Abstract
Glycerol-3-phosphate (G3P) is an important component of carbohydrate and lipid metabolic processes. In this article, we provide evidence that G3P levels in plants are associated with defense to a hemibiotrophic fungal pathogen Colletotrichum higginsianum. Inoculation of Arabidopsis (Arabidopsis thaliana) with C. higginsianum was correlated with an increase in G3P levels and a concomitant decrease in glycerol levels in the host. Plants impaired in utilization of plastidial G3P (act1) accumulated elevated levels of pathogen-induced G3P and displayed enhanced resistance. Furthermore, overexpression of the host GLY1 gene, which encodes a G3P dehydrogenase (G3Pdh), conferred enhanced resistance. In contrast, the gly1 mutant accumulated reduced levels of G3P after pathogen inoculation and showed enhanced susceptibility to C. higginsianum. Unlike gly1, a mutation in a cytosolic isoform of G3Pdh did not alter basal resistance to C. higginsianum. Furthermore, act1 gly1 double-mutant plants were as susceptible as the gly1 plants. Increased resistance or susceptibility of act1 and gly1 plants to C. higginsianum, respectively, was not due to effects of these mutations on salicylic acid- or ethylene-mediated defense pathways. The act1 mutation restored a wild-type-like response in camalexin-deficient pad3 plants, which were hypersusceptible to C. higginsianum. These data suggest that G3P-associated resistance to C. higginsianum occurs independently or downstream of the camalexin pathway. Together, these results suggest a novel and specific link between G3P metabolism and plant defense.
Plants have evolved highly specific mechanisms to resist pathogens. The most studied of these involves deployment of resistance (R) proteins, which, in most cases, are effective against specific races of pathogens carrying corresponding avirulence genes (gene-for-gene interactions; Flor, 1971). Several lines of evidence support the existence of an intricate signaling network involving various plant hormones that facilitates fine tuning of all of these resistance mechanisms and influences the amplitude of various signals derived from the various defense response pathways. Significant progress has been made, particularly in the model cruciferous plant Arabidopsis (Arabidopsis thaliana), in identifying components of the major defense signaling pathways (Feys and Parker, 2000; Glazebrook, 2001; Thomma et al., 2001; Durrant and Dong, 2004; Kachroo and Kachroo, 2007).
The establishment and maintenance of a metabolic sink is a crucial aspect of plant pathogenesis that has received very little attention in comparison to signaling involved during initial recognition of a pathogen (Asahi et al., 1979; Pennypacker, 2000; Solomon et al., 2003; Oliver and Ipcho, 2004). Colonization by a fungal pathogen is associated with multiple metabolic changes in the plant host and especially with increases in the expression of many genes encoding proteins involved in nutrient cycling and in sugar, amino acid, and mineral transport (AbuQamar et al., 2006). Primary metabolites are known to have important roles as signaling molecules in plants (Rolland et al., 2002). Because the efficient movement of nutrients from host to pathogen is an essential component of pathogenicity (Hancock and Huisman, 1981), it seems reasonable to suspect that primary metabolic pathways and metabolic signaling in both plants and pathogens could interface with disease-related signaling.
Recent evidence has suggested that components of primary metabolism can indeed act as signals regulating various aspects of plant defense (Schaaf et al., 1995; Ehness et al., 1997; Scheideler et al., 2002; Price et al., 2003; Lipka et al., 2005; Chandra-Shekara et al., 2007). For example, Glc-6-P (Gl6P) dehydrogenase (Gl6Pdh) catalyzes a rate-limiting step in the pentose phosphate pathway, and the activity of this enzyme appears to be essential for the proper localization of NPR1, a key component of the salicylic acid (SA) defense pathway in Arabidopsis (Dong, 2004). The pentose phosphate and glycolytic pathways exert regulatory control over the shikimic acid pathway, which provides precursors for synthesis of SA, Trp, and auxin (Taiz and Zeiger, 2006). As another example, vitamin B1 and Suc induce resistance to pathogens of Arabidopsis and rice (Oryza sativa), respectively (Ahn et al., 2005; Gomez-Ariza et al., 2007). Recent evidence has also shown that both fatty acid (FA) and carbohydrate metabolism play important roles in plant defense and are involved in cross-talk with various phytohormones, including SA, jasmonic acid (JA), and abscisic acid (Schaaf et al., 1995; Ehness et al., 1997; Scheideler et al., 2002; Kachroo et al., 2003, 2005; Price et al., 2003; Schaarschmidt et al., 2007). The observation that metabolic defects, resulting in an impaired cuticle or cell wall, compromise R-gene-mediated resistance to bacterial pathogens (Tang et al., 2007) and basal resistance to fungal pathogens (Bessire et al., 2007; Huckelhoven, 2007), respectively, further emphasizes the key role of metabolic pathways in plant defense.
Glycerol, a polyalcohol produced during the breakdown of Glc, proteins, pyruvate, triacylglycerols, and other glycerolipids, is a common cellular metabolite present in a wide range of organisms. Glycerol metabolism is initiated upon its conversion to glycerol-3-P (G3P), which can be derived via the glycerol kinase (GK)-mediated phosphorylation of glycerol or via G3P dehydrogenase (G3Pdh)-mediated reduction of dihydroxyacetone phosphate (DHAP; see Fig. 1). The fundamentally important role of glycerol metabolism is underscored by the high degree of sequence conservation among proteins catalyzing these reactions from evolutionary diverse organisms (Brisson et al., 2001). It has been suggested that glycerol is a primary transferred carbon metabolite during intercellular growth of Colletotrichum gloesporioides in its host, round-leaved mallow (Malva pusilla; Wei et al., 2004). This, together with the observation that glycerol metabolism participates in host defense (Kachroo et al., 2004, 2005, 2008; Chandra-Shekara et al., 2007), suggested a role for glycerol and its metabolites in both host defense and pathogenesis.
Figure 1.
A condensed scheme of glycerol metabolism in plants. Glycerol is phosphorylated to G3P by GK (GLI1). G3P can also be generated by G3Pdh via the reduction of DHAP in both the cytosol and the plastids (represented by the oval). G3P generated by this reaction can be transported between the cytosol and plastidial stroma. In the plastids, G3P is acylated with oleic acid (18:1) by the ACT1-encoded G3P acyltransferase. This ACT1-utilized 18:1 is derived from the stearoyl-acyl carrier protein (ACP)-desaturase (SSI2)-catalyzed desaturation of stearic acid (18:0). The 18:1-ACP generated by ACT1 either enters the prokaryotic lipid biosynthetic pathway through acylation of G3P or is exported out of the plastids as a CoA-thioester to enter the eukaryotic lipid biosynthetic pathway. Lyso-PA, Acyl-G3P; PA, phosphatidic acid; PG, phosphatidylglycerol; MGD, monogalactosyldiacylglycerol; DGD, digalactosyldiacylglycerol; SL, sulfolipid; DAG, diacylglycerol.
The hemibiotrophic fungus Colletotrichum higginsianum is pathogenic to Arabidopsis (Narusaka et al., 2004; O'Connell et al., 2004). Hemibiotrophs, like true biotrophs, establish an intimate intracellular contact with their host cells during the initial phases of infection. The defining characteristic of necrotrophic pathogens is that they kill host tissues in advance of, or concurrent with, colonization, and feed on the dead cells (Williams, 1979; Yoder and Turgeon, 2001; Schulze-Lefert and Panstruga, 2003). Examples include Alternaria brassicicola and Botrytis cinerea, both of which have been studied as pathogens of Arabidopsis (Glazebrook, 2005). Because they undergo both biotrophic and necrotrophic development at different points in the disease cycle, Colletotrichum fungi are uniquely suitable for studies aimed at uncovering unifying principles underlying both types of interaction (Perfect et al., 1999). In this study, we have evaluated the response of various Arabidopsis mutants that are altered in glycerol metabolism to C. higginsianum. We show that the glycerol metabolic activities in the host, leading to synthesis of G3P, are important for basal defense against C. higginsianum.
RESULTS
Mutations in Arabidopsis G3P Synthesis Enzymes Are Associated with Enhanced Susceptibility to Colletotrichum
In vitro growth experiments showed that Glc and glycerol supported similar growth of C. higginsianum (data not shown), suggesting that the fungus was capable of using glycerol as a sole source of carbon. To determine whether high endogenous glycerol levels in the host also supported more growth of the pathogen, we evaluated the response of gli1 plants, which are unable to convert glycerol to G3P (Fig. 1) and, consequently, accumulate approximately 6-fold higher levels of glycerol (Fig. 2A). In comparison to wild type, the gli1 plants showed increased susceptibility to the pathogen (Fig. 2, B and C). Although this and the in vitro data suggested that high levels of glycerol in gli1 might be responsible for enhanced susceptibility, an alternative possibility was that it was the reduced G3P levels in gli1 that were important. To address this, we determined glycerol levels and pathogen response in gly1 plants, which are impaired in conversion of DHAP to G3P (Fig. 1; Kachroo et al., 2004). Although the gly1 mutant accumulated wild-type-like levels of glycerol (Fig. 2A), this mutant was even more susceptible than gli1 (Fig. 2, B and C). Because both gly1 and gli1 plants are affected in steps leading to synthesis of G3P, these results suggested that G3P levels were important for basal defense to C. higginsianum.
Figure 2.
Glycerol levels and pathogen response in C. higginsianum-inoculated plants. A, Basal glycerol levels in 4-week-old Col-0, gly1, and gli1 leaves. B, Disease symptoms on Col-0, gly1, and gli1 plants spray inoculated with 106 spores/mL of C. higginsianum. C, Lesion size in spot-inoculated genotypes. The plants were spot inoculated with 106 spores/mL of C. higginsianum and the lesion size was measured from 20 to 30 independent leaves at 6 dpi. Statistical significance was determined using Student's t test. Asterisks indicate data statistically significant from that of control (Col-0; P < 0.05). Error bars indicate sd.
We next determined levels of glycerol in Arabidopsis plants inoculated with C. higginsianum and found that the glycerol levels were reduced to approximately 35% at 4 d postinoculation (dpi) in comparison to controls (Fig. 3A). A similar phenomenon observed in C. gloesporioides-inoculated round-leaved mallow plants was interpreted as pathogen utilization of host glycerol (Wei et al., 2004). An alternative possibility was that host glycerol was decreasing because it was being metabolized to G3P by the plant in response to pathogen infection. We measured the G3P levels in mock- and pathogen-inoculated plants and, indeed, C. higginsianum-inoculated plants accumulated approximately 4-fold higher levels of G3P than controls (Fig. 3B). However, a stoichiometric correlation was not observed between the decrease in glycerol and the increase in G3P. This could be because there are at least two enzymes, GLI1 (NHO1) and GLY1, in Arabidopsis that contribute to the synthesis of G3P (Fig. 1). GLI1 (At1g80460)-encoded GK uses glycerol as a substrate (Kang et al., 2003), whereas GLY1 (At2g40690)-encoded G3Pdh uses DHAP (Kachroo et al., 2004).
Figure 3.
Levels of glycerol and G3P in C. higginsianum-inoculated plants. A, Glycerol levels in mock (water)- or C. higginsianum-inoculated wild-type (Col-0) plants. Plants were spray inoculated (106 spores/ml) and samples were collected at indicated times. B, G3P levels in mock (water)- or C. higginsianum-inoculated plants. Plants were spray inoculated and samples were collected at indicated times.
Increased G3P Levels Are Associated with Enhanced Resistance to C. higginsianum
We further tested the possibility that the observed increase in G3P, rather than the decrease in glycerol, was important for basal resistance of Arabidopsis to C. higginsianum by evaluating the response of act1 plants to pathogen inoculations. ACT1 (At1g32200) catalyzes the acylation of oleic acid (18:1) on the G3P backbone (Fig. 1; Kunst et al., 1988). Because act1 plants accumulate 18:1, they would also be expected to accumulate G3P. Basal levels of G3P were slightly increased in act1 compared to wild type (Fig. 4A). The act1 plants were more resistant to C. higginsianum than wild-type plants (Fig. 4, B and C). In planta infection assays indicated that there was a significant reduction in the number of primary infection hyphae that were successfully established in act1 leaves as compared to wild type (Fig. 4D). In contrast, gly1 leaves, with lower basal levels of G3P (Fig. 4A), supported the establishment of approximately 2-fold more primary infection hyphae than wild-type leaves (Fig. 4D). Increased symptoms on gly1 plants were further correlated with increased fungal growth by evaluating the β-tubulin transcript levels (Fig. 4E). cDNA prepared from the wild-type, gly1, and act1 plants were normalized for fungal β-tubulin transcript and then assessed for the host β-tubulin transcript levels using reverse transcription (RT)-PCR. In comparison to wild type, much less, or an excess of, host total RNA from gly1 and act1 plants, respectively, was required to detect the same transcript levels of the fungal β-tubulin. These data suggested that the appearance of symptoms correlated with fungal growth.
Figure 4.
G3P and neutral sugar levels, in planta growth, and pathogen response in C. higginsianum-inoculated plants. A, Basal levels of G3P in 4-week-old Col-0, gly1, and act1 plants. B, Disease symptoms on Col-0, gly1, act1, and gly1 act1 plants spray inoculated with 106 spores/mL of C. higginsianum. C, Lesion size in spot-inoculated genotypes. The plants were spot inoculated with 106 spores/mL C. higginsianum and the lesion size was measured from 20 to 30 independent leaves at 6 dpi. Statistical significance was determined using Student's t test. Asterisks indicate data statistically significant from that of control (Col-0; P < 0.05). Error bars indicate sd. D, Percentage established primary hyphae of C. higginsianum on Col-0, gly1, or act1 petioles. Statistical significance was determined using Student's t test. Asterisks indicate data statistically significant from that of control (Col-0; P < 0.05). E, RT-PCR analysis showing levels of host (Arabidopsis) and fungal (C. hig) β-tubulin in C. higginsianum-inoculated Col-0, gly1, and act1 plants. F, Time-course measurement of G3P in Col-0, act1, or gly1 plants inoculated with C. higginsianum. G, Levels of neutral sugars in C. higginsianum-inoculated Col-0 plants. Plants were spray inoculated and samples were collected at indicated times.
To test whether the observed effect was due to some other function of ACT1 independent of G3P levels, we generated act1 gly1 double-mutant plants and inoculated them with C. higginsianum. Because the G3P utilized by the ACT1-catalyzed reaction is derived via GLY1 (Miquel et al., 1998; Kachroo et al., 2004), G3P levels in act1 gly1 plants should be similar to those in gly1 plants. Like gly1 plants, the act1 gly1 plants were more susceptible to C. higginsianum than wild-type plants (Fig. 4, B and C). Next, we measured changes in G3P levels in C. higginsianum-inoculated wild-type, act1, and gly1 plants. Because gly1 plants were nearly killed within 96 h of pathogen inoculation, G3P levels were monitored only up to 72 h postinoculation (hpi). In comparison with wild-type plants, pathogen inoculation resulted in approximately 2-fold higher levels of G3P in act1 and approximately 2-fold reduced levels of G3P in gly1 plants at 72 hpi (Fig. 4F).
We found that, in addition to G3P, the levels of Glc and Fru also increased significantly in C. higginsianum-inoculated wild-type plants (Fig. 4G). In contrast, Suc levels decreased, whereas sorbitol levels increased only marginally, and Gal levels did not change significantly. We compared the levels of these sugars in water- and pathogen-treated wild-type, gly1, and act1 genotypes to evaluate whether they were also associated with the pathogen response, like G3P. Pathogen-inoculated leaves of wild-type, gly1, and act1 plants accumulated similar amounts of Fru and Gal (Supplemental Fig. S1A) and varying levels of Glc, Suc, and sorbitol (Supplemental Fig. S1B). However, unlike G3P, the levels of Glc, Suc, and sorbitol did not correlate with the increased susceptibility and resistance of the gly1 and act1 plants, respectively. Together, these data provide further evidence that induced increases in levels of G3P are specifically associated with increased resistance to the pathogen.
To address the question of whether the relative changes in G3P levels in act1 and gly1 plants were a result versus a cause of the final disease outcome, we used a pharmacological approach. In comparison to water treatment, exogenous application of G3P in wild-type plants resulted in enhanced resistance to C. higginsianum (Fig. 5A). Because exogenous application of glycerol also increases endogenous G3P levels (Aubert et al., 1994), we next evaluated pathogen response in water- and glycerol-treated plants. Similar to G3P, exogenous application of glycerol also resulted in enhanced resistance in both wild-type and gly1 plants (Fig. 5B). In contrast, gli1 plants, which are unable to convert glycerol to G3P (Kang et al., 2003; Eastmond, 2004), did not show enhanced resistance as a result of glycerol treatment. These results suggest that increased G3P causes increased resistance.
Figure 5.
Pathogen response in plants pretreated with G3P or glycerol. A, Lesion size in spot-inoculated Col-0 plants treated with water or G3P. The lesion size was measured from approximately 20 independent leaves at 9 dpi. Error bars indicate sd. B, Lesion size in spot-inoculated Col-0, gly1, or gli1 plants treated with water or glycerol. The lesion size was measured from 20 to 30 independent leaves at 7 dpi. Error bars indicate sd.
Overexpression of GLY1 Confers Enhanced Resistance to C. higginsianum
As a further test of the hypothesis that increased G3P levels enhance resistance to C. higginsianum, we overexpressed GLY1 in wild-type (Columbia [Col-0] ecotype) plants under the control of the cauliflower mosaic virus 35S promoter. Transgenic plants overexpressing GLY1 were morphologically similar to wild-type plants and different lines showed low, moderate, or high expression levels of the transgene (data not shown). Pathogen inoculations showed that the T2 plants overexpressing high levels of GLY1 were resistant, but lines expressing low or moderate levels were as susceptible as wild type (Fig. 6; data not shown). Spot inoculation of leaves of the 35S-GLY1 plants expressing high levels of GLY1 transcript resulted in smaller lesions in comparison with inoculated wild-type plants (Fig. 6, A–C). Fungal mycelia did not proliferate beyond the site of inoculation on 35S-GLY1 plants, whereas wild-type plants supported extensive colonization that continued to spread beyond the initial inoculation site (Fig. 6D).
Figure 6.
Analysis of transgenic lines overexpressing GLY1. A, Expression of the GLY1 gene in wild-type or two independent T1 transgenic lines. Both transgenic lines shown here expressed highest levels of the GLY1 transgene. RNA gel-blot analysis was performed on approximately 7 μg of total RNA. Ethidium bromide staining of rRNA was used as a loading control. B, Disease symptoms in mock (M)- or C. higginsianum (C. hig)-inoculated Col-0 or 35S-GLY1 plants at 5 dpi. The plants were spot inoculated with 105 spores/mL of C. higginsianum. C, Lesion size in spot-inoculated genotypes. The plants were spot inoculated with water or 105 spores/mL and the lesion size was measured from 20 to 30 independent leaves at 6 dpi. Statistical significance was determined using Student's t test. Asterisks indicate data that are statistically significant from that of control (Col-0; P < 0.05). Error bars indicate sd. D, Microscopy of trypan blue-stained leaves from Col-0 and 35S-GLY1 plants inoculated with C. higginsianum. The leaves were harvested at 5 dpi. Dark spots marked by arrows are appressoria.
To test whether overexpression of GLY1 would enhance resistance in the absence of GLI1 function, we mobilized the gli1 mutation into the 35S-GLY1 background and analyzed gli1 35S-GLY1 plants for their response to C. higginsianum. The gli1 35S-GLY1 plants were significantly more resistant than the gli1 plants, but more susceptible than 35S-GLY1 (Fig. 6C). This suggests that the GLY1- and GLI1-catalyzed reactions have additive effects, but that GLY1 is the more important player. Based on all of the evidence together, we concluded that the accumulation of G3P or of a G3P-derived metabolite has an important role in basal resistance to C. higginsianum in Arabidopsis.
A Mutation in the Cytosolic Isoform of G3Pdh Does Not Impair Resistance to C. higginsianum
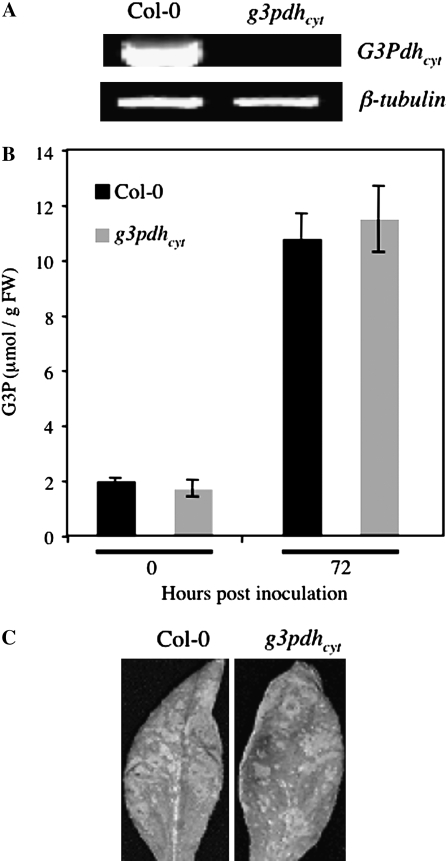
In addition to GLY1, the Arabidopsis genome encodes four other G3Pdh isoforms that are predicted to localize to cytosol (At2g41540, At3g07690), mitochondria (At3g10370), or plastids (At5g40610; Wei et al., 2001; Shen et al., 2003). To test whether enhanced susceptibility to C. higginsianum was specific to a mutation in the GLY1 isoform of G3Pdh, we isolated a knockout (KO) mutation in another G3Pdh isoform (At2g41540; designated g3pdhcyt), which, like GLY1, is predicted to localize to the cytosol and was recently shown to contribute to the total G3P pool (Shen et al., 2006). The KO plants did not amplify any detectable G3Pdhcyt transcript (Fig. 7A) and were morphologically similar to wild-type (Col-0) or gly1 plants (data not shown). The basal levels of G3P in g3pdhcyt plants were consistently higher than in gly1 and only marginally reduced as compared to wild-type plants (Figs. 4A and 7B). In contrast with the gly1 mutant, pathogen-associated G3P increases in g3pdhcyt plants were similar to wild-type plants (Figs. 4F and 7B). Because reduced G3P levels in gly1 plants lead to a reduction in hexadecatrienoic acid (16:3) FA (Miquel et al., 1998; Kachroo et al., 2004), we compared the FA profiles of wild-type, gly1, and g3pdhcyt plants to determine the effect of g3pdhcyt on the plastidial G3P pool. Unlike gly1 plants, the g3pdhcyt plants had wild-type-like levels of all FAs, including 16:3 (Table I). This suggests that, in contrast to GLY1, G3Pdhcyt does not contribute significantly to the plastidial G3P pool. