Cell polarity is one of the fundamental properties of multicellular organisms and is tightly linked with processes such as cell division, differentiation, cellular signaling, and intercellular communication. Polarities of individual cells, transmitted by cell divisions, are reflected at the tissue and organ levels and contribute to the overall shape of the multicellular organism. In addition, in plants, cell polarity provides means by which they maintain developmental continuity and adapt their development to optimally conform to environmental conditions by flexible redefinition of cell polarities. Thus, the mechanisms underlying the establishment and maintenance of cell polarity belong to the important themes of developmental and cell biology.
At the level of the individual cell, polarity is mirrored by the asymmetric distribution of intracellular components, such as organelles, cytoskeletal strands, and single proteins. This asymmetric distribution of intracellular components defines functionally and/or morphologically distinct domains (Bonifacino and Lippincott-Schwartz, 2003). Mechanisms that underlie the sorted delivery of intracellular cargos to these domains and thus contribute to the generation or maintenance of cell polarity have been extensively studied in different model organisms, including mammals, flies, worms, and yeast (Knoblich, 2000; Irazoqui and Lew, 2004; Nance, 2005). Animal epithelial cells are a preferred model system, because their plasma membrane (PM) is divided into two distinct domains, the apical domain facing the lumen and the basolateral domain (Mostov et al., 2003; Janssens and Chavrier, 2004). Selective recruitment of apical and basolateral cargos is achieved by their targeted delivery to these domains and results mainly from three processes: (1) newly synthesized proteins are sorted on their way to the PM (mainly in the trans-Golgi network) into vesicles that discriminately deliver them to the apical or basolateral surface; (2) other proteins are selectively retained at the PM polar domain; and (3) proteins that are not retained are rapidly endocytosed and either recycled back or, alternatively, delivered to a different polar PM domain by a process called transcytosis (Rodriguez-Boulan et al., 2005).
Comparable knowledge on the cellular mechanisms underlying the polar localization of proteins in plant cells is lacking, but conceptually similar modes of polar delivery can be assumed. Plant and animal cells might differ fundamentally in the manner by which the polar-competent cargos are kept in their polar domains. In animal cells, anchored protein complexes, called tight junctions, form a physical barrier and limit lateral diffusion of proteins between adjacent polar PM domains (Brown and Stow, 1996). So far, no indications for analogical structures in plant cells have been found; therefore, it remains unclear how lateral diffusion of polar cargos is limited in the plant PM. Furthermore, despite significant advances in recent years, the subcellular trafficking pathways in plants are still only rudimentarily sketched; many destinations of secretory and endocytic pathways as well as their interconnections are vaguely defined, and findings are contradictory. These deficiencies in the basic cell biology knowledge of plants restrict our understanding on where and how the main decision on sorting and delivery of polar cargos occurs. The availability of necessary tools and technologies along with enough attention paid to these topics guarantee a rapid advancement in coming years.
POLARITY IN PLANTS AND AUXIN
For the first time, the term polarity was applied to plants by Hermann von Vöchting in 1878, when he showed that pieces of willow (Salix species) stems form roots and shoots at the corresponding ends irrespective of the orientation toward gravity (for review, see Mohr and Schopfer, 1995). Plant polarity is determined at the cellular level and is tightly connected to the polarity of tissues and organs. Better than other multicellular systems, plants can redefine their cellular and tissue polarity based on the influence of many factors. The plant hormone auxin (indole-3-acetic acid) has been identified as an important factor mediating tissue and organ polarity in plants, mainly on account of its strictly directional (polar) flow through plant tissues. The existence of polar auxin transport together with its physiological and developmental roles, for example in the growth reorientation in response to environmental stimuli, has led to the hypothesis that a combination of environmental and endogenous factors regulate the auxin flow in different plant tissues and, thus, provide the vectorial information for defining the cellular behavior (Went, 1974; Friml, 2003). The important advancement in auxin biology was the formulation of the chemiosmotic model (Rubery and Sheldrake, 1974; Raven, 1975) that proposed the existence of PM-localized auxin carrier proteins that facilitate auxin uptake and auxin efflux out of the cells. The model also proposed that the asymmetric localization of the efflux carriers at one side of the transporting cells determines the direction of the intercellular auxin movement within the field of cells. This remarkable insight, which was later verified experimentally, connected polarities at the cellular and tissue levels.
