Abstract
The fate of sucrose (Suc) supplied via the phloem to developing oilseed rape (Brassica napus) seeds has been investigated by supplying [14C]Suc to pedicels of detached, developing siliques. The method gives high, sustained rates of lipid synthesis in developing embryos within the silique comparable with those on the intact plant. At very early developmental stages (3 d after anthesis), the liquid fraction that occupies most of the interior of the seed has a very high hexose-to-Suc ratio and [14C]Suc entering the seeds is rapidly converted to hexoses. Between 3 and 12 d after anthesis, the hexose-to-Suc ratio of the liquid fraction of the seed remains high, but the fraction of [14C]Suc converted to hexose falls dramatically. Instead, most of the [14C]Suc entering the seed is rapidly converted to products in the growing embryo. These data, together with light and nuclear magnetic resonance microscopy, reveal complex compartmentation of sugar metabolism and transport within the seed during development. The bulk of the sugar in the liquid fraction of the seed is probably contained within the central vacuole of the endosperm. This sugar is not in contact with the embryo and is not on the path taken by carbon from the phloem to the embryo. These findings have important implications for the sugar switch model of embryo development and for understanding the relationship between the embryo and the surrounding endosperm.
Most of the carbon for seed growth is supplied as Suc, imported from the maternal tissues of the plant. In oilseed rape seeds (Brassica napus) and legumes, two phases of Suc utilization by the growing seed can be recognized. During the first phase, much of the Suc entering the seed is converted to hexoses, which accumulate in the endosperm that occupies most of the internal volume of the seed. This coincides with a rapid increase in seed volume. The hydrolysis of Suc to hexoses probably contributes to this increase, by providing a high level of osmoticum to drive water uptake by the seed. In this first phase, the embryo occupies a small fraction of the internal volume of the seed and its growth is primarily by cell division. During the second phase, Suc rather than hexose becomes the major sugar in the seed. This change coincides with a fall in acid invertase activity in the seed. Embryo cell division ceases and cell expansion accelerates. Storage product synthesis in the embryo becomes the major fate for Suc entering the seed (legumes [Weber et al., 1995; Borisjuk et al., 2003]; oilseeds [Baud et al., 2002; Hill et al., 2003]).
The coincidence between the fall in the hexose-to-Suc ratio in the seed and the switch from cell division to expansion and storage product accumulation in the embryo has led to the suggestion that the two are causally related (Weber et al., 1996a, 1997, 2005; Borisjuk et al., 1998, 2002). Weber and colleagues propose that the high hexose-to-Suc ratio in the seed early in development promotes cell division and represses storage product accumulation in the embryo. As the hexose-to-Suc ratio falls, cell division ceases and the capacity for storage product synthesis increases. There is ample evidence that Suc and hexoses can indeed modulate expression of genes encoding enzymes involved in carbohydrate metabolism and in the biosynthesis of hormones implicated in seed development (León and Sheen, 2003; Koch, 2004; Rook et al., 2006). Further support for this sugar switch hypothesis for seeds comes from two sources. First, studies of bean seeds (Vicia spp.) show that the developmental switch from cell division to storage product accumulation in the embryo is tightly spatially correlated with hexose-to-Suc ratios through development (Borisjuk et al., 1998, 2002). Second, manipulation of the seed hexose-to-Suc ratio influences embryo development in transgenic plants and in tissue culture. In tissue culture, hexose-based medium represses storage protein and starch synthesis in embryos and maintains cell division rates, whereas Suc-based medium promotes starch accumulation and the transcription of storage protein genes (Weber et al., 1996a, 1996b).
Studies of developing oilseeds have generally supported the ideas outlined above in that the hexose-to-Suc ratio of the seed falls as the transition to storage product accumulation occurs in the embryo. However, two studies suggest that this is not a causal relationship. First, expression of yeast (Saccharomyces cerevisiae) invertase in the cytosol of the embryo and endosperm of developing tobacco (Nicotiana tabacum) seeds abolished the rise in Suc levels during development, resulting in a very high ratio of hexoses to Suc in the mature seed, but had no effect on storage product composition (Tomlinson et al., 2004). Second, the observed changes in sugar composition and invertase activity in the liquid fraction of developing oilseed rape seeds could not be explained by a simple model in which the embryo takes up sugars from this fraction (Hill et al., 2003). Hill et al. (2003) suggested that the major hexose pool generated by invertase in the liquid fraction of the seed may not be in direct contact with the embryo.
Little is known about the pathway via which carbon from Suc reaches the developing embryo in oilseeds (Baud et al., 2005). In both Arabidopsis (Arabidopsis thaliana) and oilseed rape, the phloem extends through the funiculus that links the developing seed to the silique, then terminates in a region of the integument lying below the chalazal endosperm. Symplastic unloading of Suc into the outer integument of the seed occurs at this point (Stadler et al., 2005), but there is little information about the routes by which it subsequently reaches the embryo.
The aim of our work was to investigate whether the change during development in the hexose-to-Suc ratio of oilseed rape seeds constitutes a sugar switch that triggers the transition from cell division to expansion and storage product accumulation in the embryo. Most previous metabolic studies of oilseed rape seeds have assumed that the embryo is bathed in the endosperm liquid that occupies most of the interior of the seed during the first phase of development (Fowler and Downey, 1970; King et al., 1997; Schwender and Ohlrogge, 2002, 2003), hence that the composition of this liquid reflects the environment surrounding the embryo. To test this idea, we fed [14C]Suc to whole, detached siliques and then analyzed separately the rates of conversion of Suc to hexoses in the liquid fraction of seeds and to storage products in embryos. The results indicate that the environment surrounding the seed has a different sugar content from the bulk endosperm liquid. They are not consistent with the idea that the embryo receives carbon from the bulk endosperm liquid or that the sugar composition of this liquid has a direct influence on embryo development. They reveal important complexity in the compartmentation of sugars in the developing seed: We used imaging techniques to study this compartmentation further. The implications of these results for understanding of development and nutrition of oilseed rape embryos are discussed.
