 88-kD subunits. The literature indicates that Sus usually exists as a tetramer with a native molecular mass between 540 kD (Gross and Pharr, 1982) and 320 kD (Sebkova et al., 1995). Our results differ to some extent because they suggest a dimeric form of the pollen enzyme. However, a recent study found that specific conditions are required for the enzyme to associate in tetrameric form: in the absence of Suc, the enzyme exists prevalently in dimeric form; the tetramer forms in the presence of Suc (Duncan and Huber, 2007).
The 88-kD band resolved into two distinct spots by proteomic assay. Plants generally express different isoforms of Sus. For example, the genome of Arabidopsis contains six genes that are expressed differentially in tissues and during plant development (Bieniawska et al., 2007). In Arabidopsis, three isoforms (SUS1, SUS3, and SUS5) are expressed in the flower. Although the two spots detected by 2-D electrophoresis in pollen tubes may be the result of multiple gene expression, we cannot exclude the possibility that one protein spot is the consequence of posttranslational modifications. Evidence that one isoform is phosphorylated suggests that phosphorylation of Ser residues is the mechanism used to generate distinctly functional Sus isoforms. The two pollen tube isoforms are presumably localized differently (the phosphorylated isoform being more abundant in the cytosol and cell wall, the nonphosphorylated isoform being associated prevalently with the plasma membrane) and therefore may have different functions.
Since the two isoforms are not separated by chromatographic techniques, the enzyme activity of Sus was tested on a mixture of both. The results indicate that pollen Sus functions in both directions (Suc cleavage and synthesis) as in pea (Pisum sativum; Dejardin et al., 1997). Under experimental conditions optimal for maize Sus, the prevalent activity of the pollen protein was Suc degradation. The enzyme assays were used mainly to confirm that cross-reacting bands were definitely Sus, but we did not perform a detailed enzyme analysis.
Pollen Sus was localized in two distinct protein pools: the soluble and membrane pools. These results are in agreement with the literature. Soluble Sus has been described extensively in different plants (Winter and Huber, 2000) and may be involved in metabolic activities related to glycolysis and energy production. Soluble Sus exists in equilibrium with the membrane-associated form, as in tobacco (Matic et al., 2004), cotton (Gossypium hirsutum; Amor et al., 1995), tomato (Solanum lycopersicum) fruits (Anguenot et al., 2006), and maize (Carlson and Chourey, 1996). Plasma membrane-associated Sus probably provides metabolites for both callose synthase and cellulose synthase. Sus was not found in the pollen tube plasma membrane preparation obtained by phase partitioning; however, this method is probably not the best for the isolation of plasma membrane proteins of pollen tubes, as shown for callose synthase (Turner et al., 1998). Nevertheless, the presence of Sus in the pollen tube plasma membrane is suggested by its association with Triton-extracted cell wall proteins, which indicates that the putative membrane-associated enzyme remains attached to the wall matrix during cell lysis. The protein also coelutes with organelle fractions enriched in P-ATPase activity (a marker of plasma membrane), is localized at the interface between the pollen tube cytoplasm and the cell wall by immunoelectron microscopy, and colocalizes with the plasma membrane-associated 6C6-reacting polypeptide (Cai et al., 1996).
We are convinced that Sus located in the cell wall was not contaminated by plasma membrane-associated Sus. The cell wall protein fraction was the result of extensive washing necessary to remove contaminating proteins. Additional Triton washing also separated proteins that bind to cell wall-associated membranes. In addition, most cell wall-associated Sus is recovered after washing with SDS (indicating strong binding affinity with the cell wall). The association of Sus with the plasma membrane (or generally with membranes) was supported by the immunolocalization experiments.
A partial sequence of a 1,265-bp mRNA coding for the putative Sus of tobacco pollen tubes was obtained in our laboratory (accession no. EU148354). The sequence has 96% identity with tobacco mRNA for Sus (partial coding sequence) and 89% identity with tomato and potato mRNA for Sus.
Localization of Sus in Pollen Tubes Indicates a Pathway from the Golgi to the Cell Wall
Our results indicate that pollen Sus is also associated with the Golgi membranes. Association of Sus with the Golgi fraction has already been described in maize (Buckeridge et al., 1999) and linked to the synthesis of cellulose polymers. The presence of Sus in Golgi vesicles and the cell wall suggests that the enzyme is transferred to the cell wall by a secretion mechanism involving the Golgi vesicles. This model is supported by the immunofluorescence pattern in the apical domain as well as by immunogold localization of the protein inside vesicles. Association of Sus with the cell wall has already been described in other cells. In cotton fibers, the protein accumulates in the thickenings of the secondary cell wall associated with higher concentrations of microtubules and actin filaments (Salnikov et al., 2001, 2003). Cell wall-associated Sus is unlikely to be an artifact because it was also observed in cryogenically fixed and freeze-substituted cotton fibers (Salnikov et al., 2003; Ruan, 2007). The association of Sus with the cell wall has always been enigmatic; our results here suggest that its accumulation may be driven by a Golgi-mediated secretion mechanism. Exocytosis-mediated transport of Sus toward the cell wall is confirmed by treatment with brefeldin A, which interferes with anterograde protein transport from the endoplasmic reticulum to the Golgi apparatus (Nebenfuhr et al., 2002). After treatment with brefeldin A, Sus is found in association with large membrane-bound structures that are not detected in untreated pollen tubes. These aberrant structures are similar to the already described vesicle-like structures resulting from the fusion of Golgi bodies and the endoplasmic reticulum (Rutten and Knuiman, 1993; Geitmann et al., 1996).
