Innate immune receptors in plants detect the presence of microbial pathogens and trigger defense responses to terminate or restrict pathogen growth. The molecular mechanisms of receptor-mediated nonself recognition and subsequent intracellular signaling pathways have received much attention in the past. Less is known about the cellular mechanisms that contribute to the execution of immune responses. Recent studies revealed the existence of a secretory machinery that becomes engaged in the execution of extracellular immune responses. At least two vesicle-associated and SNARE protein-mediated exocytosis pathways appear to drive focal and/or nondirectional secretion of antimicrobial cocktails comprising proteins, small molecules, and cell wall building blocks into the apoplastic space. Both pathways have additional functions in plant development and might have been coopted for immune responses. Bacteria and fungi appear to have evolved counterdefense molecules that intercept the secretion machinery by blocking vesicle formation from intracellular membranes. Independently from this, plant plasma membrane ATP-binding cassette-type (ABC) transporters act in parallel defense pathways and serve as efflux pumps for the targeted delivery of antimicrobials and/or agents promoting chemical cross-linking of plant cell wall polymers.
All multicellular eukaryotic organisms are exposed to the danger of microbial pathogens. Unlike animals, plants lack specialized and mobile immune cells to engulf and dismantle microbial intruders. Plants resist microbial attack using elaborate nonself surveillance systems consisting of a repertoire of cell surface and intracellular immune sensors. These receptors detect the presence of parasites and trigger powerful immune responses (for review, see Jones and Dangl, 2006). Microbial parasites have evolved diverse strategies to enter hosts for nutrient retrieval and multiplication. It is conceivable that plants have invented execution mechanisms for immune responses that are fine tuned to microbial entry routes. For example, most microbial pathogens such as bacteria and fungi first come into contact with plant epidermal cells (Fig. 1). In contrast to many bacterial pathogens of vertebrates that multiply inside host cells, the majority of plant pathogenic bacteria remain in the intercellular space (apoplast) after entering the interior of plant organs through natural openings such as stomata (Fig. 1). Similarly, some fungal parasites such as Cladosporium fulvum infect plant leaves by growth of fungal hyphae in the apoplastic space (Thomma et al., 2005). Thus, plants must have evolved defense mechanisms to terminate extracellular colonization attempts. Even for parasitic fungi that enter host cells through direct penetration of the plant cell wall, pathogenesis is often terminated prior to invasive growth at the host cell periphery (Johnson et al. 1982; Hoogkamp et al., 1998), indicating the existence of apoplastic defense.
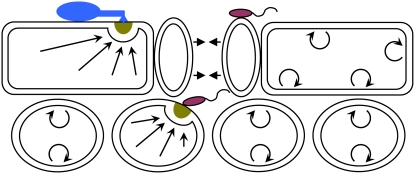
Figure 1.
Dual function of secretory pathways in plant immune responses and plant development. Leaf cross section showing epidermal and mesophyll cell layers. Single plant cells target immune responses to contact sites of fungal or bacterial pathogens, leading to papilla formation (dark yellow semicircle) in the paramural space. The indicated flagellate bacteria (red) enter the leaf interior through stomata and remain in the apoplastic space for multiplication. The indicated fungal parasite (blue) attempts to penetrate the plant cell wall to access nutrients from an epidermal cell. Stomatal closure (denoted by short arrows) is one resistance mechanism against bacterial ingress (Melotto et al., 2006). Known vesicle-associated and SNARE protein-mediated exocytosis pathways drive focal (long straight arrows) as well as nondirectional (semicircles with arrowheads) secretion of antimicrobial cargo into the apoplastic space. A second function of these secretory pathways in plant development might involve constitutive nondirectional secretion of cell wall building blocks and cell wall modifying enzymes during cell growth.
The exterior of plant cells such as the cell wall or leaf cuticle can be considered as preformed physical barriers to invasive pathogens. Indeed, cell wall-penetrating fungal species in general secrete hydrolytic enzymes to degrade the cuticles and cell walls of plant cells and frequently form specialized pad-like infection structures, called appressoria, to initiate entry into plant cells (Kolattukudy, 1985; Mendgen and Deising, 1993; Knogge, 1996). In many cases plant cells respond to such entry attempts by de novo cell wall biosynthesis and by local deposition of the newly synthesized cell wall material, including the (1→3)-β-d-glucan callose, into the paramural space between the cell wall and the plasma membrane (Aist, 1976; Belanger et al., 2002). This localized thickening is called a papilla and is thought to represent an inducible reinforcement of the cell wall against pathogen entry. However, callose-rich papillae are also found at contact sites between plant cells and bacteria that do not penetrate plant cell walls (Bestwick et al., 1995), thereby pointing to additional more complex roles in plant defense.
One remarkable feature of pathogen-induced papillae is their spatial confinement to microbial contact sites (typically representing a small circular area at the periphery of a single plant cell), suggesting directional delivery of cell wall precursors and/or cell wall-synthesizing enzymes to incipient infection sites. Focal redistribution of the actin cytoskeleton and the congregation of cellular organelles such as the nucleus, Golgi, the endoplasmic reticulum (ER), peroxisomes, and vesicle-like structures beneath microbial contact sites suggest that papilla formation may represent one outcome of a complex microbe-induced cell polarization process (Bestwick et al., 1997; Schmelzer, 2002; Takemoto et al., 2003; Koh et al., 2005). Pharmacological or genetic disruption of actin polymerization in plant cells impaired papilla formation and concomitantly enhanced fungal entry rates (Kobayashi et al., 1997; Schultheiss et al., 2003; Opalski et al., 2005; Miklis et al., 2007), thereby revealing the relevance of host cytoskeleton dynamics for immune responses at the cell periphery. Of particular note, the bacterial effector AvrPtoB of Pseudomonas syringae, which is delivered inside plant cells during extracellular bacterial colonization to suppress plant immune responses through its E3 ubiquitin ligase activity (Abramovitch et al., 2006; Rosebrock et al., 2007), is sufficient to inhibit papilla formation and disease resistance to P. syringae pv phaseolicola when expressed alone in transgenic plants (de Torres et al., 2006). However, specific depletion of papillary callose in Arabidopsis (Arabidopsis thaliana) pmr4/gsl5 mutant plants, which lack one of 12 callose synthase-like family members, enhances salicylic acid (SA)-dependent resistance responses to the powdery mildew fungi Erysiphe cruciferarum and Golovinomyces orontii (Jacobs et al., 2003; Nishimura et al., 2003). This hints at the existence of an unexpected negative feedback system between particular papilla components and plant defense signaling.