RESULTS
Relationship between Sugar Composition of the Endosperm and Embryo Development
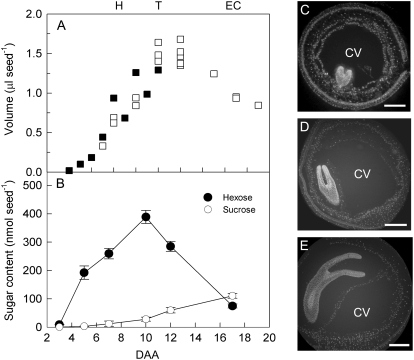
We first established the relationship between embryo development up to the early-cotyledon stage and the concentration of sugars in the liquid fraction of the seed as a baseline for 14C feeding experiments. The liquid fraction was collected by puncturing the integuments immediately after harvesting and opening siliques (Hill et al., 2003). The volume of the seed occupied by the liquid fraction was estimated both gravimetrically by difference, following seed dissection, and by estimating internal volume based on seed size and integument thickness (from light microscope images). The two methods gave similar results (Fig. 1A).
Figure 1.
Sugar content in the liquid fraction of the seed during early development. A, Total volume of the liquid fraction was calculated gravimetrically (white symbols) or by estimation of internal volume (black symbols) based on seed size and integument thickness (taken from micrographs as in C–E). The heart, torpedo, and early-cotyledon stages of embryo development are indicated by H, T, and EC. B, Suc (○) and hexose (•) content per seed was derived from measurements of sugar content per microliter of liquid fraction multiplied by the volumes in A. C to E, Light micrographs of wax-embedded sections of seeds containing embryos at the heart (C), torpedo (D), and early-cotyledon stages (E). Scale bars = 300, 300, and 400 μm, respectively.
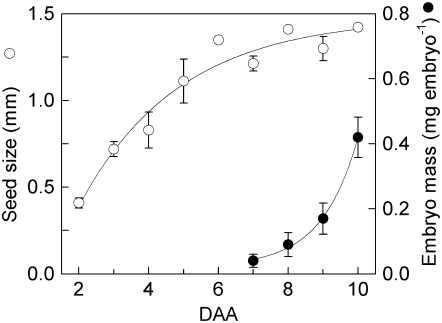
The volume of the liquid fraction increased to a maximum of 1.6 μL at 10 to 12 d after anthesis (DAA; Fig. 1A), at which point the seed had reached its maximal size (Fig. 2) and embryos were at the early-torpedo stage (Fig. 1D). Embryo mass increased exponentially up to this point (Fig. 2). After this point, the volume of the liquid fraction declined as the mass of the embryo continued to increase so that, by 19 DAA, it was less than 1.0 μL (Fig. 1A).
Figure 2.
Seed and embryo size during early development. Seed size was measured as the longest diameter of the seed on three seeds from the center of a silique. Embryo weight was estimated from the average fresh weight of all embryos in a silique. Values are the mean ± se of measurements on three replicate siliques.
Up to 10 DAA, the hexose content of the liquid fraction rose in parallel with the increase in volume so that the hexose concentration remained relatively constant. After 10 DAA, the hexose concentration fell from about 250 mm to less than 100 mm by 15 DAA. Suc concentration was very low until 7 DAA and then it rose so that, by 17 DAA, it was greater than the hexose concentration (Fig. 1B). These data are generally consistent with previous reports on changes in sugar concentrations through development in oilseed rape seeds (King et al., 1997; Schwender and Ohlrogge, 2002; Hill et al., 2003).
Development of a Method for Supplying [14C]Suc to Intact Siliques
To study the fate of Suc in intact, attached seeds, we supplied [14C]Suc to the pedicels of intact siliques. Siliques were severed from racemes at the base of the pedicel and incubated in the light with the severed ends of the pedicels in medium containing 20 mm [14C]Suc. The 14C content of seeds was measured after incubation by opening siliques and immediately puncturing seed integuments to obtain the liquid fraction or rapidly dissecting seeds to obtain embryos.
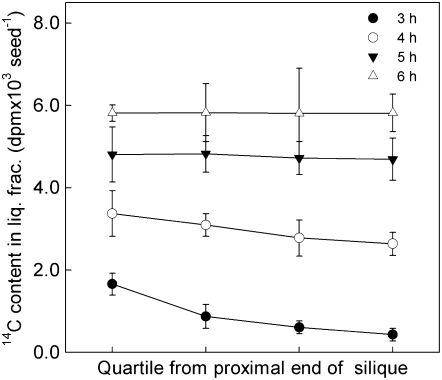
The 14C content of the liquid fraction of the seed was dependent upon the incubation time and in the first 3 to 4 h it was also dependent on the position of seeds in the silique (Fig. 3). After 4 h, differences in 14C content of the liquid fraction between seeds at the proximal and distal ends of the silique were negligible. All subsequent experiments used incubations of 4 h or more and each sample consisted of all of the harvestable seeds from a single silique.
Figure 3.
Appearance of 14C in the liquid fraction of seeds during incubation of excised siliques with [14C]Suc. Siliques containing seeds with embryos at the torpedo stage of development (11 DAA) were supplied with [14C]Suc and the amount of 14C was measured in the liquid fraction (liq. frac.) after 4 to 7 h of incubation. Individual siliques were divided into quarters lengthwise and seeds were pooled from each quarter to give four samples. Values are the mean ± se of measurements made on seeds from three siliques.
To discover whether excision and incubation of siliques affect the sugar composition of the liquid fraction, we compared seeds containing embryos at the heart, torpedo, or early-cotyledon stage after incubations of either 4 or 7 h. At all three stages and both incubation times, the ratio of hexose to Suc and the ratio of Glc to Fru in the liquid fraction were the same as for seeds taken from freshly harvested, unincubated siliques at the same developmental stage (data not shown).
We next investigated whether metabolism by embryos in seeds in excised siliques resembled that in seeds on the intact plant by measuring rates of fatty acid synthesis. Siliques containing embryos at three different developmental stages representing the start, middle, and end of the main phase of lipid accumulation (starting at approximately mid-cotyledon stage, equivalent to stages B, B/C, and C defined by Eastmond and Rawsthorne (2000)] were supplied for 12 h in the light or dark with [14C]Suc, then the total 14C content of the embryo, the specific radioactivity of the Glc-6-P pool within the embryo, and the 14C content of the fatty acid fraction of the embryo were determined. The 14C content of the embryos was low up to 6 or 7 h and increased linearly thereafter. At 12 h, fatty acids typically accounted for 40% to 50% of the 14C in embryos (data not shown). The absolute rate of fatty acid synthesis was calculated from measurements of the amount of 14C in fatty acids and the specific radioactivity of Glc-6-P based on the assumption that the acetyl-CoA and glycerol used for fatty acid synthesis are derived from these pools. A representative dataset is shown in Supplemental Table S1. Typically, the mean rate of fatty acid synthesis across the three developmental stages was 100 nmol acetate units h−1 embryo−1 in the light and 25 nmol acetate units h−1 embryo−1 in the dark. These rates correspond well to those we calculated previously for lipid synthesis in vivo using the same cultivar and growth conditions: the maximal rate was 0.36 nmol malonyl CoA synthesized min−1 mg−1 fresh weight (Kang et al., 1994), corresponding to 77 nmol acetate units h−1 embryo−1.
Collectively, these data indicate that the incubation conditions do not perturb seed metabolism; hence, the fate of [14C]Suc in seeds in excised siliques is likely to reflect its fate in seeds on intact plants.
Suc Metabolism in the Liquid Fraction of Developing Seeds
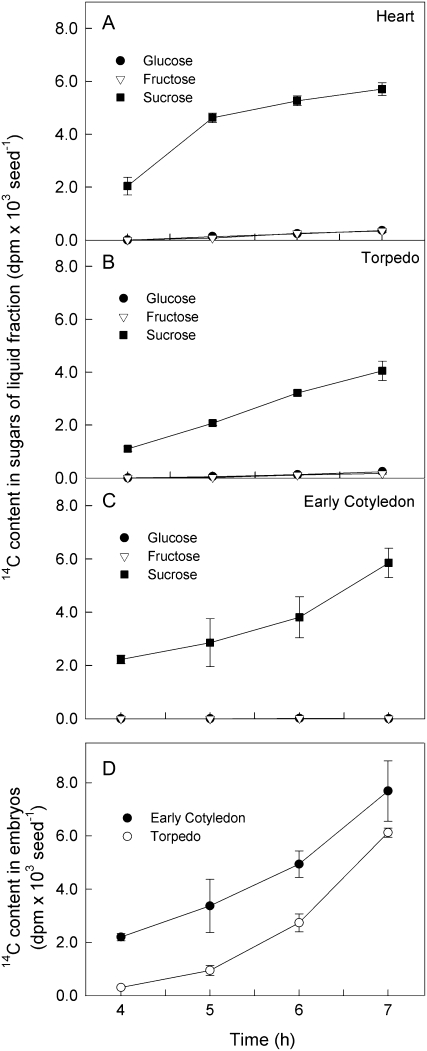
To investigate the metabolism of Suc entering developing seeds, we monitored the fate of 14C from [14C]Suc supplied to siliques containing embryos at the heart, torpedo, or early-cotyledon stages. In the liquid fraction, most of the 14C was in Suc after 7-h incubation at all three developmental stages (Fig. 4). 14C appeared in Glc and Fru only very slowly. At the end of the incubations, [14C]hexoses accounted for less than 10% of the total labeled sugars in the heart- and torpedo-stage seeds and an even smaller percentage in the early-cotyledon-stage seeds.
Figure 4.
Metabolism of [14C]Suc by seeds during incubation of excised siliques with [14C]Suc. The 14C in Suc, Fru, and Glc was measured in the liquid fraction after 4 to 7 h of incubation. Experiments were carried out at heart (8 DAA; A), torpedo (11 DAA; B), and early-cotyledon (17 DAA; C) stages of development. At the two later stages, the amount of 14C in embryos (D) was also determined. Values are the mean ± se of measurements made on seeds from four siliques.
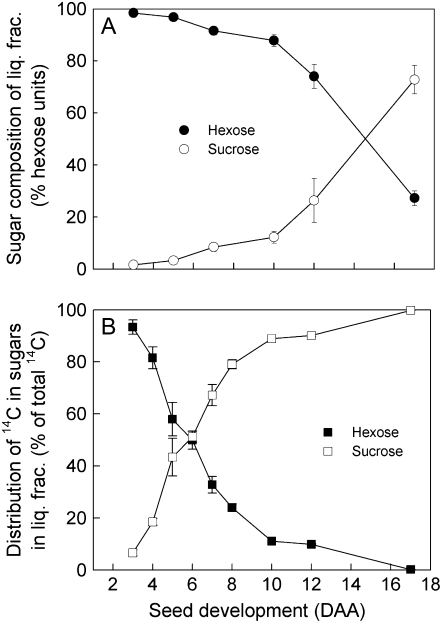
The pattern of labeling in these experiments showed that, although the liquid fraction has a high hexose-to-Suc ratio, particularly at the heart and torpedo stage, Suc entering this fraction is converted to hexoses only very slowly at all three developmental stages. To investigate further the relationship between Suc metabolism and sugar content in the liquid fraction, we repeated this experiment over a wider developmental window (from 3–17 DAA) and measured both the fate of [14C]Suc and the sugar composition in the liquid fraction. During this time, the total molarity of sugars (Glc + Fru + Suc) in the liquid fraction did not change significantly and averaged 330 ± 12 mm (mean ± se of data from Fig. 5). As in the experiment shown in Figure 1, hexoses were the major sugars during most of this period, with Suc becoming the major sugar only in the last few days (Fig. 5A).
Figure 5.
Effect of development on sugar metabolism in seeds. Excised siliques were supplied with [14C]Suc and the liquid fraction was collected, by puncture, after 7 h. The proportion of Suc, Glc, and Fru (as hexose units) in this fraction was measured (A), as was the distribution of 14C in these sugars (B). Each value is the mean ± se of measurements made on at least three replicate siliques.
Analysis of the 14C in sugars in the liquid fraction of seeds revealed the same developmental trend as the sugar composition, but with a different temporal pattern (Fig. 5B). At the earliest stage analyzed (3 DAA) [14C]Suc entering the liquid fraction of the seed was almost all hydrolyzed to Glc and Fru. After a 7-h incubation, over 90% of the 14C was in Glc and Fru, in equimolar amounts. The remainder was in Suc. Over the next 7 d of development, the proportion of Suc that was converted to Glc and Fru in the liquid fraction during the incubation declined. By 10 DAA, only about 10% of the Suc entering the liquid fraction was converted to hexose and this declined to almost undetectable levels by 17 DAA. No labeled compounds other than Suc, Glc, and Fru were detected. The results for 8 to 17 DAA (heart to early-cotyledon stages) are essentially identical to those shown in Figure 4 from a separate batch of plants, demonstrating the reproducibility of results from the silique incubation technique.