Phosphorylation analysis indicated that the phosphorylated Sus isoform is relatively more abundant in the cytoplasm and in cell walls, while the nonphosphorylated isoform is associated with the plasma membrane. This evidence suggests that the protein is not subject to extensive posttranslational modifications during transfer to the cell wall, whereas dephosphorylation of Ser residues is necessary for the association of Sus with the plasma membrane. The literature does not contain any indications about posttranslational modification of cell wall-associated Sus, only about the phosphorylation mechanisms presumed to regulate the association of Sus with the plasma membrane (Komina et al., 2002). Evidence similar to that obtained in pollen tubes has also been found in maize, in which phosphorylation of Ser-15 affects the catalytic activity of Sus and its association with membranes (Hardin et al., 2004). Even in soybean (Glycine max) nodules, microsomal Sus is hypophosphorylated with respect to soluble Sus (Komina et al., 2002), and phosphorylation of Sus decreases its surface hydrophobicity, causing its release from membranes (Winter et al., 1997).
The different distribution of Sus between pollen tubes grown in Suc- and glycerol/PEG-based media suggests that the type of osmoticum influences the expression of Sus or its distribution within the cell. Glycerol and PEG cannot be used by the pollen tube as a carbon source, and pollen tube elongation ceases after 1.5 to 2 h of growth in these media. This is indirect confirmation that pollen tubes mainly utilize internal sugars in initial growth stages and external carbohydrates (in growth media or in the style) during later growth stages (Ylstra et al., 1998). The distribution and relative quantity of Sus with respect to different growth media draw attention to the regulation of enzyme expression and distribution. Sugar availability seems to affect how Sus is distributed in pollen tubes, as in cotton. In cotton cells, sugar availability favors the growth phase by increasing the levels of membrane Sus, whereas scarcity of nutrients switches Sus to the soluble form, which is involved in the optimization of Suc storage (Haigler et al., 2001).
Some Hypotheses on the Role of Sus in Pollen Tubes
Comparing our results with the literature allows us to formulate some hypotheses on Sus activity in pollen tubes. The association of Sus with the plasma membrane and cell wall suggests that the enzyme is involved in the construction of cellulose microfibrils and callose. This is largely supported by the current literature (Chourey et al., 1998). The cellulose levels are usually low in pollen tubes but may be critical for proper tube shaping and growth (Anderson et al., 2002). Unfortunately, the localization of cellulose synthase in pollen tubes is unknown. Staining with Calcofluor White suggests that cellulose-like components start to be synthesized in the apical domain of pollen tubes and accumulate massively approximately 10 to 15 μm from the apex. Sus is most abundant in the same region, which could enable efficient synthesis of cellulose microfibrils. The same reasoning seems appropriate for the synthesis of callose. The association between callose synthase and Sus has been hypothesized in several cases (Amor et al., 1995; Hong et al., 2001; Salnikov et al., 2003). When the distribution of Sus and callose has been studied, the enzyme has been localized in the exoplasmic space (practically, in the cell wall; Salnikov et al., 2003). The abundant presence of Sus in these cell walls suggests that the extracellular protein is involved in the synthesis of callose in the plasma membrane and remains attached to callose after its synthesis; this could be an alternative extracellular system of callose synthesis (Salnikov et al., 2003). Therefore, an association of Sus with the pollen tube cell wall is not atypical. Callose synthase of pollen tubes is probably localized in the plasma membrane (Turner et al., 1998) and has been postulated to cooperate with Sus (Li et al., 1999).
Another interesting possibility is that Sus has a role in the synthesis of xyloglucans. Sus has already been localized in Golgi membranes (Buckeridge et al., 1999) and could provide UDP-Glc for the synthesis of xyloglucans (which occurs in Golgi bodies). As support for this hypothesis, a UDP-Glc transporter has been localized in association with Golgi vesicles and involved in the synthesis of polysaccharides (Munoz et al., 1996). This hypothesis is fascinating, although levels of xyloglucans in pollen tubes are typically low, like those of cellulose.
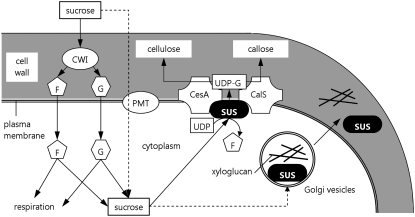
On the basis of our observations, we propose a model in which external Suc is converted into Glc and Fru through the activity of a cell wall invertase (Ylstra et al., 1998). We cannot totally exclude the possibility that Suc penetrates directly into the pollen tube through specific transporters (Aoki et al., 2003; Johnson et al., 2004) or that cell wall-associated Sus may do the same job as invertase. Once in the pollen tube cytoplasm, Glc and Fru take part in energy production. At the same time, Glc and/or Suc may enhance the expression of Sus (Karrer and Rodriguez, 1992), and the availability of sugars may regulate the equilibrium between soluble and membrane-associated Sus by altering the phosphorylation status of Sus isoforms (Hardin et al., 2006). The activity of the differential phosphorylation and export of Sus through the secretory apparatus associates the enzyme with the plasma membrane and cell wall (Fig. 10). The presence of cell wall-associated Sus suggests that high enzyme activity is required for the production of substantial quantities of callose, as described in cotton fibers (Salnikov et al., 2003). Synthesis of new cell walls and energy production are critical prerequisites for the growth of pollen tubes. Accumulation of Sus in cell walls seems to depend on Golgi vesicle-mediated transport. This finding is partially different from the hypothesis for somatic cells, in which Sus accumulates mainly in the plasma membrane. However, the tip growth of pollen tubes differs from the diffuse growth of most plant cells, because expansion is localized essentially at the tube apex and presumably depends on the balance between intracellular turgor pressure and cell wall loosening (Zonia et al., 2006), being governed by actin-mediated vesicle transport to the expanding tip (Kropf et al., 1998). The presence of a callose layer in the cell wall could also require differential localization of Sus.
Figure 10.