POLAR-COMPETENT PROTEINS IN PLANT CELLS
More than 20 years after the chemiosmotic model had been formulated, the hypothetical molecular components were identified. Molecular genetics and physiological studies in Arabidopsis (Arabidopsis thaliana) led to the discovery of genes coding for auxin influx and efflux carriers.
The PINFORMED (PIN) proteins have been identified and characterized as key regulators of a multitude of auxin-mediated developmental processes, including tropic growth (Chen et al., 1998; Luschnig et al., 1998; Müller et al., 1998; Utsuno et al., 1998; Friml et al., 2002b), axis formation in embryogenesis (Friml et al., 2003), postembryonic organogenesis (Benková et al., 2003; Reinhardt et al., 2003), root meristem maintenance (Friml et al., 2002a; Blilou et al., 2005), and vascular tissue differentiation and regeneration (Sauer et al., 2006; Scarpella et al., 2006). PIN proteins are PM proteins that act as auxin efflux carriers (Petrášek et al., 2006) and have mainly a polar localization that correlates with and is required for the direction of auxin flow (Wiśniewska et al., 2006). The Arabidopsis PIN family consists of eight members, most of which have been functionally characterized and found to be localized polarly at different sides of the various cell types (Vieten et al., 2007). For example, during embryogenesis, PIN1, PIN4, and PIN7 show polar localizations and act together to specify the apical-basal axis of the embryo (Fig. 1). Postembryonically, PIN proteins have different PM localizations, most being localized at the basal (root apex-facing) side of the vasculature and stele cells, such as PIN1, PIN3, PIN4, and PIN7, whereas some localize also apically (shoot apex-facing side), such as PIN1 in the shoot apex epidermis or PIN2 in the lateral root cap and epidermis cells. In the shoot endodermis and root pericycle cells, PIN3 localizes also at the inner lateral side, whereas it has a symmetric localization in columella cells (Fig. 2). Thus, PIN proteins constitute prominent cell polarity markers in plants. Furthermore, polar targeting of PIN proteins has a clear developmental output, because the polarity of the PIN localization at the single-cell level determines the direction of intercellular auxin transport and the directional signaling to neighboring cells (Wiśniewska et al., 2006).
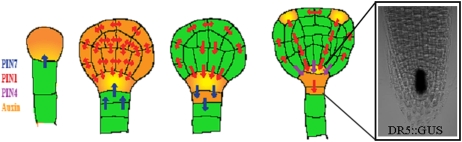
Figure 1.
Establishment of apical-basal polarity during Arabidopsis embryogenesis. At early stages, PIN7 localizes to the apical sides of suspensor cells mediating auxin flow into the proembryo, where PIN1 is localized first in a nonpolar manner. At the middle globular stage, PIN1 basal polarity in provascular cells is established, followed by PIN7 relocation to the basal side of suspensor cells. These PIN polarity rearrangements result in redirection of the auxin flow to the basal part of the embryo, where auxin accumulation contributes to root meristem specification. At later stages, PIN4 expression in the central root meristem aids the establishment of local auxin accumulation in the center of developing root meristems. Furthermore, PIN1 relocates at the apical surface of the proembryo to establish two symmetrically positioned auxin accumulation foci marking sites of future embryonic leaves.
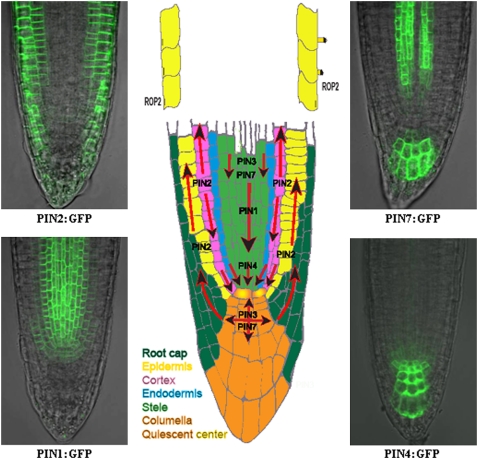
Figure 2.