The 14C Content of Embryos
At both the torpedo and the early-cotyledon stage, the 14C content of the embryo increased more rapidly than that of the liquid fraction during the latter 3 h of the incubation (compare Fig. 4, B and C with Fig. 4D). The 14C content of the embryos at 7 h exceeded that of the liquid fraction even though the embryo had a much smaller volume than the liquid fraction. We were not able to make robust measurements of the 14C content of embryos at the heart stage.
Compartmentation in the Endosperm
The results described above are not compatible with a simple model in which the embryo receives sugars directly from a compartment represented by the bulk liquid fraction of the seed. The patterns of labeling are best explained by models in which the liquid fraction consists of more than one sugar-containing compartment (see “Discussion”). Published studies of the anatomy of seeds of oilseed rape and the closely related species Arabidopsis provide the following picture of the compartmentation of the interior of the seed (Van Lammeren et al., 1996; Brown et al., 1999, 2003; Sørensen et al., 2002). Throughout the developmental period under study, most of the internal volume of the seed is occupied by the endosperm. At the earliest stages of development, this consists of a large central vacuole surrounded by a thin layer of cytoplasm containing nuclei (the syncytium). The embryo is surrounded by the syncytium, except where it is attached to the embryo sac wall by the suspensor. Cellularization of the endosperm then occurs from the embryo sac wall inward. The cellular endosperm and the embryo occupy an increasing proportion of the internal volume of the seed as development progresses and the proportion of the volume occupied by the central vacuole decreases. Importantly, the embryo is at all stages contained within an apoplastic space inside the endosperm that is separate from the central vacuole of the endosperm.
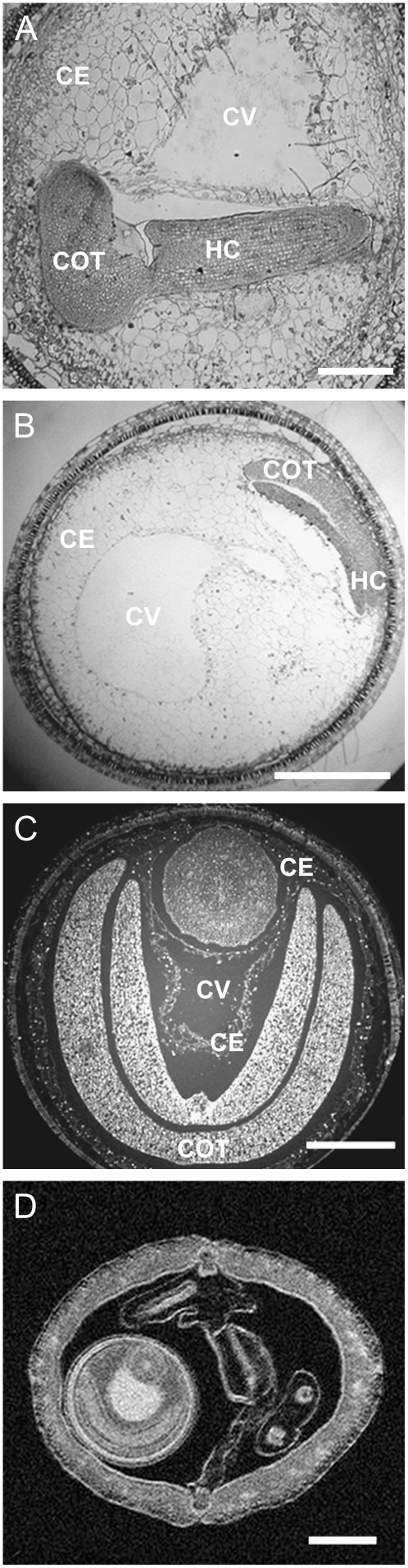
Our own light micrographs (Figs. 1 and 6) and serial sectioning (data not shown) confirm the existence of an apoplastic space around the embryo that is separate from the central vacuole. A layer of cytoplasm separates these two compartments at early stages of development (Fig. 1) and by the cotyledon stage one or more layers of endosperm cells separates the compartments (Fig. 6, A and B). To provide independent evidence about the existence of these separate compartments, free of possible artefacts introduced by fixation and dehydration, we imaged whole, freshly harvested siliques using 1H-NMR spectroscopy (Fig. 6D). No preparation of the material is required for this technique. Compartments differing in the relaxation times of the water protons show different degrees of brightness (Köckenberger, 2001a, 2001b). The reason for different relaxation times could be a difference in water mobility due to different local viscosity or different water exchange with water bound to membrane structures. The NMR images closely mirror the light micrographs from the same developmental stage (compare Fig. 6, C and D). The central vacuole is distinct and it is clear that it is separated from the embryo. Bright rings around the embryo are consistent with the existence of an apoplastic space with a relatively mobile water content surrounding the embryo (Fig. 6D).
Figure 6.
Compartmentation in developing seeds. A and B, Sections of resin-embedded seeds containing embryos at the late-torpedo to early-cotyledon stage of development (15 DAA), stained with Toluidine blue. Cellularized endosperm (CE) dividing the central vacuole (CV) from embryo-containing compartments is clearly visible. In both examples, serial sectioning confirmed the separation of these two compartments (data not shown). Scale bars = 200 and 500 μm, respectively. C, Section through a wax-embedded seed at mid-cotyledon stage (20 DAA) stained with DAPI. The embryo is seen as two U-shaped, folded cotyledons (COT) and the hypocotyl (HC). The central vacuole and the embryo are in separate compartments that are separated by a thin layer of cellularized endosperm. Scale bar = 500 μm. D, NMR microscopy image of an intact, living silique containing a seed at a similar stage of development to C. The brightness of the image reflects relative water mobility. The large bright area in the center of the seed is the central vacuole. A fine halo of brightness, distinct from the central vacuole, surrounds the embryo. Scale bar = 1 mm.
DISCUSSION
Summary of Sugar Content and Metabolism in Relation to Seed Development up to 17 DAA
In the first 6 DAA, the seed expands rapidly and the embryo represents only a tiny fraction of the total volume (pre-heart stage). The interior of the seed is occupied primarily by the central vacuole of the endosperm, surrounded by a thin layer of cytoplasm (Fig. 1C). Most of the sugar in the liquid fraction of the seed is hexose (Fig. 1B) and invertase activity in this fraction is high (Hill et al., 2003). During the first 3 DAA, Suc entering the liquid fraction of the seed during silique incubations is almost completely hydrolyzed to hexoses. As development progresses up to 6 DAA, the proportion of Suc hydrolyzed during incubations falls to about 50% (Fig. 5).