Hypothetical function of Sus in the construction of pollen tube cell walls. External disaccharides (mainly Suc) are hydrolyzed to Glc (G) and Fru (F) by a cell wall invertase (CWI; Ylstra et al., 1998). Suc may also be imported directly by plasma membrane transporters (PMT). Glc and Fru take part in energy production (respiration) but may be reconverted into Suc (hypothetically by cytoplasmic Sus). The disaccharide is then degraded by membrane Sus into UDP-Glc (UDP-G), which is used for the production of cell wall polysaccharides by cellulose synthase (CesA) and callose synthase (CalS). The availability of internal sugars may trigger (dotted arrows) the accumulation of Golgi vesicle Sus, which could take part in the synthesis of xyloglucans and/or enhance the production of cellulose and callose. Golgi vesicle Sus would then be recovered in the cell wall.
MATERIALS AND METHODS
Reagents
Tobacco (Nicotiana tabacum) plants and other species used in this research were grown in the Botanical Gardens of Siena University. Three different batches (K2, K3, and K4) of Sus antiserum were kindly provided by Dr. Wolfgang Werr (University of Köln, Institut für Entwicklungsbiologie); the characteristics of the antibody are described in the literature (Heinlein and Starlinger, 1989). The 6C6 antibody (which recognizes a plasma membrane-associated protein in tobacco pollen tubes) has been described by Cai et al. (1996). Unless specified otherwise, reagents were obtained from Sigma Chemicals.
Pollen Germination and Growth
Tobacco pollen was progressively thawed from storage at −20°C and hydrated overnight in a humid chamber. It was germinated in BK medium [1.62 mm H3BO3, 1.25 mm Ca(NO3)2·4H2O, 2.97 mm KNO3, and 1.65 mm MgSO4·7H2O; Brewbaker and Kwack, 1963] containing 15% Suc. After 3 h of germination at 25°C, pollen tubes were generally 200 μm long. Tobacco pollen was also germinated in BK medium containing 0.32 m glycerol or 0.1 m PEG-400 (O'Kelley, 1955) to verify the effect of different carbon sources and osmotica on the presence and distribution of Sus. Brefeldin A was used at a concentration of 2 μm for 20 to 30 min, during which pollen tubes still showed partial growth (Geitmann et al., 1996; Parton et al., 2001). Therefore, we are confident that the Sus localization results depend on the effects of brefeldin A rather than on growth arrest.
Extraction of Proteins from Tobacco Pollen Tubes
After germination for 3 h, the pollen was collected by centrifugation at 1,000g for 5 min at 25°C and was then washed twice with BRB25 buffer (25 mm HEPES, pH 7.5, 2 mm EGTA, and 2 mm MgCl2) + 15% Suc. The pollen was resuspended in lysis buffer (BRB25 containing 2 mm dithiothreitol [DTT], 1 mm phenylmethylsulfonyl fluoride [PMSF], 10 μL/mL protease inhibitors, 1 mm NaN3, and 10% mannitol) and then lysed on ice using a motor-driven Potter-Elvehjem homogenizer. After lysis, samples were centrifuged at 1,000g for 10 min at 4°C, and the supernatant was centrifuged again at 100,000g for 45 min at 4°C over a 20% (w/v) Suc cushion. The supernatant (soluble fraction) was collected. The pellet, containing the membrane fraction of pollen tubes (PNS), was resuspended in resuspension buffer (BRB25 plus 10% mannitol, 1 mm DTT, and 1 mm PMSF) for subsequent procedures.
Extraction of Proteins from Different Plant Sources
Proteins were extracted from leaves of maize (Zea mays), rice (Oryza sativa), and tobacco and from whole flowering plants of Arabidopsis (Arabidopsis thaliana). Approximately 1 to 3 g of leaves (or plants in the case of Arabidopsis) was ground in liquid nitrogen, and the powder was mixed 1:2 with extraction buffer (0.1 m Tris-HCl, pH 8, 10 mm MgCl2, 2 mm EGTA, 1% mannitol, 1 mm PMSF, 10 μL/mL protease inhibitors, 1 mm DTT, and 1% polyvinylpolypyrrolidone). Samples were incubated on ice for 15 min and centrifuged at 1,000g for 10 min at 4°C. The pellet was discarded, and the supernatant was centrifuged at 100,000g for 1 h at 4°C. The resulting supernatant (containing soluble proteins) and pellet (containing membrane-bound organelles) were resuspended directly in sample buffer for 1-D electrophoresis (Laemmli, 1970).
Isolation of Sus from Maize Kernels and Pollen Tubes
Sus was isolated from maize kernels using a combination of chromatography techniques performed with an AKTA Purifier system (GE HealthCare). The method is based on protocols used for the isolation of Sus from different plant species (Sebkova et al., 1995; Huang and Wang, 1998; Tanase and Yamaki, 2000; Schafer et al., 2005). Briefly, maize kernels were ground in liquid nitrogen, and proteins were extracted by incubation in extraction buffer (50 mm imidazole, pH 7.5, 1 mm EDTA, 1 mm PMSF, 1% polyvinylpyrrolidone-360, 2 mm DTT, and 10 μg/mL protease inhibitors) for 45 min at 4°C. Samples were centrifuged at 15,000g for 20 min (4°C), and the resulting low-speed supernatant was further centrifuged at 50,000g for 90 min (4°C). The high-speed supernatant was loaded onto a Resource-Q column (GE HealthCare) equilibrated in starting buffer (50 mm imidazole, pH 7.5, 1 mm EDTA, 1 mm PMSF, and 2 mm DTT) and was eluted at 1 mL/min with a linear gradient from 0% to 100% elution buffer (starting buffer plus 1 m KCl) over 20 column volumes. All fractions were analyzed by immunoblot with K2-K3-K4 antibodies, and cross-reacting fractions were pooled. Pools were then fractionated through GPC onto a Sephacryl S-300 column (GE HealthCare) equilibrated with starting buffer. Elution was done at 1 mL/min over 1.5 column volume. Fractions (2 mL each) were analyzed by immunoblot. Positive fractions were pooled and diluted 1:10 with HIC buffer [50 mm imidazole, pH 7.5, 1 mm EDTA, 1 mm PMSF, 2 mm DTT, and 2.5 m (NH4)2SO4], then loaded onto a Resource-Phe column (GE HealthCare), which was eluted at 1 mL/min with a linear gradient from HIC buffer to starting buffer over 20 column volumes. After immunoblotting, positive fractions were collected and dialyzed overnight against storage buffer (50 mm MES, pH 6, 1 mm DTT, 1 mm EDTA, and 20% glycerol) using a Mini Dialysis Kit with an 8-kD cutoff (GE HealthCare).