Polar localization of PIN proteins in the Arabidopsis root tip. The directionality of auxin transport (arrows) is determined by the polar, subcellular localization of PIN proteins. PIN1 is localized at the basal (root apex-facing) side of the root vasculature; PIN2 at the basal side of the cortical cells and at the apical (shoot apex-facing) side of the epidermal and root cap cells; PIN3 in an apolar manner in the columella cells of the root; PIN4 at the basal side of cells in the central root meristem and with less pronounced polarity in the cells of the quiescent center; and PIN7 at the basal side of the stele cells and apolar in the columella cells. ROP2 is also asymmetrically localized, associated with the places of root hair formation. Examples of PIN1:GFP, PIN2:GFP, PIN4:GFP, and PIN7:GFP expression in the root are depicted.
Besides PIN proteins, other polarly localized components are involved in auxin transport. The AUXIN RESISTANT1/LIKE AUX1 (AUX1/LAX) proteins are PM-localized auxin influx carriers (Bennett et al., 1996; Yang et al., 2006; Swarup et al., 2008). The AUX1 protein has a polar localization in some cells, such as the protophloem and the shoot apical meristem (Swarup et al., 2001; Reinhardt et al., 2003; Kleine-Vehn et al., 2006; Fig. 3). Other auxin transport proteins from the ATP-binding cassette multidrug resistance/P-glycoprotein family are localized mainly symmetrically, but polar localization in some cells has been reported as well (Geisler et al., 2005; Terasaka et al., 2005).
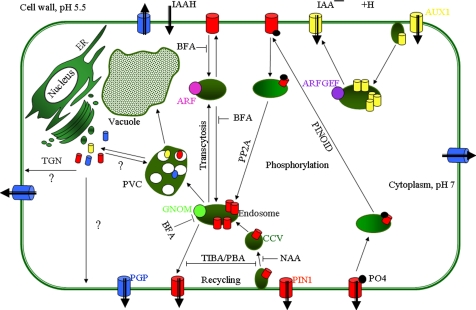
Figure 3.
Overview of the subcellular trafficking routes in a polarized plant cell. Auxin influx and efflux (black thick arrows) are mediated by AUX1 and PIN proteins, respectively. PGP ATP-binding cassette-type transporters are also involved in auxin efflux. According to the chemiosmotic model, auxin in the protonated form can also enter the cell passively. Basally localized PIN1 recycles in a BFA-sensitive and GNOM-dependent manner between endosomes and the PM. Constitutive cycling of vesicles between these two compartments is disrupted by 2,3,5-triiodobenzoic acid (TIBA) and 1-pyrenoylbenzoic acid (PBA), drugs that inhibit auxin transport and actin cytoskeleton dynamics (Dhonukshe et al., 2008). AUX1 also displays constitutive cycling to and from the apical PM that is dependent of an unknown BFA-insensitive ARF GEF. Auxin, such as α-naphthaleneacetic acid (NAA), inhibits PIN internalization, increasing the amount and activity of PINs at the PM (Paciorek et al., 2005). PID kinase and PP2A phosphatase mediate reversible PIN phosphorylation, thus contributing to the decision on the apical-basal PIN targeting. Phosphorylated and dephosphorylated PIN proteins are preferentially sorted into the apical and basal targeting pathways, respectively (Michniewicz et al., 2007). PIN1 is able to translocate between the basal and apical cell sides by a combination of constitutive endocytic cycling and alternative recruitment by distinct ARF GEF-dependent apical and basal targeting machineries (Kleine-Vehn et al., 2008).
Other plant proteins, whose functions are not directly connected to auxin transport, also have been found to be localized asymmetrically in plant cells, namely at the inner lateral side, outer lateral side, and both longitudinal sides of the cells (Roudier et al., 2005; Ma et al., 2006, 2007; Miwa et al., 2007). Examples of laterally localized proteins include transporters for boron (BOR1 and BOR4) and for low silicon (LSI1 and LSI2) in rice (Oryza sativa) and the regulator of the anisotropic cell expansion (COBRA). A special asymmetric localization has been detected for a small G protein of the Rho family ROP2 that localizes to a distinct position close to the basal end of the outer membrane of elongating epidermis cells and marks the initiation position of the outgrowing root hair (Molendijk et al., 2001). This unprecedented diversity of polar cargos that localize to different sides of plant cells suggests that plants possess more diverse polar targeting machineries than are known from other eukaryotic systems.