Between 7 and 10 DAA, seed expansion stops and rapid embryo growth begins. Development from the heart-to-torpedo stage occurs and embryo volume increases about 40-fold (Fig. 2). The central vacuole still occupies most of the interior of the seed, but the volume of cellularized endosperm increases (Figs. 1D and 6). Over 90% of the sugar in the liquid fraction of the seed is hexose throughout this period (Figs. 1B and 5). However, the proportion of Suc converted to hexose during silique incubations decreases to only 10% by 10 DAA (torpedo stage; Fig. 5). At this stage, 14C from Suc appears in embryos after about 4 h of incubation and the 14C content of embryos increases rapidly up to 7 h so that the amount of 14C in the embryo exceeds that in the liquid fraction of the seed (Fig. 4).
Between 11 and 17 DAA, embryo expansion continues and the volume of the central vacuole declines (Fig. 1E). In the liquid fraction, hexoses fall from 90% of the total sugar to about 30% at 17 DAA (Fig. 5). Throughout this period, 14C entering the liquid fraction of the seed during silique incubations remains as Suc rather than being converted to hexose (Fig. 5). At 17 DAA, as at 10 DAA, the 14C content in the embryo increases more rapidly and reaches a higher level during the incubation than the 14C content of the liquid fraction of the seed (Fig. 4).
Microscopic and 1H-NMR spectroscopic studies (Fig. 6) reveal that the liquid fraction that exudes when the seed is punctured probably contains material from three main compartments. These are the central vacuole of the endosperm, the apoplastic space surrounding the embryo, and the cytoplasmic/cellular part of the endosperm. The liquid fraction may also contain material from the apoplastic space that lies between the endosperm and the integuments. The relative volumes of these compartments change through development, with the vacuole representing a progressively smaller fraction as development proceeds.
The Embryo Is Not in Contact with the Major Sugar-Containing Compartment of the Seed
The developmental switch from cell division to cell expansion and storage product accumulation in the embryo roughly coincides with the large fall in the hexose-to-Suc ratio in the liquid fraction of the seed. Up to the torpedo stage, when embryo cells are dividing, the hexose-to-Suc ratio is very high. Beyond this point, when embryo growth is primarily by expansion and lipid accumulation accelerates, the hexose-to-Suc ratio in the liquid fraction falls rapidly. However, this change in sugar composition in the liquid fraction is unlikely to be directly responsible for the developmental switch in the embryo. Consistent with our modeling of sugar concentrations in the seed (Hill et al., 2003), our data show that the embryo is not in contact with the major sugar-containing compartment of the seed and receives its carbon via a route that does not involve this compartment. The reasoning behind these conclusions is as follows.
In incubations of siliques containing embryos at the torpedo and early-cotyledon stages, Suc entering the seed entered a compartment in the liquid fraction in which it was not rapidly hydrolyzed to hexose. The specific activity of the bulk hexose pool in the liquid fraction of the seed was vanishingly small, whereas that of the bulk Suc pool was high. The 14C content of the embryo increased rapidly during the later stages of the incubations; hence, 14C reaching the embryo cannot have passed through the bulk hexose pool.
The Main Sugar-Containing Compartment Is Probably the Central Vacuole of the Endosperm
We suggest that the main hexose-containing compartment of the liquid fraction of the seed is the central vacuole of the endosperm, which accounts for most of the internal volume of the seed during its expansion. The high and fairly constant molarity of hexose in the liquid fraction up to 10 DAA suggests that formation of hexose from Suc (presumably via a vacuolar acid invertase) is important in vacuole filling and hence seed expansion over this period. After 11 DAA the vacuole shrinks as the endosperm cellularizes and the embryo grows. Consistent with the idea that the vacuole is the main location of hexose in the liquid fraction, the hexose content of the seed falls as the vacuole volume decreases. The hexoses lost from the seed after 11 DAA may be available to the embryo, but they can account for only a small fraction of its growth. Net hexose loss from the seed between 10 and 17 DAA is about 55 μg per seed, whereas the embryo increases in weight from 400 to 1,000 μg over this period.
Early in seed development, the endosperm consists of the central vacuole and a syncytium that lines the embryo sac. The syncytium has a tiny volume compared with the central vacuole. The rapid conversion of [14C]Suc entering the seed into hexose in very young seeds can thus be explained by assuming that Suc entering the syncytium from the integuments transfers very rapidly across this thin layer into the central vacuole, where it is immediately hydrolyzed by acid invertase. Three factors may contribute to the decrease through development in the rate at which Suc entering the seed is converted to hexose. First, as development proceeds, the endosperm cellularizes. Both the distance and the volume of cellular material between the integuments and the central vacuole increase as a result; hence, the time taken for 14C entering the seed to reach the central vacuole will increase. The impact of cellularization on Suc transport is likely to be particularly striking at the chalazal pole of the endosperm, which lies directly between the end of the phloem and the central vacuole. Up to the heart stage, this endosperm is a syncytium penetrated by extensive ramifications of the central vacuole, giving a very large area of tonoplast over which sugars entering the endosperm may be transferred into the central vacuole. At the heart stage, the chalazal endosperm cellularizes, and these vacuolar ramifications are lost (Brown et al., 2004). Second, the cellularization of the endosperm and the expansion of the embryo provide increasingly strong sinks for carbon from Suc, hence a decreasing proportion of the carbon from Suc entering the seed will be partitioned to the vacuole. Third, invertase activity in the liquid fraction declines rapidly after the first 8 DAA (Hill et al., 2003). Suc entering the central vacuole may be converted less rapidly to hexose than at the earliest stages of development when invertase activity is high.
The Path of Sugar from the Phloem to the Embryo Is Likely to Involve Several Distinct Compartments
There are several possible routes via which Suc could move from the phloem to the embryo. It may traverse the integuments from the phloem to the base of the micropylar endosperm adjacent to the radicle of the embryo and then traverse the apoplastic space between the integuments and the endosperm, the tissue of the micropylar endosperm, and the apoplastic space surrounding the embryo. At early developmental stages when the suspensor is probably symplastically contiguous with the embryo and is attached to the embryo sac wall (Van Lammeren et al., 1996; Stadler et al., 2005), Suc may reach the embryo directly from the integuments via the suspensor. Alternatively, Suc may reach the apoplastic space surrounding the embryo by moving from the integuments to the chalazal endosperm, then diffusing via the syncytium/cellularized endosperm to the micropylar region. The much faster appearance of 14C in the embryo than in the central vacuole at the torpedo and early-cotyledon stages tends to suggest the former routes rather than the longer path via the chalazal endosperm.
There is support for the idea that metabolic events important to embryo nutrition occur in the region of the endosperm adjacent to the embryo (the micropylar endosperm or embryo-surrounding region [ESR]). In Arabidopsis, expression of the AtSUC5 Suc transporter is confined to this region at early stages of development (up to the torpedo stage) and is necessary for normal rates of embryo development (Baud et al., 2005). Thus, the embryo may receive much of its carbon in the form of Suc transported out of immediately adjacent endosperm cells into the apoplastic space via AtSUC5 up to this stage. Beyond the torpedo stage, AtSUC5 is not essential for normal development: Suc must be delivered by an alternative transporter or a different route. In maize (Zea mays), the apoplastic invertase inhibitor ZmINVINH1 is localized to the ESR. This protein can inhibit cell wall invertases from maize endosperm and is itself inhibited by Suc (Bate et al., 2004). It could thus influence the ratio of Suc to hexose in the apoplastic space surrounding the embryo. As well as a potential role in embryo nutrition, the ESR is also thought to be important in embryo development. For example, the extracellular subtilisin-like protease ALE1 is strongly expressed in the ESR in Arabidopsis seeds and is required for normal development of the embryo cuticle (Tanaka et al., 2001). Other chemical changes in the immediate environment of the embryo may also influence its metabolism and development. For example, the oxygen concentration in developing oilseed rape, sunflower (Helianthus annuus), and legume seeds changes through development and is sufficiently low to have a major impact on metabolism and, in the case of oilseed rape and sunflower, to restrict storage product synthesis (Rolletschek et al., 2002, 2007; Vigeolas et al., 2003). Growth of reproductive structures of Brassicaceae at subambient oxygen concentrations affects both the composition and the developmental progression of the embryo, perhaps in part through effects on phytohormone synthesis (Porterfield et al., 1999; Ramonell et al., 2002). Thus, it seems likely that a network of metabolic and developmental signals in the immediately adjacent endosperm play a significant role in embryo development and nutrition.
Taken as a whole, our data do not support the view that the embryo exists initially in a high-hexose environment and later in a high-Suc environment. Although the composition of the liquid fraction of the seed changes from high hexose to high Suc as development proceeds, most of the hexose is probably in the central vacuole of the endosperm, which is neither in direct contact with the embryo nor part of the pathway by which sugar from the phloem reaches the embryo. Instead, as discussed above, the young embryo may receive its sugar via localized transport of Suc from the integuments into the micropylar endosperm and thence into the embryo apoplastic space. Some hydrolysis of Suc may occur along this pathway, perhaps via invertases in the apoplastic spaces between the outer and the inner integuments (there are few symplastic connections between the two integuments in Arabidopsis seeds [Stadler et al., 2005]) or the inner integument and the endosperm or the endosperm and the embryo. Thus, the possibility that the space around the embryo has a high concentration of hexose at some stages of development cannot be ruled out.
The current model of the effect of sugars on embryo development is based largely on studies of legume seeds. It uses a simple representation of the interior of the seed in which Suc is unloaded from the inner layer of the testa into the endospermal cavity, then directly taken up by the embryo (Weber et al., 1997; Wobus and Weber, 1999; Borisjuk et al., 2003). It is clear that the situation in the interior of oilseed rape seeds is much more complex. There are at least five distinct compartments within which Suc transport and metabolism can occur (the apoplastic space between the integuments and the endosperm, the central vacuole of the endosperm, the apoplastic space between the endosperm and the embryo, the embryo itself, and the syncytium/cellularized portion of endosperm). A full understanding of the carbon nutrition and sugar environment of the embryo through development in oilseed rape and Arabidopsis seeds will require analysis of sugar metabolism and transport in each of these compartments. This will be a major task: the Arabidopsis genome encodes at least 21 enzymes capable of metabolizing Suc (six Suc synthases [Bieniawska et al., 2007], nine neutral invertases [Ji et al., 2005], and eight acid invertases [Link et al., 2004]), at least two invertase inhibitors (Link et al., 2004), nine Suc transporters (Schulze et al., 2003), and substantial numbers of hexose transporters (Sherson et al., 2003; Aluri and Büttner, 2007). Most of these proteins are expressed in developing seeds.
It remains possible that the transition from cell division to expansion and storage product accumulation is triggered by a sugar switch not in the immediate environment of the embryo but within the embryo itself. In legume seeds, a switch from a hexose- to a Suc-dominated sugar pool occurs in both the bulk liquid of the seed and within the tissues of the embryo during development (Borisjuk et al., 1998, 2002). Direct and accurate measurement of the in vivo sugar composition of early-stage oilseed rape embryos is rendered impossible by the very high levels of hexose contamination that occur during their isolation (M. Pike, unpublished data). However, our previous measurements of enzymes of Suc catabolism in oilseed rape embryos through development provide no particular support for the idea that the embryo accumulates hexoses at early developmental stages. We showed that Suc synthase activity is comparable with or higher than invertase activity from early-torpedo stage onward and that fructokinase activity exceeds glucokinase activity (Hill et al., 2003). Metabolism of Suc via Suc synthase and fructokinase produces a cytosolic hexose phosphate pool for respiration and biosynthesis rather than an accumulation of free hexose.
Our results are relevant to studies in which carbon fluxes in developing oilseed embryos are analyzed in a culture system. In devising incubation medium for embryo culture, researchers have assumed that the embryo in vivo is surrounded by a medium with the composition of the bulk liquid fraction of the seed (Schwender and Ohlrogge, 2002). Our results suggest that, particularly during the early stages of development, the embryo is almost certainly exposed to a much lower ratio of hexose to Suc than that found in the bulk endosperm. However, at later stages, when the major proportion of the oil is synthesized, the sugar concentration of the bulk endosperm (dominated by Suc) is more likely to resemble that to which the embryo is exposed in vivo.
MATERIALS AND METHODS
Plant Growth
Plants of oilseed rape (Brassica napus) ‘Topas’ were grown in a glasshouse with supplementary illumination from September to March (Kang and Rawsthorne, 1994).
Gravimetric Estimation of Volume
Seeds were weighed and then opened with a razor blade under a dissecting microscope. The integuments were blotted with fine absorbent tissue and immediately reweighed. The volume of the liquid fraction was taken as the difference between these weights.
Incubation of Detached Siliques
Siliques were excised from plants at the base of the pedicel adjacent to the stem using a razor blade under Murashige and Skoog medium containing 20 mm Suc (the incubation medium). This medium was chosen because it is commonly used for tissue culture and was therefore likely to minimize deterioration of the severed pedicel. Pedicels were inserted through holes in the lid into 1.5-mL plastic tubes containing 0.5 mL of incubation medium and [U-14C]Suc (30 MBq mol−1; Amersham International). Incubations were at approximately 25°C with a gentle continuous airflow and 300 μmol quanta photosynthetically active radiation m−2 s−1 from a horizontally mounted halogen lamp. A heat sink consisting of a clear Perspex box with a continuous flow of cold water was placed between the lamp and the siliques.
To measure 14C in the liquid fraction of the seed, siliques were opened along the replum with a razor blade. Seeds were punctured with a hypodermic needle and the liquid exuding under pressure was collected in a 10-μL pipette tip (Hill et al., 2003) and added to 30 μL 80% ethanol (v/v) in 50 mm HEPES, pH 7.0, at 80°C. As many seeds as possible from a single silique were pooled to give a single sample per silique for analysis.
To measure the incorporation of 14C into products within the embryo, embryos were dissected from seeds and washed with ice-cold incubation medium, without Suc, on filter paper in a Buchner filtration device. Embryos from a single silique were frozen in liquid nitrogen in preweighed plastic tubes prior to further analysis.
Sugar Content and 14C Distribution in the Liquid Fraction of Seeds
Sugars in the ethanolic fractions from punctured seeds were assayed enzymatically (Hill et al., 2003). The distribution of 14C in sugars in these fractions was determined by thin-layer chromatography and phosphor imager analysis as described by Chia et al. (2005), except that the solvent system was 10:3.5:2.5 (v/v) ethyl acetate:pyridine:water, and plates were run five times successively.
14C Content of Embryos
Frozen embryos were homogenized in tissue solubilizer prior to liquid scintillation counting.
Light Microscopy
Seeds were fixed by brief vacuum infiltration and then overnight incubation in 2.5% (v/v) glutaraldehyde in 0.05 m sodium cacodylate, pH 7.3. After three 10-min washes in 0.05 m sodium cacodylate, seeds were postfixed in 1% (w/v) OsO4 in 0.05 m sodium cacodylate for 1 h at room temperature. After three further 10-min washes in water, seeds were dehydrated in the following ethanol concentrations (30%, 50%, 70%, and 95% [v/v] for 20 min each, then 100% for 1 h). For resin embedding, samples were infiltrated with LR White resin (London Resin Company) by successive changes of resin-ethanol mixes over 24 h at room temperature (1:1 for 1 h, 2:1 for 1 h, 3:1 for 1 h, then three changes of 100% resin for 1, 16, and 6 h), then transferred into capsules of fresh resin at 60°C for 16 h. Wax embedding was according to Vitha et al. (2000). Sectioning was with a glass knife using an ultramicrotome. Sections of approximately 0.5-μm thickness were dried onto glass slides and stained with 0.5% (w/v) Toluidine blue “O” in 0.5% (w/v) borax.
NMR Microscopy
Siliques from well-watered plants were sealed in small tubes and inserted into standard 4-mm resonators of a 400-MHz (9.4 T) spectrometer equipped with a BRUKER microimaging accessory and gradient amplifiers capable of producing 60-A shaped gradient pulses. Images were calculated from conventional three-dimensional spin echo experiments. Usually 256 × 256 complex data points were acquired in the transverse plane with a field of view of 6 mm × 6 mm and with 128 increments covering a section of 12 mm in longitudinal direction, thus resulting in 128 images with a nominal spatial resolution of 23 μm × 23 μm and a slice thickness of 47 μm. Typically, the repetition time TR between separate excitations was 0.8 s, the echo time TE was 4.5 ms, and two averages were acquired for each increment of the phase encoding gradients.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Results from a representative experiment to measure the rate of fatty acid synthesis in detached siliques incubated with Suc.
Supplementary Material
Acknowledgments
We thank Kay Denyer, Matthew Hills, and Stanislav Kopriva for valuable discussions. Seed of oilseed rape ‘Topas’ was a kind gift of Dalgety Agriculture (Essex, UK).
This work was supported by a Core Strategic Grant from the UK Biotechnology and Biological Sciences Research Council to the John Innes Centre and, at the University of Nottingham, by a University Research Fellowship of the Royal Society to W.K.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alison M. Smith (alison.smith@bbsrc.ac.uk).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aluri S, Büttner M (2007) Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering. Proc Natl Acad Sci USA 104 2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate NJ, Niu X, Wang Y, Reimann KS, Helentjaris TG (2004) An invertase inhibitor from maize localizes to the embryo surrounding region during early kernel development. Plant Physiol 134 246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Boutin JP, Miquel M, Lepiniec L, Rochat C (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype Ws. Plant Physiol Biochem 40 151–160 [Google Scholar]
- Baud S, Wuillème S, Lemoine R, Kronenberger J, Caboche M, Lepiniec L, Rochat C (2005) The AtSUC5 sucrose transporter specifically expressed in the endosperm is involved in early seed development in Arabidopsis. Plant J 43 824–836 [DOI] [PubMed] [Google Scholar]
- Bieniawska Z, Barratt DHP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49 810–828 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Wobus U, Weber H (2003) Differentiation of legume cotyledons as related to metabolic gradients and assimilate transport into seeds. J Exp Bot 54 503–512 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Walenta S, Rolletschek H, Mueller-Klieser W, Wobus U, Weber H (2002) Spatial analysis of plant metabolism: sucrose imaging within Vicia faba cotyledons reveals specific developmental patterns. Plant J 29 521–530 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Walenta S, Weber H, Mueller-Klieser W, Wobus U (1998) High resolution histographical mapping of glucose concentrations in developing cotyledons of Vicia faba in relation to mitotic activity and storage processes: glucose as a possible metabolic trigger. Plant J 15 583–591 [Google Scholar]
- Brown RC, Lemmon BE, Nguyen H (2003) Events during the first four rounds of mitosis establish three developmental domains in the syncytial endosperm of Arabidopsis. Protoplasma 222 167–174 [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Nguyen H (2004) Comparative anatomy of the chalazal endosperm cyst in seeds of the Brassicaceae. Bot J Linn Soc 144 375–394 [Google Scholar]
- Brown RC, Lemmon BE, Nguyen H, Olsen OA (1999) Development of endosperm in Arabidopsis thaliana. Sex Plant Reprod 12 32–42 [Google Scholar]
- Chia TYP, Pike MJ, Rawsthorne S (2005) Storage oil breakdown during embryo development of Brassica napus (L.). J Exp Bot 56 1285–1296 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, Rawsthorne S (2000) Coordinate changes in carbon partitioning and plastidial metabolism during the development of oilseed rape embryos. Plant Physiol 122 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DB, Downey RK (1970) Lipid and morphological changes in developing rapeseed, Brassica napus. Can J Plant Sci 50 233–247 [Google Scholar]
- Hill LM, Morley-Smith ER, Rawsthorne S (2003) Metabolism of sugars in the endosperm of developing seeds of oilseed rape. Plant Physiol 131 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Van den Ende W, Van Laere A, Cheng S, Bennett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60 615–634 [DOI] [PubMed] [Google Scholar]
- Kang F, Rawsthorne S (1994) Starch and fatty acid synthesis in plastids from developing embryos of oilseed rape (Brassica napus L.). Plant J 6 795–805 [Google Scholar]
- Kang F, Ridout CJ, Morgan CL, Rawsthorne S (1994) The activity of acetyl-CoA carboxylase is not correlated with the rate of lipid synthesis during development of oilseed rape (Brassica napus L.) embryos. Planta 193 320–325 [Google Scholar]
- King SP, Lunn JE, Furbank RT (1997) Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiol 114 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7 235–246 [DOI] [PubMed] [Google Scholar]
- Köckenberger W (2001. a) Functional imaging of plants by magnetic resonance experiments. Trends Plant Sci 6 286–292 [DOI] [PubMed] [Google Scholar]
- Köckenberger W (2001. b) Nuclear magnetic resonance micro-imaging in the investigation of plant cell metabolism. J Exp Bot 52 641–652 [DOI] [PubMed] [Google Scholar]
- León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8 110–116 [DOI] [PubMed] [Google Scholar]
- Link M, Rausch T, Greiner S (2004) In Arabidopsis thaliana, the invertase inhibitors AtC/VIF1 and 2 exhibit distinct target enzyme specificities and expression profiles. FEBS Lett 573 105–109 [DOI] [PubMed] [Google Scholar]
- Porterfield DM, Juang A, Smith PJS, Crispi ML, Musgrave ME (1999) Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot 77 1439–1446 [PubMed] [Google Scholar]
- Ramonell KM, McClure G, Musgrave ME (2002) Oxygen control of ethylene biosynthesis during seed development in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ 25 793–801 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53 1099–1107 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Sánchez-García A, Gotor C, Romero LC, Martínez-Rivas JM, Mancha M (2007) Temperature-dependent endogenous oxygen concentration regulates microsomal oleate desaturase in developing sunflower seeds. J Exp Bot 58 3171–3181 [DOI] [PubMed] [Google Scholar]
- Rook F, Hadingham SA, Li Y, Bevan MW (2006) Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ 29 426–434 [DOI] [PubMed] [Google Scholar]
- Schulze WX, Reinders A, Ward J, Lalonde S, Frommer WB (2003) Interactions between co-expressed Arabidopsis sucrose transporters in the split-ubiquitin system. BMC Biochem 4 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender J, Ohlrogge JB (2002) Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiol 130 347–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender J, Ohlrogge JB (2003) A flux model of glycolysis and the oxidative pentosephosphate pathway in developing Brassica napus embryos. J Biol Chem 278 29442–29453 [DOI] [PubMed] [Google Scholar]
- Sherson SM, Alford HL, Forbes SM, Wallace G, Smith SM (2003) Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J Exp Bot 54 525–531 [DOI] [PubMed] [Google Scholar]
- Sørensen MB, Mayer U, Lukowitz W, Robert H, Chambrier P, Jürgens G, Somerville C, Lepiniec L, Berger F (2002) Cellularisation in the endosperm of Arabidopsis is coupled to mitosis and shares multiple components with cytokinesis. Development 129 5567–5576 [DOI] [PubMed] [Google Scholar]
- Stadler R, Lauterbach C, Sauer N (2005) Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol 139 701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y (2001) A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128 4681–4689 [DOI] [PubMed] [Google Scholar]
- Tomlinson KL, McHugh S, Labbe H, Grainger JL, James LE, Pomeroy KM, Mullin JW, Miller SS, Dennis DT, Miki BLA (2004) Evidence that the hexose-to-sucrose ratio does not control the switch to storage product accumulation in oilseeds: analysis of tobacco seed development and effects of overexpressing apoplastic invertase. J Exp Bot 55 2291–2303 [DOI] [PubMed] [Google Scholar]
- Van Lammeren AAM, Kieft H, Ma F, Van Veenendaal WLH (1996) Light microscopical study of endosperm formation in Brassica napus L. Acta Soc Bot Polon 65 267–272 [Google Scholar]
- Vigeolas H, van Dongen JT, Waldeck P, Hühn D, Geigenberger P (2003) Lipid storage metabolism is limited by the prevailing low oxygen concentrations within developing seeds of oilseed rape. Plant Physiol 133 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, Baluška F, Jasik J, Volkmann D, Barlow P (2000) Steedman's wax for F-actin visualization. In CJ Staiger, ed, Actin: A Dynamic Framework for Multiple Plant Cell Functions. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 619–636
- Weber H, Borisjuk L, Heim U, Buchner P, Wobus U (1995) Seed coat-associated invertases of fava bean control both unloading and storage functions: cloning of cDNAs and cell type-specific expression. Plant Cell 7 1835–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U (1996. a) Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J 10 823–834 [Google Scholar]
- Weber H, Borisjuk L, Wobus U (1997) Sugar import and metabolism during seed development. Trends Plant Sci 2 169–174 [Google Scholar]
- Weber H, Borisjuk L, Wobus U (2005) Molecular physiology of legume seed development. Annu Rev Plant Biol 56 253–279 [DOI] [PubMed] [Google Scholar]
- Weber H, Buchner P, Borisjuk L, Wobus U (1996. b) Sucrose metabolism during cotyledon development of Vicia faba L. is controlled by the concerted action of both sucrose-phosphate synthase and sucrose synthase: expression patterns, metabolic regulation and implications for seed development. Plant J 9 841–850 [DOI] [PubMed] [Google Scholar]
- Wobus U, Weber H (1999) Sugars as signal molecules in plant seed development. Biol Chem 380 937–944 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.