Sus was isolated from 3-h-old tobacco pollen tubes using the method described above for maize Sus but with a final additional separation step on a Mini-Q column (GE HealthCare). Briefly, after HIC, the fraction pool was desalted using a PC3.2/10 Fast Desalting column (GE HealthCare), then loaded on the Mini-Q column, which was eluted at 1 mL/min with 20 column volumes of the same buffers as for the AEC on Resource-Q. Fractions positive to immunoblot were pooled and dialyzed overnight against storage buffer.
Analysis of Enzyme Activity
The synthesis direction of Sus is enhanced by alkaline pH (>8; Romer et al., 2004), while the degradation pathway is favored by slightly acidic pH (Ross and Davies, 1992). Therefore, the enzyme activity of pollen Sus was assayed in both the cleavage and synthesis directions. For analysis of Suc cleavage, we used the method set up for the analysis of maize Sus (Echt and Chourey, 1985). The assay volume consisted of 5 μg of pollen or maize Sus, 200 mm Suc, 5 mm UDP, 2 mm MgCl2, and 50 mm MES buffer (pH 6.5). Samples were incubated for 15 min at 30°C, and then reactions were stopped by boiling at 100°C for 2 min. Blanks consisted of protein (5 μg) mixed with 200 mm Suc or 5 mm UDP, 2 mm MgCl2, and 50 mm MES.
The synthesis pathway was assayed as outlined in the literature (Dejardin et al., 1997; Tanase and Yamaki, 2000). Briefly, the assay volume consisted of 5 μg of protein, 20 mm Fru, 3 mm UDP-Glc, and 50 mm Tris buffer (pH 8.5). Samples were incubated for 15 min at 30°C and stopped by boiling at 100°C for 2 min. In blank samples, either Fru or UDP-Glc was omitted.
Determination of Suc and Fru in all samples was performed by HPLC analysis on a Waters LC1 system using a Waters 2410 refractometric detector. The column was a Supelco C-611 (300 × 7.8 mm, 10-μm pore size) at a constant temperature of 60°C. The mobile phase consisted of 10−4 m NaOH at a flow rate of 0.5 mL/min. The volume of injected samples was 20 μL. The instrument was calibrated with known amounts of Suc and Fru, separately or together. The amount of sugars produced in the assays was calculated by integrating the corresponding peak areas of the chromatograms.
Fractionation of Pollen Tube Organelles on Suc Gradients
Organelles (the PNS) from 3-h-old pollen tubes were fractionated on 15% to 65% linear Suc gradients in gradient buffer (50 mm imidazole, pH 7.5, 2 mm EDTA, 1 mm PMSF, and 1 mm DTT) using a Hoefer SG30 gradient former (GE HealthCare). The PNS was loaded on top of 10-mL Suc gradients prepared in 13-mL Beckman tubes (Beckman Coulter). Samples were centrifuged in a Beckman Optima L-80 ultracentrifuge at 100,000g for 8 h (4°C) and separated into 20 fractions of 0.5 mL each. Fractions were assayed for protein concentration, Sus, and specific organelle markers.
Isolation of Plasma Membrane Proteins from Pollen Tubes
The plasma membrane fraction of tobacco pollen tubes grown for 3 h was isolated according to known methods (Carlson and Chourey, 1996) and adapted to pollen tubes (Cai et al., 2005). Briefly, approximately 1 mL of PNS from 3-h-old pollen tubes was added to a “two-phase” mixture (3.4 g of 20% Dextran T-500, 1.7 g of 40% PEG-3350, and 2.15 g of TSK buffer [50 mm Tris-HCl, pH 7.5, 1 m Suc, and 8 mm KCl] to 9 g with water). The sample was mixed vigorously and centrifuged for 5 min at 1,500g. The upper phase was removed and added to the lower phase obtained by centrifuging a blank sample. After mixing, the sample was centrifuged for 5 min at 1,500g. The upper phase was collected and diluted 1:1 with resuspension buffer (50 mm Tris-HCl, pH 7.5, 250 mm Suc, 1 mm EDTA, and 1 mm DTT). The sample was centrifuged for 120 min at 100,000g to pellet the plasma membrane. The pellet was resuspended in storage buffer (50 mm Tris-HCl, pH 7.5, 1 mm DTT, and 15% glycerol).
Isolation of Cell Wall Proteins
Cell wall proteins from 3-h-old tobacco pollen tubes were isolated as described in the literature (Li et al., 1983). We added two additional washes (with Triton X-100 and SDS) to fractionate cell wall protein content into three final samples: Triton-solubilized proteins, which should contain loosely bound proteins (presumably plasma membrane-associated); NaCl-solubilized proteins matching the sample obtained by Li et al. (1983); and SDS-solubilized proteins, which are strongly bound cell wall proteins.
Immunofluorescence Microscopy
Indirect immunofluorescence microscopy was performed according to Del Casino et al. (1993). Pollen was germinated for 3 h in BK medium and then fixed for 30 min with 3% formaldehyde in PM buffer (50 mm PIPES, pH 6.9, 1 mm EGTA, and 1 mm MgCl2). After washing in PM buffer, the cell wall was digested in 1.5% cellulysin (Calbiochem) in PM buffer for 7 min in the dark. After washing in PM buffer, samples were incubated with antibodies to Sus diluted 1:100 in phosphate-buffered saline (PBS; pH 7.5) for 1 to 2 h at room temperature. The secondary antibody was Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen), which was used for 1 h after dilution at 1:150 in PBS. Callose was stained with decolorized Aniline Blue and crystalline wall material was stained with Calcofluor White according to the literature (Derksen et al., 2002). Samples were observed with a Zeiss Axiophot optical microscope equipped with a 63× objective. Images were captured with a MRc5 AxioCam video camera using AxioVision software. Samples were also analyzed with a Bio-Rad MicroRadiance confocal microscope using a 63× objective. Pollen tubes of different lengths were recorded. In controls, the primary antibodies were omitted or preimmune serum was used. Fluorescence intensity was measured along the curvature of the pollen tube from the extreme tip down to the cell border using ImageJ software (http://rsb.info.nih.gov/ij/; Calibrate and Plot Profile commands). Measures are averages of 10 different pollen tubes at the same length.
Immunoelectron Microscopy
Immunogold labeling on tobacco pollen tubes was performed according to the protocol described by Li et al. (1995). Briefly, samples of pollen tubes after 3 h of growth were fixed in 1.5% formaldehyde and 0.75% glutaraldehyde in phosphate buffer, pH 6.9, for 30 min at room temperature. After fixation, the samples were rinsed with phosphate buffer and dehydrated gradually in a series of increasing concentrations of ethanol (from 10% to 100%). Samples were embedded in LR White resin and polymerized for 2 d at 40°C. Samples were then cut into 600-Å sections using a Leica Ultracut R microtome. The primary Sus antibodies were used after dilution at 1:50 in PBS, while the 6C6 antibody was diluted 1:150. Secondary goat anti-rabbit Ig 15-nm gold-conjugated and goat anti-mouse Ig 10-nm gold-conjugated antibodies were used at a dilution of 1:50 (BioCell). Sample images were captured with a Philips Morgagni 268D transmission electron microscope operating at 80 kV and equipped with a MegaView II CCD camera (Philips Electronics). For quantitative analysis, gold particles were counted in random selected images (approximately 30 images from different experiments). Gold particles were analyzed and calculated using ImageJ software (http://rsb.info.nih.gov/ij/) with the command Analyze → Particle (Threshold option). In controls, the primary antibodies were omitted or preimmune serum was used.
Kinetic Analysis of Sus during Pollen Tube Growth
The relative quantity of Sus was estimated in three different cell compartments during pollen tube growth. We considered the cytoplasmic (soluble) fraction, the PNS fraction, and the SDS-extracted cell wall proteins of pollen tubes grown for fixed times (0, 30, 60, 90, 180, and 360 min) in both Suc- and glycerol-based media. These fractions were collected as described previously, and then equal amounts of proteins were immunoblotted with Sus antibody. Signals from at least three different experiments were quantified using Quantity One software.
1-D and 2-D Electrophoresis and Immunoblotting
1-D electrophoresis was carried out as described (Laemmli, 1970) using 7.5% acrylamide gels. All gels were stained with BioSafe Coomassie (Bio-Rad). Molecular mass standards of the Precision series were purchased from Bio-Rad.
For 2-D electrophoresis, samples were prepared using the ReadyPrep 2-D Cleanup Kit from Bio-Rad. Total proteins from germinated pollen tubes were extracted using the Total Protein Extraction Kit (Bio-Rad) and the ReadyPrep 2-D Cleanup Kit. Proteins were separated in the first dimension by isoelectric focusing using a Multiphor II apparatus (GE HealthCare) according to the manufacturer's instructions. Immobiline DryStrip gels (7 cm long; GE HealthCare) contained a pH gradient of 3 to 10 (for the first screening) or 4 to 7 (for sharper separation). Gels were run at 200 V (2 mA, 5 W) for 1 min, 3,500 V for 1.5 h, and 3,500 V for a further 1.5 h. Samples were included in the rehydration buffer. After running, gels were equilibrated in equilibration buffer (prepared as indicated by the manufacturer) for 15 min or, alternatively, frozen and stored. Proteins were separated in the second dimension by SDS gel electrophoresis on a Bio-Rad Mini-Protean II using 1.0-mm-thick 10% acrylamide gels. At least three gels were run for each protein fraction. Parallel unstained gels were blotted onto nitrocellulose membranes and probed with antibodies.
Immunoblotting was performed as described (Towbin et al., 1979) using a Mini Trans-Blot Cell (Bio-Rad) and nitrocellulose membranes (GE HealthCare). After blotting, membranes were washed in TBS-T (Tris-buffered saline containing 0.1% Tween 20) and blocked with 5% powdered milk in TBS-T. Primary antibodies were diluted 1:1,000 in TBS-T and incubated for 2 h. After several washings in TBS-T, goat anti-rabbit horseradish peroxidase-conjugated and goat anti-mouse horseradish peroxidase-conjugated secondary antibodies (GE HealthCare) were diluted 1:3,000 in TBS-T and incubated for 1 h. Immune reaction was visualized using the ECL Plus system from GE HealthCare.
For visualizing the phosphorylation status of Sus, we performed double immunoblot experiments using the DyeChrome Double Western Blot Stain Kit (Invitrogen). For these experiments, extraction of protein samples was performed as described previously with the addition of 1 mm Na3VO4 in all buffers. Proteins were blotted on polyvinylidene difluoride membranes and labeled as described in the Double Western Blot Kit manual. Proteins were simultaneously labeled with the Sus antibody and with a monoclonal antibody against phospho-Ser (Sigma-Aldrich). In control experiments, samples were prepared without 1 mm Na3VO4 but were pretreated with 20 units of alkaline phosphatase (Sigma-Aldrich; Tanase et al., 2002). In addition to anti-phospho-Ser, we also tested monoclonal antibodies against phospho-Tyr and phospho-Thr (both from Sigma-Aldrich). Peppermint (Mentha piperita) phosphoprotein molecular mass standards were purchased from Invitrogen.
Image Analysis of Gels and Blots
Images of Coomassie Brilliant Blue-stained gels were captured using the Fluor-S MultiImager from Bio-Rad. The molecular mass of proteins was evaluated using Quantity One software (Bio-Rad). The immunoblot membranes were developed directly in the Fluor-S MultiImager using Quantity One software; images of blots were captured at different times and then superimposed on images of prestained standards obtained using the same membrane. Protein content was analyzed in each blot (from three different experiments) using the Volume Analysis Report of Quantity One; the volumes of reactive bands were calculated as Adjusted Volumes (volume minus the background volume of each blot). Analysis of 2-D gels was done with PDQuest software (Bio-Rad) after storing gel images with the Fluor-S MultiImager, while pI and molecular mass were estimated by comparison with 2-D standards (Bio-Rad). At least three different blots from independent experiments were analyzed. Visualization of DyeChrome double staining was achieved with the Fluor-S MultiImager using UV epifluorescence illumination of membranes and 530BP and 610LP filters. Images were compared using the MultiViewer command of PD Quest software.
Analytical Techniques
Protein concentration was evaluated using the colorimetric method of Bradford (1976). Protein concentrations in samples for 2-D electrophoresis were calculated using the 2-D Quant Kit from GE HealthCare. The following organelle marker enzymes were analyzed: IDPase activity for Golgi vesicles (in the presence and absence of Triton X-100), P-ATPase activity for plasma membrane (in the presence and absence of vanadate), cytochrome c reductase activity for endoplasmic reticulum, and cytochrome c oxidase activity for mitochondria (Turner et al., 1998; Robinson and Hinz, 2001). Enzyme activities were always expressed as percentages of maximum activity. Three different membrane fractionations were used for the analysis of organelle markers. Free inorganic phosphate was determined using the colorimetric assay from Cytoskeleton.
The GenBank accession number for the partial 1,265-bp mRNA for Sus of tobacco pollen tubes is EU148354.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. 1-D DyeChrome double immunoblot analysis of different pollen tube extracts.
Supplemental Figure S2. Distribution (and relative control) of Sus at the onset of germination in tobacco pollen tubes.
Supplementary Material
Acknowledgments
We express our gratitude to Dr. Yong-Ling Ruan (Commonwealth Scientific and Industrial Research Organization Plant Industry, Canberra, Australia) for critically revising and commenting on the manuscript and to Dr. Massimo Nepi, Mr. Massimo Guarnieri, and Dr. Daniele Artesi (Dipartimento Scienze Ambientali, University of Siena) for the analysis of sugars by HPLC. We also thank Prof. Wolfgang Werr (Institut für Entwicklungsbiologie, Koln University, Koln, Germany) for kindly providing the antibodies against Sus. We are grateful to the gardeners of the Dipartimento di Scienze Ambientali of Siena University for growing plants and for pollen collection.
This work was supported by grants under the Human Frontier Science Program (http://www.hfsp.org/) and the Research Athenaeum Program of Siena University.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Giampiero Cai (cai@unisi.it).
The online version of this article contains Web-only data.
References
- Albrecht G, Mustroph A (2003) Localization of sucrose synthase in wheat roots: Increased in situ activity of sucrose synthase correlates with cell wall thickening by cellulose deposition under hypoxia. Planta 217 252–260 [DOI] [PubMed] [Google Scholar]
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92 9353–9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Barnes WS, Bedinger P (2002) 2,6-Dichlorobenzonitrile, a cellulose biosynthesis inhibitor, affects morphology and structural integrity of petunia and lily pollen tubes. J Plant Physiol 159 61–67 [Google Scholar]
- Anguenot R, Nguyen-Quoc B, Yelle S, Michaud D (2006) Protein phosphorylation and membrane association of sucrose synthase in developing tomato fruit. Plant Physiol Biochem 44 294–300 [DOI] [PubMed] [Google Scholar]
- Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT (2003) The sucrose transporter gene family in rice. Plant Cell Physiol 44 223–232 [DOI] [PubMed] [Google Scholar]
- Baroja-Fernandez E, Munoz FJ, Saikusa T, Rodriguez-Lopez M, Akazawa T, Pozueta-Romero J (2003) Sucrose synthase catalyzes the de novo production of ADPglucose linked to starch biosynthesis in heterotrophic tissues of plants. Plant Cell Physiol 44 500–509 [DOI] [PubMed] [Google Scholar]
- Bieniawska Z, Paul Barratt DH, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49 810–828 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50 859–865 [Google Scholar]
- Brownfield L, Ford K, Doblin MS, Newbigin E, Read S, Bacic A (2007) Proteomic and biochemical evidence links the callose synthase in Nicotiana alata pollen tubes to the product of the NaGSL1 gene. Plant J 52 147–156 [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Vergara CE, Carpita NC (1999) The mechanism of synthesis of a mixed-linkage (1→3),(1→4)beta-D-glucan in maize: evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiol 120 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Moscatelli A, Del Casino C, Chevrier V, Mazzi M, Tiezzi A, Cresti M (1996) The anti-centrosome mAb 6C6 reacts with a plasma membrane-associated polypeptide of 77-kDa from the Nicotiana tabacum pollen tubes. Protoplasma 190 68–78 [Google Scholar]
- Cai G, Ovidi E, Romagnoli S, Vantard M, Cresti M, Tiezzi A (2005) Identification and characterization of plasma membrane proteins that bind to microtubules in pollen tubes and generative cells of tobacco. Plant Cell Physiol 46 563–578 [DOI] [PubMed] [Google Scholar]
- Cardenas L, McKenna ST, Kunkel JG, Hepler PK (2006) NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol 142 1460–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS (1996) Evidence for plasma membrane-associated forms of sucrose synthase in maize. Mol Gen Genet 252 303–310 [DOI] [PubMed] [Google Scholar]
- Chourey PS, Taliercio EW, Carlson SJ, Ruan YL (1998) Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol Gen Genet 259 88–96 [DOI] [PubMed] [Google Scholar]
- Cresti M, VanWent JL (1976) Callose deposition and plug formation in Petunia pollen tubes in situ. Planta 133 35–40 [DOI] [PubMed] [Google Scholar]
- Dejardin A, Rochat C, Maugenest S, Boutin JP (1997) Purification, characterization and physiological role of sucrose synthase in the pea seed coat (Pisum sativum L.). Planta 201 128–137 [DOI] [PubMed] [Google Scholar]
- Del Casino C, Li Y, Moscatelli A, Scali M, Tiezzi A, Cresti M (1993) Distribution of microtubules during the growth of tobacco pollen tubes. Biol Cell 79 125–132 [Google Scholar]
- Derksen J, Knuiman B, Hoedemaekers K, Guyon A, Bonhomme S, Pierson ES (2002) Growth and cellular organization of Arabidopsis pollen tubes in vitro. Sex Plant Reprod 15 133–139 [Google Scholar]
- Derksen J, Li YQ, Knuiman B, Geurts H (1999) The wall of Pinus sylvestris L. pollen tubes. Protoplasma 208 26–36 [Google Scholar]
- Duncan KA, Huber SC (2007) Sucrose synthase oligomerization and F-actin association are regulated by sucrose concentration and phosphorylation. Plant Cell Physiol 48 1612–1623 [DOI] [PubMed] [Google Scholar]
- Echt CS, Chourey PS (1985) A comparison of two sucrose synthatase isozyme from normal and shrunken-1 maize. Plant Physiol 79 530–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria E, Gonzalez P (2003) Evidence for a tonoplast-associated form of sucrose synthase and its potential involvement in sucrose mobilization from the vacuole. J Exp Bot 54 1407–1414 [DOI] [PubMed] [Google Scholar]
- Ferguson C, Teeri TT, Siika-aho M, Read SM, Bacic A (1998) Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206 452–460 [Google Scholar]
- Geitmann A, Steer M (2006) The architecture and properties of the pollen tube cell wall. In R Malho, ed, The Pollen Tube. Springer-Verlag, Berlin, pp 177–200
- Geitmann A, Wojciechowicz K, Cresti M (1996) Inhibition of intracellular pectin transport in pollen tubes by monensin, brefeldin A and cytochalasin D. Bot Acta 109 373–381 [Google Scholar]
- Gross KC, Pharr DM (1982) Cucumber fruit sucrose synthetase isozymes. Phytochemistry 21 1241–1244 [Google Scholar]
- Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47 29–51 [PubMed] [Google Scholar]
- Hardin SC, Duncan KA, Huber SC (2006) Determination of structural requirements and probable regulatory effectors for membrane association of maize sucrose synthase 1. Plant Physiol 141 1106–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin SC, Winter H, Huber SC (2004) Phosphorylation of the amino terminus of maize sucrose synthase in relation to membrane association and enzyme activity. Plant Physiol 134 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein M, Starlinger P (1989) Tissue- and cell-specific expression of the two sucrose synthase isoenzymes in developing maize kernels. Mol Gen Genet 215 441–446 [Google Scholar]
- Herrero M, Dickinson HG (1980) Pollen tube growth following compatible and incompatible intraspecific pollinations in Petunia hybrida. Planta 148 217–221 [DOI] [PubMed] [Google Scholar]
- Hong Z, Zhang Z, Olson JM, Verma DP (2001) A novel UDP-glucose transferase is part of the callose synthase complex and interacts with phragmoplastin at the forming cell plate. Plant Cell 13 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DY, Wang AY (1998) Purification and characterization of sucrose synthase isozymes from etiolated rice seedlings. Biochem Mol Biol Int 46 107–113 [DOI] [PubMed] [Google Scholar]
- Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, Levin JZ, Preuss D (2004) Arabidopsis hapless mutations define essential gametophytic functions. Genetics 168 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer EE, Rodriguez RL (1992) Metabolic regulation of rice alpha-amylase and sucrose synthase genes in planta. Plant J 2 517–523 [PubMed] [Google Scholar]
- Komina O, Zhou Y, Sarath G, Chollet R (2002) In vivo and in vitro phosphorylation of membrane and soluble forms of soybean nodule sucrose synthase. Plant Physiol 129 1664–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T, Ohmiya Y, Hayashi T (2004) Evidence that sucrose loaded into the phloem of a poplar leaf is used directly by sucrose synthase associated with various beta-glucan synthases in the stem. Plant Physiol 134 1146–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf DL, Bisgrove SR, Hable WE (1998) Cytoskeletal control of polar growth in plant cells. Curr Opin Cell Biol 10 117–122 [DOI] [PubMed] [Google Scholar]
- Labarca C, Loewus F (1972) The nutritional role of pistil exudate in pollen tube wall formation in Lilium longiflorum. I. Utilization of injected stigmatic exudate. Plant Physiol 50 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Lennon KA, Lord EM (2000) In vivo pollen tube cell of Arabidopsis thaliana. I. Tube cell cytoplasm and wall. Protoplasma 214 45–56 [Google Scholar]
- Li H, Bacic A, Read SM (1999) Role of a callose synthase zymogen in regulating wall deposition in pollen tubes of Nicotiana alata Link et Otto. Planta 208 528–538 [Google Scholar]
- Li YQ, Croes AF, Linskens HF (1983) Cell-wall proteins in pollen and roots of Lilium longiflorum: extraction and partial characterization. Planta 158 422–427 [DOI] [PubMed] [Google Scholar]
- Li YQ, Faleri C, Geitmann A, Zhang HQ, Cresti M (1995) Immunogold localization of arabinogalactan proteins, unesterified and esterified pectins in pollen grains and pollen tubes of Nicotiana tabacum L. Protoplasma 189 26–36 [Google Scholar]
- Lovy-Wheeler A, Cardenas L, Kunkel JG, Hepler PK (2006) Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motil Cytoskeleton 64 217–232 [DOI] [PubMed] [Google Scholar]
- Matic S, Akerlund HE, Everitt E, Widell S (2004) Sucrose synthase isoforms in cultured tobacco cells. Plant Physiol Biochem 42 299–306 [DOI] [PubMed] [Google Scholar]
- Munoz P, Norambuena L, Orellana A (1996) Evidence for a UDP-glucose transporter in Golgi apparatus-derived vesicles from pea and its possible role in polysaccharide biosynthesis. Plant Physiol 112 1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenfuhr A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kelley JC (1955) External carbohydrates in growth and respiration of pollen tubes in vitro. Am J Bot 42 322–327 [Google Scholar]
- Pacini E, Guarnieri M, Nepi M (2006) Pollen carbohydrates and water content during development, presentation, and dispersal: a short review. Protoplasma 228 73–77 [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A (2005) More than a leak sealant: the mechanical properties of callose in pollen tubes. Plant Physiol 137 274–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ (2001) Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci 114 2685–2695 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Hinz G (2001) Organelle isolation. In C Hawes, B Satiat-Jeunemaitre, eds, Plant Cell Biology. Oxford University Press, Oxford, pp 295–323
- Romer U, Schrader H, Gunther N, Nettelstroth N, Frommer WB, Elling L (2004) Expression, purification and characterization of recombinant sucrose synthase 1 from Solanum tuberosum L. for carbohydrate engineering. J Biotechnol 107 135–149 [DOI] [PubMed] [Google Scholar]
- Ross HA, Davies HV (1992) Purification and characterization of sucrose synthase from the cotyledons of Vicia faba L. Plant Physiol 100 1008–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y (2007) Rapid cell expansion and cellulose synthesis regulated by plasmodesmata and sugar: insights from the single-celled cotton fibre. Funct Plant Biol 34 1–10 [DOI] [PubMed] [Google Scholar]
- Ruan YL, Chourey PS (1998) A fiberless seed mutation in cotton is associated with lack of fiber cell initiation in ovule epidermis and alterations in sucrose synthase expression and carbon partitioning in developing seeds. Plant Physiol 118 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Chourey PS, Delmer DP, Perez-Grau L (1997) The differential expression of sucrose synthase in relation to diverse patterns of carbon partitioning in developing cotton seed. Plant Physiol 115 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Llewellyn DJ, Furbank RT (2003) Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell 15 952–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten TL, Knuiman B (1993) Brefeldin A effects on tobacco pollen tubes. Eur J Cell Biol 61 247–255 [PubMed] [Google Scholar]
- Salnikov VV, Grimson MJ, Delmer DP, Haigler CH (2001) Sucrose synthase localizes to cellulose synthesis sites in tracheary elements. Phytochemistry 57 823–833 [DOI] [PubMed] [Google Scholar]
- Salnikov VV, Grimson MJ, Seagull RW, Haigler CH (2003) Localization of sucrose synthase and callose in freeze-substituted secondary-wall-stage cotton fibers. Protoplasma 221 175–184 [DOI] [PubMed] [Google Scholar]
- Schafer WE, Rohwer JM, Botha FC (2005) Partial purification and characterisation of sucrose synthase in sugarcane. J Plant Physiol 162 11–20 [DOI] [PubMed] [Google Scholar]
- Schlupmann H, Bacic A, Read SM (1994) Uridine diphosphate glucose metabolism and callose synthesis in cultured pollen tubes of Nicotiana alata Link et Otto. Plant Physiol 105 659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebkova V, Unger C, Hardegger M, Sturm A (1995) Biochemical, physiological, and molecular characterization of sucrose synthase from Daucus carota. Plant Physiol 108 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase K, Shiratake K, Mori H, Yamaki S (2002) Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit. Physiol Plant 114 21–26 [DOI] [PubMed] [Google Scholar]
- Tanase K, Yamaki S (2000) Purification and characterization of two sucrose synthase isoforms from Japanese pear fruit. Plant Cell Physiol 41 408–414 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Bacic A, Harris PJ, Read SM (1998) Membrane fractionation and enrichment of callose synthase from pollen tubes of Nicotiana alata Link et Otto. Planta 205 380–388 [DOI] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1997) Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett 420 151–155 [DOI] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1998) Identification of sucrose synthase as an actin-binding protein. FEBS Lett 430 205–208 [DOI] [PubMed] [Google Scholar]
- Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol 35 253–289 [DOI] [PubMed] [Google Scholar]
- Wittich PE, Willemse MTM (1999) Sucrose utilization during ovule and seed development of Gasteria verrucosa (Mill.) H. Duval as monitored by sucrose synthase and invertase localization. Protoplasma 208 136–148 [Google Scholar]
- Yen SF, Su JC, Sung HY (1994) Purification and characterization of rice sucrose synthase isozymes. Biochem Mol Biol Int 34 613–620 [PubMed] [Google Scholar]
- Ylstra B, Garrido D, Busscher J, van Tunen AJ (1998) Hexose transport in growing Petunia pollen tubes and characterization of a pollen-specific, putative monosaccharide transporter. Plant Physiol 118 297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L, Muller M, Munnik T (2006) Hydrodynamics and cell volume oscillations in the pollen tube apical region are integral components of the biomechanics of Nicotiana tabacum pollen tube growth. Cell Biochem Biophys 46 209–232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.