ROLE OF PHOSPHORYLATION IN POLAR TARGETING
An important and yet unsolved question concerns how the different polar-competent proteins are recognized and delivered to the correct side of the cell. In animal systems, polar cargo proteins carry signals that determine their residence at different polar domains (Dugani and Klip, 2005; Rodriguez-Boulan et al., 2005). Similar concepts of polarity determinants in the protein sequence also apply to plants, because different polar cargos, such as PIN1 or PIN2, localize to different polar domains of the same cell, necessitating some identification mechanism. Furthermore, by inserting a GFP tag at a specific position of the PIN1 sequence, the basal targeting of PIN1 is disrupted in epidermal cells, leading to apical localization (Wiśniewska et al., 2006). This observation implies a PIN1 sequence-based signal for decision on the PIN1 subcellular localization. Several findings suggest that the PIN polarity signals are related to the phosphorylation sites found in the PIN sequences. One of the major decision regulators on the PIN polarity are the Ser/Thr protein kinase PINOID (PID; Christensen et al., 2000; Benjamins et al., 2001; Friml et al., 2004) and the protein phosphatase 2A (PP2A; Michniewicz et al., 2007). High levels of PIN phosphorylation as achieved by overexpression of PID or inhibition of PP2A lead to a preferential apical PIN targeting, whereas low phosphorylation levels in the pid mutants result in a preferential basal PIN targeting (Friml et al., 2004; Treml et al., 2005). Importantly, PID has been shown to directly phosphorylate the hydrophilic loop of PIN proteins in vivo and in vitro and PP2A phosphatase has been shown to antagonize this action (Michniewicz et al., 2007). The available data are thus consistent with the model: when dephosphorylated, PIN is preferentially recruited by the basal targeting machinery, and phosphorylated PIN is trafficked by the apical pathway (Fig. 3). Such a model also has implications for the conditional regulation of PIN polarity and directional auxin fluxes by different signaling pathways that act upstream of the PID-dependent PIN phosphorylation. Other polar-competent proteins probably possess different types of also phosphorylation-unrelated polarity signals that have yet to be identified.
ROLE OF STEROLS IN POLAR TARGETING
Plant sterols are essential components of plant membranes. Their chemical structure resembles that of animal cholesterol, whose cellular functions are supposed to be similar. Depletion of cholesterol, the main animal sterol, decreases the polar delivery of target proteins (Keller and Simons, 1998), while depletion of plant sterols leads to cell polarity defects followed by reduced auxin transport and auxin-related developmental defects. Studies carried out on sterol-deficient orc and cyclopropylsterol isomerase1 (cpi1) mutants have detected defects in the polar localization of PIN and AUX1 proteins, indicating that the polar delivery of cargos in plants also depends on the sterol composition of the PM (Souter et al., 2002; Grebe et al., 2003; Willemsen et al., 2003; Kleine-Vehn et al., 2006). Recent work has highlighted the essential role of sterols in the reiteration of PIN polarity after the division of polarized cells (Men et al., 2008). PIN proteins have been shown to be targeted to the forming cell plate during cell division (Geldner et al., 2001). This poses a problem that, after the fusion of the cell plate to the PM, PIN will be present at both apical and basal sides of one of the daughter cells. In order to maintain the polarity of the mother cell in both daughter cells, there must be a mechanism that stabilizes the polar cargo at one side and retrieves it from the opposite side of the newly formed cell wall. Little is known of how that is achieved and which cellular and molecular mechanisms are involved, but sterols seem to play a crucial role. The cpi1 sterol-deficient mutants have impaired endocytosis and show depositions of PIN2 at both apical and basal PMs in postcytokinetic cells (Men et al., 2008), suggesting that sterol-dependent endocytosis is required to retrieve PINs from the “wrong” side of the cell after cell division. In summary, these observations indicate that sterol-enriched PM microdomains related to the so-called lipid rafts (Martin et al., 2005) are important also in plant cells for different membrane-related trafficking and signaling processes, including the regulation of cell polarity.
ROLE OF SECRETION AND RECYCLING IN POLAR TARGETING
Direct delivery of secreted proteins and other cargos to distinct polar domains at the PM is one of the basic possibilities for generating an asymmetric distribution at the cell surface. Indeed, in animal epithelial cells, different cargos are secreted directly to the apical and basolateral domains (Mostov et al., 2003). In plants, no data are available on such a mode of polar secretion. On the contrary, PIN proteins, as tested for the basally localized PIN1 or apically localized PIN2, seem to be delivered originally in a nonpolar fashion after the de novo synthesis, and their apical or basal polarity is then established in the next step involving internalization from the PM and polar recycling (Dhonukshe et al., 2008). Thus, the secretion, clathrin-dependent endocytosis (Dhonukshe et al., 2007), and subsequent recycling are important processes in the generation of the PIN polar localization.
The delivery of PIN proteins to the PM is sensitive to brefeldin A (BFA), a known inhibitor of secretion and subcellular trafficking (Steinmann et al., 1999; Geldner et al., 2001). A molecular target of the BFA action is GNOM, an endosomal exchange factor for ARF GTPases (ARF GEF). GNOM functions as the GDP/GTP exchange factor for the small G proteins of the ARF class that mediate vesicle budding processes at different subcellular compartments (Shevell et al., 1994; Geldner et al., 2001). In the presence of BFA, PIN1 largely disappears from the PM and can be found internalized in so-called BFA compartments. The PIN1 internalization is completely reversible and occurs also when de novo protein synthesis is inhibited, indicating constitutive endocytosis and recycling of PIN proteins (Geldner et al., 2001). The repeated cycles of PIN endocytosis and recycling to the PM were also visualized with a green-to-red photoconvertible fluorescent PIN2 version (Dhonukshe et al., 2007). These findings, together with the analysis of the gnom mutant (also called emb30), have shown that GNOM is a BFA-sensitive regulator of PIN trafficking from the endosomes back to the PM. GNOM seems to be more crucial for basal polar targeting, because the apical PM localization of PIN proteins and AUX1 is not strongly affected when GNOM function is inhibited (Kleine-Vehn et al., 2008). Thus, the apical cargos utilize a different targeting pathway that might require another, possibly BFA-insensitive, ARF GEF. These observations and, in particular, the PIN polarity defects in the gnom loss-of-function mutants tightly link endocytic recycling and polar targeting in plant cells.
The GNOM-dependent, BFA-sensitive recycling pathway applies mainly to the polar PIN targeting in the interphase cells that depend on the actin cytoskeleton. On the contrary, in dividing cells, PIN proteins are delivered to the forming cell plate by the microtubule-dependent pathway (Geldner et al., 2001; Dhonukshe et al., 2006). These observations demonstrate the diversity of different trafficking pathways for the polar delivery of PIN proteins and other polar cargos.
TRANSCYTOSIS MECHANISM FOR PIN POLARITY SWITCHES
A prominent mechanism for polar delivery of cargos in animal cells is transcytosis, which involves trafficking of polar cargos from one side of the cell to the other. In the animal epithelium, this process is crucial for the polar delivery of multiple cargos (Rodriguez-Boulan et al., 2005; Leibfried and Bellaïche, 2007).
In plants, the directional translocation of PIN1 protein from the basal to the apical PM can be induced by BFA treatment, as has been directly visualized with photoconvertible PIN2 versions. After BFA removal, the basal localization of PIN proteins is restored by translocation in the opposite direction from the apical to the basal cell side (Kleine-Vehn et al., 2008). These results demonstrate that apical and basal targeting pathways in plants are interconnected and can be used by PIN proteins to move between the apical and basal sides of cells. Thus, the transcytosis mechanism in plant cells is realized by a combination of constitutive endocytic recycling and alternative recruitment of cargos by distinct ARF GEF-dependent apical and basal targeting machineries. It remains unclear to what extent the transcytosis mechanism contributes to the establishment of polar PIN localization in different cells and developmental contexts; nonetheless, it is likely that the rapid PIN polarity switches, which can be observed during different developmental processes, are realized by the transcytosis mechanism.
DEVELOPMENTAL AND ENVIRONMENTAL MODULATION OF PIN POLARITY
The connection between constitutive endocytic recycling and polar PIN localization via transcytosis provides a plausible mechanism for the quick changes in the polarity of the PIN proteins that are utilized to integrate various signals at the single-cell level and translate them into redirecting the intercellular auxin flow through the tissues, ultimately modifying the developmental program of the given tissue or organ.
The earliest known switches in PIN polarity occur during embryogenesis and contribute to the specification of the root pole and the initiation of root meristem development (Fig. 1). At the early embryogenesis stages, PIN7 is found at the apical side of the suspensor cells, where it mediates the auxin flow toward the small apical cell to specify it as a proembryo. PIN1 is expressed at this stage in a nonpolar manner in the proembryo. At the defined moment of embryo development, PIN1 polarizes toward the basal side of provascular cells and PIN7 changes its localization to the basal side of the suspensor cells. These PIN polarity rearrangements redirect the auxin flow toward the area of the future root meristem, where auxin accumulates and contributes to the specification of the root meristem (Friml et al., 2003; Weijers et al., 2005). Similarly, during postembryonic organogenesis, illustrated by the formation of lateral roots or leaves and flowers at the shoot apical meristem, PIN polarity undergoes rearrangements that are important for determining both the position of the future organ relative to the preexisting ones and the new growth axis of the organ primordium (Benková et al., 2003; Reinhardt et al., 2003; Heisler et al., 2005).
Other examples of PIN polarity changes are related to the canalization hypothesis, which assumes that auxin, by a positive feedback, can induce the capacity and polarity of its own transport within the field of cells that form the auxin channels preceding the flexible formation of the vasculature (Sachs, 1981). Indeed, predicted rearrangements of PIN polarity have been observed during leaf vasculature formation (Scarpella et al., 2006) and during vasculature regeneration after wounding (Sauer et al., 2006). The underlying mechanism is unknown, but feedback regulations of PIN polarity by auxin itself are the indispensable parts of models describing different auxin-dependent patterning processes, such as the phylotactic pattern of organ initiation (Barbier de Reuille et al., 2006; Jönsson et al., 2006; Smith et al., 2006).
Besides the changes in PIN polarity in response to internal signals, PIN polarity switches can occur also in response to environmental stimuli. After gravistimulation, PIN3 has been found to relocate to the lower side of columella cells, thus rerouting the auxin flow to the lower side of the root and triggering root bending (Friml et al., 2002b; Harrison and Masson, 2008). It is possible, although not demonstrated, that other environmental signals, such as light, trigger PIN polarity changes for mediating other developmental responses. These polarity modulations in response to various signals illustrate how the integration of signals at the level of subcellular dynamics of individual cells can be translated into the directional signaling at the tissue level and contribute to the unique plasticity of plant development.
Acknowledgments
We apologize to all authors whose important work was not cited due to space constraints. We are grateful to Martine De Cock for help in preparing the manuscript.
This work was supported by the Odysseus Programme of the Research Foundation-Flanders (grant no. G091608) and the Young Investigator Programme of the European Molecular Biology Organization.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jiří Friml (jiri.friml@psb.ugent.be).
References
- Barbier de Reuille P, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, Traas J (2006) Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc Natl Acad Sci USA 103 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067 [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273 948–950 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433 39–44 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J (2003) Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol 4 409–414 [DOI] [PubMed] [Google Scholar]
- Brown D, Stow JL (1996) Protein trafficking and polarity in kidney epithelium: from cell biology to physiology. Physiol Rev 76 245–297 [DOI] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson P (1998) The Arabidopsis thaliana AGRAVITROPIC1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA 95 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100 469–478 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17 520–527 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Baluška F, Schlicht M, Hlavacka A, Šamaj J, Friml J, Gadella TWJ Jr (2006) Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell 10 137–150 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hašek J, Paciorek T, Petrášek J, Seifertová D, Tejos R, et al (2008) Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA 105 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugani CB, Klip A (2005) Glucose transporter 4: cycling, compartments and controversies. EMBO Rep 6 1137–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6 7–12 [DOI] [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, et al (2002. a) AtPIN4 mediates sink driven auxin gradients and root patterning in Arabidopsis. Cell 108 661–673 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426 147–153 [DOI] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002. b) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415 806–809 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PBF, Ljung K, Sandberg G, et al (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306 862–865 [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44 179–194 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413 425–428 [DOI] [PubMed] [Google Scholar]
- Grebe M, Xu J, Möbius W, Ueda T, Nakano A, Geuze HJ, Rook MB, Scheres B (2003) Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr Biol 13 1378–1387 [DOI] [PubMed] [Google Scholar]
- Harrison BR, Masson PH (2008) ARL2, ARG1 and PIN3 define a gravity signal transduction pathway in root statocytes. Plant J 53 380–392 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15 1899–1911 [DOI] [PubMed] [Google Scholar]
- Irazoqui JE, Lew DJ (2004) Polarity establishment in yeast. J Cell Sci 117 2169–2171 [DOI] [PubMed] [Google Scholar]
- Janssens B, Chavrier P (2004) Mediterranean views on epithelial polarity. Nat Cell Biol 6 493–496 [DOI] [PubMed] [Google Scholar]
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E (2006) An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA 103 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K (1998) Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol 140 1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer P, Wiśniewka J, Paciorek T, Benková E, Friml J (2008) ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol 18 526–531 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J (2006) Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA (2000) Epithelial polarity: the ins and outs of the fly epidermis. Curr Biol 10 R791–R794 [DOI] [PubMed] [Google Scholar]
- Leibfried A, Bellaïche Y (2007) Functions of endosomal trafficking in Drosophila epithelial cells. Curr Opin Cell Biol 19 446–452 [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448 209–212 [DOI] [PubMed] [Google Scholar]
- Martin SW, Glover BJ, Davies JM (2005) Lipid microdomains: plant membranes get organized. Trends Plant Sci 10 263–265 [DOI] [PubMed] [Google Scholar]
- Men S, Boutté Y, Ikeda Y, Li X, Palme K, Stierhof YD, Hartmann MA, Moritz T, Grebe M (2008) Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol 10 237–244 [DOI] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130 1044–1056 [DOI] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T (2007) Plants tolerant of high boron levels. Science 318 1417. [DOI] [PubMed] [Google Scholar]
- Mohr H, Schopfer P (1995) Plant Physiology. Springer-Verlag, Berlin
- Molendijk AJ, Bischoff F, Rajendrakumar CSV, Friml J, Braun M, Gilroy S, Palme K (2001) Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J 20 2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M (2003) Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol 5 287–293 [DOI] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J (2005) PAR proteins and the establishment of cell polarity during C. elegans development. Bioessays 27 126–135 [DOI] [PubMed] [Google Scholar]
- Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, et al (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435 1251–1256 [DOI] [PubMed] [Google Scholar]
- Petrášek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wiśniewska J, Tadele Z, Kubeš M, Čovanová M, et al (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312 914–918 [DOI] [PubMed] [Google Scholar]
- Raven JA (1975) Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol 74 163–172 [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426 255–260 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Müsch A (2005) Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6 233–247 [DOI] [PubMed] [Google Scholar]
- Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GHH, Schindelman G, Song S, Baskin TI, Dupree P, Wasteneys GO, et al (2005) COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell 17 1749–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery PH, Sheldrake AR (1974) Carrier-mediated auxin transport. Planta 118 101–121 [DOI] [PubMed] [Google Scholar]
- Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9 151–162 [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wišniewska J, Reinöhl V, Friml J, Benková E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feed-back regulation of PIN polarity. Genes Dev 20 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell DE, Leu WM, Gillmor CS, Xia G, Feldmann KA, Chua NH (1994) EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell 77 1051–1062 [DOI] [PubMed] [Google Scholar]
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P (2006) A plausible model of phyllotaxis. Proc Natl Acad Sci USA 103 1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K (2002) hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 14 1017–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286 316–318 [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol (in press) [DOI] [PubMed]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, Lee OR, Richards EL, Murphy AS, Sato F, et al (2005) PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17 2922–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treml BS, Winderl S, Radykewicz R, Herz M, Schweizer G, Hutzler P, Glawischnig E, Torres Ruiz RA (2005) The gene ENHANCER OF PINOID controls cotyledon development in the Arabidopsis embryo. Development 132 4063–4074 [DOI] [PubMed] [Google Scholar]
- Utsuno K, Shikanai T, Yamada Y, Hashimoto T (1998) AGR, an Agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol 39 1111–1118 [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12 160–168 [DOI] [PubMed] [Google Scholar]
- Weijers D, Sauer M, Meurette O, Friml J, Ljung K, Sandberg G, Hooykaas P, Offringa R (2005) Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17 2517–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW (1974) Reflections and speculations. Annu Rev Plant Physiol 25 1–26 [Google Scholar]
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B (2003) Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15 612–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewska J, Xu J, Seifertová D, Brewer PB, Ruzicka K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312 883. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16 1123–1127 [DOI] [PubMed] [Google Scholar]