Studies in Arabidopsis and Nicotiana benthamiana have provided deeper insights in the ability of plant cells to focally direct extracellular immune responses and revealed several components of the underlying secretion machinery. The dicotyledonous plant Arabidopsis is immune to the grass powdery mildew fungus Blumeria graminis f. sp. hordei, which in nature colonizes leaves of the host barley (Hordeum vulgare). Several immuno-compromised Arabidopsis mutants that allow increased fungal entry into leaf epidermal cells were isolated. The corresponding wild-type genes (designated PEN for penetration resistance) encode secretion- associated and efflux-associated proteins: plasma membrane-resident PEN1 syntaxin, peroxisome-associated PEN2 glycosyl hydrolase, and the plasma membrane-resident PEN3 ABC transporter (Collins et al., 2003; Lipka et al., 2005; Stein et al., 2006). PEN1 has been shown to act genetically in a separate pathway from PEN2 and PEN3 (Lipka et al., 2005; Stein et al., 2006).
PEN1 syntaxin is a member of the superfamily of soluble SNAREs that play a pivotal role in driving intracellular vesicle fusion (Jahn and Scheller, 2006). PEN1 was found to interact and form ternary SNARE complexes with synaptosomal-associated protein of 33 kD (SNAP33) and vesicle-associated membrane protein (VAMP) 721 or 722 in vitro as well as in vivo (Kwon et al., 2008a). Of the 14 Arabidopsis VAMP family members, VAMP721 is most closely related to VAMP722, sharing 96% amino acid sequence identity. Such ternary SNARE complexes are essential for various exocytosis-dependent processes in yeast, insects, and animals (Jahn and Scheller, 2006). Plants expressing GFP-tagged VAMP722 display mobile fluorescent endomembrane compartments (Kwon et al., 2008a). Focal concentration of these at fungal entry sites together with the integral plasma membrane protein PEN1 (Assaad et al., 2004; Bhat et al., 2005) and the peripheral membrane protein SNAP33 suggests that directed ternary SNARE complex-mediated exocytosis and the release of cargo from VAMP721/722 vesicle-like compartments represents one mechanism of preinvasion resistance (Kwon et al., 2008a; Fig. 2). It will be interesting to examine whether other Arabidopsis syntaxins such as KNOLLE and SYP22, which are essential for cytokinesis and gravitropism, respectively, function through the formation of similar ternary SNARE complexes, involving different SNAP and/or VAMP family members (Lukowitz et al., 1996; Yano et al., 2003). In Arabidopsis vegetative tissues, a multitude of SNARE genes is expressed, encoding four plasma membrane-resident syntaxins, two SNAPs, and five VAMPs (Uemura et al., 2004; Lipka et al., 2007). Conceptually, this provides ample opportunity for the formation of distinct ternary SNARE complexes and thereby specific secretory pathways acting in diverse plant biological processes. For example, gene disruptions of SNAP33 or VAMP721 plus VAMP722 are lethal (Heese et al., 2001; Kwon et al., 2008a), whereas pen1 syp122 dwarfed plants can still reproduce (Assaad et al., 2004; Zhang et al., 2007). This suggests that SNAP33 and VAMP721/722 can cooperate with plasma membrane syntaxins other than PEN1 and SYP122 in plant growth and development (see below).
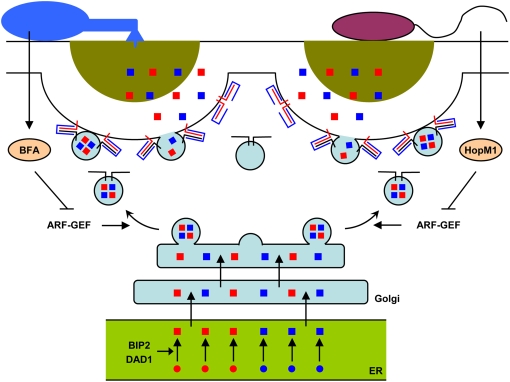
Figure 2.
Targeted vesicle-associated and SNARE protein-mediated secretion at microbial contact sites. The indicated vesicles are targeted to the plant plasma membrane beneath a bacterial (right) or fungal (left) contact site along polarized actin cables (not shown). Vesicles are thought to be loaded with cargo derived from the ER/Golgi protein secretory pathway. Vesicles contain constitutive molecules required for the maintenance of plasma membrane and cell wall functions (blue squares) and/or transport additional pathogen-inducible antimicrobial molecules (red squares), leading to the formation of a papillary cell wall scaffold containing toxic cocktails (dark yellow semicircle). At contact sites with powdery mildew fungi (left), vesicle cargo is discharged subsequent to ternary SNARE complex formation involving plasma membrane-resident PEN1 syntaxin (red line), SNAP33 (blue line), and endomembrane-resident VAMP721/722 (black line). At contact sites with bacterial pathogens (right), resistance responses require plasma membrane-resident SYP132 syntaxin (red line), and possibly SNAP33 (blue line) as well as yet unknown VAMPs (black line). During immune responses to bacteria, expansion of the protein-folding machinery including BIP2 and DAD1 is believed to reflect an increased cellular requirement to build up secreted PR proteins in the ER (green). One defense molecule secreted in a SYP132-dependent manner is the pathogen-inducible PR-1 protein. Fungi and bacteria can interfere with the host secretory pathway by inhibiting ARF-GEFs, which regulate vesicle formation, using effector molecules such as brefeldin A (BFA) or HopM1, respectively.
It has long been thought that plant cells secrete toxic cocktails of antimicrobial compounds and proteins in response to pathogen attack that kill or limit the growth of parasites. Pathogenesis-related (PR) proteins were among one of the first described induced responses of plants to microbial attack and are now known to encompass at least 17 evolutionarily conserved families (for review, see van Loon et al., 2006). Although PRs are believed to function as executioners of immune responses due to their antimicrobial activities in vitro, including lysis of microbial cell walls, restriction of microbial growth was only rarely observed when single members were constitutively expressed in transgenic plants (for review, see van Loon et al., 2006). It is possible that in planta different PR proteins act together and that the concerted action of several family members is needed for effective antimicrobial activity (Melchers and Stuiver, 2000). Although a subset of PR proteins are targeted to vacuoles, acidic forms such as PR-1 typically contain an N-terminal signal peptide for secretion. Silencing of N. benthamiana SYP132 (NbSYP132), the ortholog of the plasma membrane-resident Arabidopsis SYP132 (AtSYP132), resulted in a delay of extracellular PR-1 accumulation after inoculation with P. syringae pv tabacina (Pstab), although the PR protein was produced and detectable at high levels in tissue after extraction of apoplastic proteins (Kalde et al., 2007). Because other tested defense-associated responses were unaffected in the NbSYP132-silenced plants, this suggests a specific role for the NbSYP132 syntaxin in PR-1 secretion. Importantly, NbSYP132 silencing not only compromised resistance (R) gene-mediated (race-specific) resistance to Pstab expressing the avirulence protein AvrPto, but also impaired basal resistance to disarmed P. syringae pv tomato hrpA− mutants that cannot deliver effectors into plant cells by the type III secretion system, and blocked chemically induced systemic acquired resistance (SAR) to Pstab (Kalde et al., 2007). Thus, NbSYP132 appears to be a convergence point of these three forms of disease resistance to this bacterial pathogen. Silencing of NbSYP121, the N. benthamiana ortholog of AtPEN1, neither affected R gene-triggered resistance nor PR-1 secretion (Kalde et al., 2007). Therefore, if NbSYP132/AtSYP132 act like AtPEN1 (AtSYP121) through the formation of ternary SNARE complexes, then VAMP family members other than VAMP721/VAMP722 must be engaged in PR-1 secretion (Fig. 2). This suggests the existence of at least two distinct vesicle-associated immune response pathways and predicts that if VAMP721/VAMP722 export PR proteins, these must be different from PR-1 (Fig. 2).
The establishment of SAR in Arabidopsis requires accumulation of the endogenous signaling molecule SA as well as nuclear activity of the master regulator NPR1 and is tightly correlated with the accumulation of PR proteins (Dong, 2004). One target of nuclear NPR1 are TGA transcription factors, which in turn are directly involved in the regulation of PR genes (Zhang et al., 1999; Despres et al., 2000; Zhang et al., 2003; for review, see Fobert and Despres, 2005). NPR1 also directly and coordinately up-regulates the expression of genes in the protein secretory pathway, probably involving other, as yet unknown, transcription factor(s) that act through a common cis-acting element present in the promoters of these genes (Wang et al., 2005). In mutant plants lacking the ER-resident secretion-related proteins BIP2, SEC61α, or DAD1, SAR-mediated growth restriction of virulent P. syringae pv. maculicula was impaired and correlated with reduced PR-1 secretion (Wang et al., 2005). It is thought that the coordinated up-regulation of the secretory machinery during SAR reflects the need for cells to accommodate a massive buildup of secreted PR proteins. This finding together with the requirement of NbSYP132 for SAR and PR-1 secretion in N. benthamiana (Kalde et al., 2007) suggests a molecular process in which NPR1 expands the ER secretory capacity and, through a parallel pathway, also triggers biosynthesis of PR proteins. A subset of the latter, including PR-1, might then become destined for export along the ER/Golgi route, reaching the apoplast by vesicle-mediated transport and SYP132 syntaxin-dependent discharge at the plasma membrane (Fig. 2).
Although the above framework for gene activation and cellular routing of PR-1 secretion in SAR is now genetically underpinned, the causal relationship between extracellular PR protein accumulation and microbial growth restriction during SAR remains unclear. Ironically, although the PR-1 protein family is strongly conserved in all tested plant species and high-resolution structures of select family members exist, their in planta function is the least understood of all conserved PR families. However, PR-1 homologs in nonplant species might exhibit Ser protease activity, raising the possibility that plant family members act as extracellular substrate-specific proteases (Milne et al., 2003; Serrano et al., 2004). Given that overexpression of individual PR-1 genes in tobacco (Nicotiana tabacum) conferred slightly enhanced resistance to Peronospora tabacina and Phytophthora parasitica pv nicotianae but not to Pstab or several viruses (Linthorst et al., 1989; Alexander et al., 1993) and that constitutive expression of other single PR genes resulted in little, if any, enhanced resistance to parasites (van Loon et al., 2006), SYP132 is likely to mediate exocytosis of an antimicrobial cocktail rather than a single toxic principle. Thus, we hypothesize that PEN1 and SYP132 syntaxins are plasma membrane-resident components of two execution machineries in two secretory immune response pathways for the release of at least partly distinct antimicrobial cocktails (Fig. 2).
Neither of the abovementioned secretory pathways function exclusively in immune responses. Whereas Arabidopsis plants harboring null mutations in either PEN1 syntaxin or in its closest homolog SYP122 are macroscopically indistinguishable from the wild type, pen1 syp122 double mutants show severe dwarfism that is only partially due to deregulated SA signaling (Assaad et al., 2004; Zhang et al., 2007). Thus, PEN1 and SYP122 share overlapping and redundant functions required for normal plant growth. Unlike pen1 mutants, however, syp122 plants retain wild-type-like resistance to B. graminis f. sp. hordei, pointing to an apparent functional specialization of PEN1 in plant defense responses (Assaad et al., 2004). Gene silencing of NbSYP132 syntaxin impaired not only resistance to Pstab, but also perturbed plant developmental processes, leading to morphological aberrations such as shorter stems and petioles, thicker leaves, and partial loss of apical dominance (Kalde et al., 2007). This suggests dual functions for NbSYP132 in plant development and immune responses. Arabidopsis SNAP33, which interacts with PEN1 and VAMP721/722 in the immune secretory pathway to powdery mildews and Hyaloperonospora parasitica (see above), was originally isolated by its ability to interact with KNOLLE, a syntaxin required for cytokinesis (Heese et al., 2001). Clearly, lethality of snap33 mutants before flowering, associated with dwarfism, cytokinetic defects, and necrotic leaf lesion formation, points to additional SNAP33 engagement(s) in essential cellular processes other than immunity (Heese et al., 2001). Finally, double homozygous mutations in Arabidopsis VAMP721 and VAMP722 lead to embryo lethality, and constitutive gene silencing of both genes results in stunted growth (Kwon et al., 2008a). Together with the immunodeficiency of VAMP721+/− VAMP722−/− and VAMP721−/− VAMP722+/− plants to powdery mildews and H. parasitica that show otherwise wild-type-like morphology, this demonstrates dual functions of these VAMPs in separate biological processes.
This raises the question whether VAMP721/722 vesicles transport identical, overlapping, or distinct cargoes during plant growth/development and immune responses. A change from nondirectional to directional movement of GFP-VAMP722-tagged vesicles toward incipient B. graminis f. sp. hordei or Erysiphe pisi entry sites (Kwon et al., 2008a) suggests that plant cells reutilize the preexisting secretory machinery for focal discharge of cargoes transported by VAMP721/722 endomembrane containers. The delayed formation of papillae observed in both pen1 and VAMP721/722-silenced plants (Assaad et al., 2004; Kwon et al., 2008a) implies that cell wall precursors and/or cell wall-synthesizing enzymes are potential cargoes delivered via VAMP721/722 vesicle-like structures. Thus, a change from nondirectional to directional vesicle transport, driven by focal reorganization of the actin cytoskeleton, could explain the formation of papillae and does not necessarily involve a change of vesicle load during preinvasion resistance. However, VAMP721/722-dependent cargo can also restrict the growth of virulent G. orontii and H. parasitica that have largely overcome preinvasive immunity, thereby pointing to an additional role of these VAMPs in restricting subsequent postinvasive immune responses (Kwon et al., 2008a). Hence VAMP721/VAMP722 might also carry antimicrobial cargo that is synthesized de novo upon transcriptional reprogramming for defense (see above; Fig. 2). This predicts dynamic changes of vesicle cargo during the infection process. To date, PEN1 syntaxin has been shown to restrict fungal entry only in leaf epidermal cells, whereas N. benthamiana SYP132 syntaxin limits bacterial growth in the interior of leaves (Collins et al., 2003; Kalde et al., 2007; Kwon et al., 2008a). Thus, tissue or even cell type-specific secretory cocktails could add a further layer of complexity and might contribute to an overall spatiotemporal control of secretory processes at attempted infection sites (Fig. 1).
One evolutionarily conserved response of flowering plants to pathogenic microorganisms that distinguishes the plant from the animal immune system involves the biosynthesis of small antimicrobial molecules (Dixon, 2001; Field et al., 2006). Many of these metabolites are known to be secreted in a pathogen-inducible manner by plant roots or leaves (Zook and Hammerschmidt, 1997; Churngchow and Rattarasarn, 2001; Bais et al., 2005; Bednarek et al., 2005), suggesting that the secretion of toxic phytochemicals could have general significance for growth restriction of diverse pathogen classes. In addition, a number of plant secondary metabolites, including pathogen-inducible representatives, are covalently bound to cell wall components (McLusky et al., 1999; Hagemeier et al., 2001; Tan et al., 2004). These cell wall-bound compounds must be translocated through the plasma membrane before they reach their destination compartment, and such a process would require active transport mechanisms. All these observations make plant secondary metabolites likely components of the toxic cocktail transported in secretory vesicles to sites of incipient pathogen ingress. A striking example supporting such a hypothesis comes from studies in sorghum (Sorghum bicolor) following inoculation with the fungus Colletotrichum graminicola, which in nature colonizes maize (Zea mays). In this nonhost interaction, sorghum leaves respond to fungal attack by the accumulation of red-colored 3-deoxyanthocyanidin phytoalexins (Snyder and Nicholson, 1990; Snyder et al., 1991). Microscopic studies revealed that these compounds accumulate in inclusion bodies that are formed in the cytoplasm of attacked epidermal cells. These vesicle-like structures move toward sites of attempted penetration and focally accumulate beneath the fungal appressorium, leading to high local phytoalexin concentrations at incipient entry sites (Snyder and Nicholson, 1990; Nielsen et al., 2004). Similarly, the formation of vesicle-like structures containing autofluorescent hydroxycinnamic acid amides, feruloyl-3′-methoxytyramine and feruloyltyramine, has been reported in onion (Allium cepa) epidermal cells upon challenge inoculation with the necrotrophic fungus Botrytis allii (McLusky et al., 1999). These vesicle-like structures move directionally toward B. allii entry sites. The polarized transport correlates with a reorganization of the actin cytoskeleton and results in a chemical cell wall reinforcement that involves binding by ether linkages of vesicle-transported hydroxycinnamic acid amides (McLusky et al., 1999). These data corroborate the idea that the mechanism underlying preinvasion resistance might involve directed transport of intracellular vesicles along polarized actin cables toward pathogen contact sites.
A conceptually similar framework for defense-related focal secretion appears to have been independently invented in the vertebrate immune system. In vertebrates, mobile T cells form intimate junctions with target cells such as an antigen-presenting cells to execute immune responses (Huppa and Davis, 2003; Billadeau et al., 2007). This inducible and transient cell-cell junction is called the immunological synapse (IS). Upon recognition of nonself peptides on the cell surface of a target cell by the T-cell receptor, helper T cells secrete cytokines to activate neighboring sleeping T cells, and cytotoxic T cells (CTLs) release cytolytic molecules to kill target cells (Stinchcombe and Griffiths, 2007). The release of cytolytic molecules from CTLs is tightly controlled and involves polarization of the cytoskeleton and precise delivery of lytic granules across the IS. Indeed, genetically inherited disorders of this process are linked to mutations in genes encoding exocytosis-associated factors such as syntaxin 11, Rab27a, and Munc13-4 that drive or regulate vesicle fusion with the plasma membrane of CTLs (Menasche et al., 2000; Feldmann et al., 2003; zur Stadt et al., 2005). Directed vesicle-mediated discharge of cytolytic cargo through the IS spares neighboring cells and the CTL itself from undergoing cytolysis. Because VAMP721/722 endomembrane compartments are likely to transport toxic cargoes as well as cell wall-forming building blocks, we hypothesized that papillae in plants might provide a structural scaffold to contain released toxic molecules at high concentrations in the paramural space (Kwon et al., 2008b). By this means, papillae might serve a role as a self-protection mechanism for host cells during secretory immune responses.
Because SNARE protein function is now known to restrict the growth of different pathogen classes including bacteria, fungi, and oomycetes (Kalde et al., 2007; Kwon et al., 2008a), the plant vesicle trafficking machinery might have become prone to microbial sabotage. Indeed, the conserved P. syringae effector HopM1 interferes with the plant secretion system by association with the plant endomembrane system and by degrading an ADP ribosylation factor guanine nucleotide exchange factor (ARF-GEF), possibly through engagement of the plant ubiquitination-dependent proteasome machinery (Nomura et al., 2006; Fig. 2). Whereas GTP-binding ARFs serve a central role in vesicle trafficking, either by directly activating and inactivating vesicle coat protein recruitment and dissociation or by facilitating binding of coat protein to vesicle cargo, ARF-GEFs specifically activate ARFs by facilitating exchange of its bound GDP for GTP (for review, see Nie et al., 2003). The finding that insertion mutants in the Arabidopsis At3g43300 gene—encoding one of eight Arabidopsis ARF-GEF family members—restore bacterial growth of P. syringae mutants lacking HopM1 (Nomura et al., 2006) has implicated an ARF-GEF family member in bacterial resistance. It is possible that the HopM1-targeted ARF-GEF family member is involved in the generation of a vesicle subpopulation loaded with cargo that is subsequently discharged at the plasma membrane by SYP132 syntaxin (see above). To date, only one other Arabidopsis ARF-GEF, called GNOM, has been assigned to a biological process, mediating endosomal recycling, auxin transport, and auxin-dependent plant growth (Geldner et al., 2003). Interestingly, treatment of wild-type Arabidopsis with brefeldin A also restored bacterial growth of P. syringae mutants lacking HopM1 (Nomura et al., 2006). Brefeldin A specifically inhibits the exchange of bound GDP for GTP in ARFs by binding to ARF-GEFs such as Arabidopsis GNOM (Geldner et al., 2003). The macrolide fungal metabolite brefeldin A has been detected in a wide range of fungi, including species of Alternaria, Ascochyta, Penicillium, Curvularia, Cercospora, Phyllosticta, and Phoma, several of which are either parasites or endophytes of plants (Wang et al., 2002; Weber et al., 2004). It is thus possible that one function of brefeldin A is to suppress plant immune responses. If so, then both fungal brefeldin A and bacterial HopM1 intercept the immune response secretion machinery by inhibiting vesicle formation rather than the later stage of vesicle fusion with the plasma membrane via ternary SNARE complex formation (Fig. 2).
Experimental evidence suggests that plants evolved yet another mechanism for delivery of toxic plant secondary metabolites that might act independently from vesicle trafficking and fusion events with the plasma membrane. This mechanism appears to utilize plasma membrane ABC transporters as pumps for the release of small molecules on the cell surface. One transporter implicated in the secretion of plant antimicrobials is NpABC1/PDR1 from tobacco thought to be involved in the release of sclareol, an antifungal terpenoid, on the leaf surface of Nicotiana spp. (Jasinski et al., 2001; Fig. 3). Involvement of this particular ABC transporter in plant responses to microbial infection was inferred from the responsiveness of NpABC1/PDR1 expression upon treatment of plants with a range of pathogen-associated molecular patterns and phytopathogens (Sasabe et al., 2002; Stukkens et al., 2005). Moreover, transgenic tobacco plants in which pathogen-triggered up-regulation of NpPDR1 gene expression was blocked by gene silencing showed enhanced susceptibility to Botrytis cinerea (Stukkens et al., 2005), thus strongly supporting a functional contribution of NpPDR1 to plant immunity.
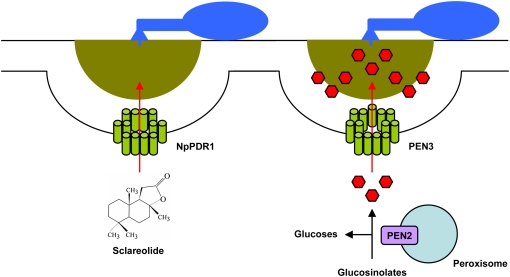
Figure 3.
Pathogen-triggered and ABC transporter-driven efflux of small molecules into the apoplast. Plants secret a wide range of secondary metabolites in response to pathogen challenge. In tobacco, the PDR1 ABC transporter is required for the translocation of sclareolide, a toxic phytochemical (left). In Arabidopsis, the PEN3 ABC transporter is required for preinvasive resistance to a broad range of fungal parasites (right). Genetic and biochemical data suggest that peroxisome-associated PEN2 glycosyl hydrolase generates toxic products from glucosinolates that are translocated into the apoplast by PEN3. Due to the self-cytotoxicity of the secreted secondary metabolites, it is likely that their generation and release may occur in direct proximity to microbial contact sites.
In Arabidopsis, the plasma membrane-resident PEN3/PDR8 transporter was found to focally accumulate beneath sites of attempted fungal entry of leaf epidermal cells. Moreover, mutant pen3 plants permit increased entry rates of the nonadapted powdery mildews Blumeria graminis or E. pisi and are hypersusceptible to the necrotrophic fungus Plectosphaerella cucumerina (Stein et al., 2006). This suggests that PEN3, similarly to NpPDR1, serves as one component of a machinery for targeted efflux of antimicrobials and/or chemical agents for cross-linking of the cell wall. Genetic evidence indicates that PEN3 is involved in the same biochemical pathway as peroxisome-associated PEN2 glucosyl hydrolase (Stein et al., 2006; Fig. 3). This protein, like PEN3, is an essential component of effective nonhost resistance to several nonadapted fungal parasites (Lipka et al., 2005; Stein et al., 2006). Upon establishment of fungal contact sites on the Arabidopsis leaf surface and invasion attempts into single leaf epidermal cells, peroxisomes together with associated PEN2 focally accumulate beneath incipient entry sites. Recently obtained data suggest that PEN2 possesses myrosinase (Grubb and Abel, 2006) activity and can cleave Glc from indole glucosinolates (P. Bednarek and P. Schulze-Lefert, unpublished data; Fig. 3). However, unlike conventional myrosinase, PEN2-dependent myrosinase activity can be triggered in vivo without tissue damage/cell death. In this context it seems likely that the enzyme might act on substrates only after its pathogen-triggered relocalization (Lipka et al., 2005), thereby releasing at high concentrations biologically active end product(s) at attempted entry sites. One hypothesis is that PEN3 translocates PEN2-myrosinase generated molecules into the apoplastic space. Thus, both proteins, most likely together with other as yet uncharacterized components, may constitute a secretory immune response pathway for small molecules with broad-spectrum antimicrobial activity (Fig. 3).
OUTLOOK
The existence of at least two secretory immune systems, acting in parallel or sequentially in the same host cell (PEN1, SNAP33, VAMP721/VAMP722, and PEN2, PEN3, respectively), raises questions regarding its functional significance. This could have the advantage of enabling cellular self-protection if the PEN2/PEN3 pathway synthesizes and translocates protoxins that are activated to toxins by apoplastic enzymes, which in turn are delivered as vesicle cargo via the SNARE protein-dependent pathway. Such extracellularly activated toxic cocktails could be contained within the papillary cell wall scaffold and, together with chemical cross-linking of the newly synthesized cell wall polymers, might form a fine-tuned chemical and structural barrier against microbial intruders. Clearly, defining the repertoire of pathogen-triggered secreted antimicrobial compounds, proteins, and cell wall building blocks, as well as their intracellular biosynthesis compartments, represents one of the next challenges. This will be key in addressing an unresolved question in plant-microbe interactions: what terminates microbial growth during plant immune responses?
Acknowledgments
We thank Richard O'Connell for critical reading of this manuscript and his helpful suggestions.
This work was supported by funds from the Max Planck Society and the Deutsche Forschungsgemeinschaft (SFB670 and SPP1212).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Paul Schulze-Lefert (schlef@mpiz-koeln.mpg.de).
References
- Abramovitch RB, Janjusevic R, Stebbins CE, Martin GB (2006) Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proc Natl Acad Sci USA 103 2851–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aist JR (1976) Papillae and related wound plugs of plant cells. Annu Rev Phytopathol 14 145–163 [Google Scholar]
- Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E, et al (1993) Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc Natl Acad Sci USA 90 7327–7331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Edwards H, Ramonell K, et al (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15 5118–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais HP, Prithiviraj B, Jha AK, Ausubel FM, Vivanco JM (2005) Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature 434 217–221 [DOI] [PubMed] [Google Scholar]
- Bednarek P, Schneider B, Svatos A, Oldham NJ, Hahlbrock K (2005) Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol 138 1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger RR, Bushnell WR, Dik AJ, Carver TLW (2002) The Powdery Mildews. The American Phytopathological Society Press, St. Paul, MN
- Bestwick CS, Bennett MH, Mansfield JW (1995) Hrp mutant of Pseudomonas syringae pv phaseolicola induces cell wall alterations but not membrane damage leading to the hypersensitive reaction in lettuce. Plant Physiol 108 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MH, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RA, Miklis M, Schmelzer E, Schulze-Lefert P, Panstruga R (2005) Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc Natl Acad Sci USA 102 3135–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billadeau DD, Nolz JC, Gomez TS (2007) Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol 7 131–143 [DOI] [PubMed] [Google Scholar]
- Churngchow N, Rattarasarn M (2001) Biosynthesis of scopoletin in Hevea brasiliensis leaves inoculated with Phytophthora palmivora. J Plant Physiol 158 875–882 [Google Scholar]
- Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, et al (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425 973–977 [DOI] [PubMed] [Google Scholar]
- de Torres M, Mansfield JW, Grabov N, Brown IR, Ammouneh H, Tsiamis G, Forsyth A, Robatzek S, Grant M, Boch J (2006) Pseudomonas syringae effector AvrPtoB suppresses basal defence in Arabidopsis. Plant J 47 368–382 [DOI] [PubMed] [Google Scholar]
- Despres C, DeLong C, Glaze S, Liu E, Fobert PR (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12 279–290 [PMC free article] [PubMed] [Google Scholar]
- Dixon RA (2001) Natural products and plant disease resistance. Nature 411 843–847 [DOI] [PubMed] [Google Scholar]
- Dong X (2004) Pathogen-induced systemic DNA rearrangement in plants. Trends Plant Sci 9 60–61 [DOI] [PubMed] [Google Scholar]
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, et al (2003) Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 115 461–473 [DOI] [PubMed] [Google Scholar]
- Field B, Jordan F, Osbourn A (2006) First encounters—deployment of defence-related natural products by plants. New Phytol 172 193–207 [DOI] [PubMed] [Google Scholar]
- Fobert PR, Despres C (2005) Redox control of systemic acquired resistance. Curr Opin Plant Biol 8 378–382 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112 219–230 [DOI] [PubMed] [Google Scholar]
- Grubb CD, Abel S (2006) Glucosinolate metabolism and its control. Trends Plant Sci 11 89–100 [DOI] [PubMed] [Google Scholar]
- Hagemeier J, Schneider B, Oldham NJ, Hahlbrock K (2001) Accumulation of soluble and wall-bound indolic metabolites in Arabidopsis thaliana leaves infected with virulent or avirulent Pseudomonas syringae pathovar tomato strains. Proc Natl Acad Sci USA 98 753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jurgens G (2001) Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol 155 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogkamp TJH, Chen WQ, Niks RE (1998) Specificity of prehaustorial resistance to Puccinia hordei and to two inappropriate rust fungi in barley. Phytopathology 88 856–861 [DOI] [PubMed] [Google Scholar]
- Huppa JB, Davis MM (2003) T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol 3 973–983 [DOI] [PubMed] [Google Scholar]
- Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15 2503–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH (2006) SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7 631–643 [DOI] [PubMed] [Google Scholar]
- Jasinski M, Stukkens Y, Degand H, Purnelle B, Marchand-Brynaert J, Boutry M (2001) A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13 1095–1107 [PMC free article] [PubMed] [Google Scholar]
- Johnson LEB, Bushnell WR, Zeyen RJ (1982) Defense patterns in nonhost higher plant species against two powdery mildew fungi. I. Monocotyledonous species. Can J Bot 60 1068–1083 [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444 323–329 [DOI] [PubMed] [Google Scholar]
- Kalde M, Nühse TS, Findlay K, Peck SC (2007) The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci USA 104 11850–11855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knogge W (1996) Fungal infection of plants. Plant Cell 8 1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H (1997) Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. Plant J 11 525–537 [Google Scholar]
- Koh S, Andre A, Edwards H, Ehrhardt D, Somerville S (2005) Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. Plant J 44 516–529 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE (1985) Enzymatic penetration of the plant cuticle by fungal pathogens. Annu Rev Phytopathol 23 223–250 [Google Scholar]
- Kwon C, Neu C, Pajonk S, Yun HS, Lipka U, Humphry M, Bau S, Straus M, Kwaaitaal M, Rampelt H, et al (2008. a) Co-option of a default secretory pathway for plant immune responses. Nature 451 835–840 [DOI] [PubMed] [Google Scholar]
- Kwon C, Panstruga R, Schulze-Lefert P (2008. b) Les liaisons dangereuses: immunological synapse formation in animals and plants. Trends Immunol 29 159–166 [DOI] [PubMed] [Google Scholar]
- Linthorst HJM, Meuwissen RLJ, Kauffmann S, Bol JF (1989) Constitutive expression of pathogenesis-related proteins PR-1, GRP, and PR-S in tobacco has no effect on virus infection. Plant Cell 1 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, et al (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310 1180–1183 [DOI] [PubMed] [Google Scholar]
- Lipka V, Kwon C, Panstruga R (2007) SNARE-ware: the role of SNARE-domain proteins in plant biology. Annu Rev Cell Dev Biol 23 147–174 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Mayer U, Jurgens G (1996) Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84 61–71 [DOI] [PubMed] [Google Scholar]
- McLusky SR, Bennett MH, Beale MH, Lewis MJ, Gaskin P, Mansfield JW (1999) Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarization, peroxidase activity and suppression of flavonoid biosynthesis. Plant J 17 523–534 [Google Scholar]
- Melchers LS, Stuiver MH (2000) Novel genes for disease-resistance breeding. Curr Opin Plant Biol 3 147–152 [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126 969–980 [DOI] [PubMed] [Google Scholar]
- Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, et al (2000) Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25 173–176 [DOI] [PubMed] [Google Scholar]
- Mendgen K, Deising H (1993) Infection structures of fungal plant pathogens—a cytological and physiological evaluation. New Phytol 124 192–213 [DOI] [PubMed] [Google Scholar]
- Miklis M, Consonni C, Bhat RA, Lipka V, Schulze-Lefert P, Panstruga R (2007) Barley MLO modulates actin-dependent and actin-independent antifungal defense pathways at the cell periphery. Plant Physiol 144 1132–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne TJ, Abbenante G, Tyndall JD, Halliday J, Lewis RJ (2003) Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J Biol Chem 278 31105–31110 [DOI] [PubMed] [Google Scholar]
- Nie Z, Hirsch DS, Randazzo PA (2003) Arf and its many interactors. Curr Opin Cell Biol 15 396–404 [DOI] [PubMed] [Google Scholar]
- Nielsen KA, Gotfredsen CH, Buch-Pedersen MJ, Ammitzboll H, Mattsson O, Duus JO, Nicholson RL (2004) Inclusions of flavonoid 3-deoxyanthocynidins in Sorghum bicolor self-organize into spherical structures. Physiol Mol Plant Pathol 65 187–196 [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301 969–972 [DOI] [PubMed] [Google Scholar]
- Nomura K, Debroy S, Lee YH, Pumplin N, Jones J, He SY (2006) A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science 313 220–223 [DOI] [PubMed] [Google Scholar]
- Opalski KS, Schultheiss H, Kogel KH, Hückelhoven R (2005) The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f. sp. hordei. Plant J 41 291–303 [DOI] [PubMed] [Google Scholar]
- Rosebrock TR, Zeng LR, Brady JJ, Abramovitch RB, Xiao FM, Martin GB (2007) A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448 370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M, Toyoda K, Shiraishi T, Inagaki Y, Ichinose Y (2002) cDNA cloning and characterization of tobacco ABC transporter: NtPDR1 is a novel elicitor-responsive gene. FEBS Lett 518 164–168 [DOI] [PubMed] [Google Scholar]
- Schmelzer E (2002) Cell polarization, a crucial process in fungal defence. Trends Plant Sci 7 411–415 [DOI] [PubMed] [Google Scholar]
- Schultheiss H, Dechert C, Kogel KH, Hückelhoven R (2003) Functional analysis of barley RAC/ROP G-protein family members in susceptibility to the powdery mildew fungus. Plant J 36 589–601 [DOI] [PubMed] [Google Scholar]
- Serrano RL, Kuhn A, Hendricks A, Helms JB, Sinning I, Groves MR (2004) Structural analysis of the human Golgi-associated plant pathogenesis related protein GAPR-1 implicates dimerization as a regulatory mechanism. J Mol Biol 339 173–183 [DOI] [PubMed] [Google Scholar]
- Snyder BA, Leite B, Hipskind J, Butler LG, Nicholson RL (1991) Accumulation of sorghum phytoalexins induced by Colletotrichum graminicola at the infection site. Physiol Mol Plant Pathol 39 463–470 [Google Scholar]
- Snyder BA, Nicholson RL (1990) Synthesis of phytoalexins in Sorghum as a site-specific response to fungal ingress. Science 248 1637–1639 [DOI] [PubMed] [Google Scholar]
- Stein M, Dittgen J, Sanchez-Rodriguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18 731–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Griffiths GM (2007) Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol 23 495–517 [DOI] [PubMed] [Google Scholar]
- Stukkens Y, Bultreys A, Grec S, Trombik T, Vanham D, Boutry M (2005) NpPDR1, a pleiotropic drug resistance-type ATP-binding cassette transporter from Nicotiana plumbaginifolia, plays a major role in plant pathogen defense. Plant Physiol 139 341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto D, Jones DA, Hardham AR (2003) GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33 775–792 [DOI] [PubMed] [Google Scholar]
- Tan JW, Bednarek P, Liu HK, Schneider B, Svatos A, Hahlbrock K (2004) Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry 65 691–699 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Van Esse HP, Crous PW, De Wit PJGM (2005) Cladosporium fulvum (syn. Passalora fulva), a highly specialized plant pathogen as a model for functional studies on plant pathogenic Mycosphaerellaceae. Mol Plant Pathol 6 379–393 [DOI] [PubMed] [Google Scholar]
- Uemura T, Ueda T, Ohniwa RL, Nakano A, Takeyasu K, Sato MH (2004) Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct Funct 29 49–65 [DOI] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44 135–162 [DOI] [PubMed] [Google Scholar]
- Wang D, Weaver ND, Kesarwani M, Dong X (2005) Induction of protein secretory pathway is required for systemic acquired resistance. Science 308 1036–1040 [DOI] [PubMed] [Google Scholar]
- Wang J, Huang Y, Fang M, Zhang Y, Zheng Z, Zhao Y, Su W (2002) Brefeldin A, a cytotoxin produced by Paecilomyces sp. and Aspergillus clavatus isolated from Taxus mairei and Torreya grandis. FEMS Immunol Med Microbiol 34 51–57 [DOI] [PubMed] [Google Scholar]
- Weber RW, Stenger E, Meffert A, Hahn M (2004) Brefeldin A production by Phoma medicaginis in dead pre-colonized plant tissue: a strategy for habitat conquest? Mycol Res 108 662–671 [DOI] [PubMed] [Google Scholar]
- Yano D, Sato M, Saito C, Sato MH, Morita MT, Tasaka M (2003) A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc Natl Acad Sci USA 100 8589–8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fan W, Kinkema M, Li X, Dong X (1999) Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc Natl Acad Sci USA 96 6523–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tessaro MJ, Lassner M, Li X (2003) Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15 2647–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Feechan A, Pedersen C, Newman MA, Qiu JL, Olesen KL, Thordal-Christensen H (2007) A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. Plant J 49 302–312 [DOI] [PubMed] [Google Scholar]
- Zook M, Hammerschmidt R (1997) Origin of the thiazole ring of camalexin, a phytoalexin from Arabidopsis thaliana. Plant Physiol 113 463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Stadt U, SchmidtS, Kasper B, Beutel K, Diler AS, Henter JI, Kabisch H, Schneppenheim R, Nürnberg P, Janka G, et al (2005) Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet 14 827–834 [DOI] [PubMed] [Google Scholar]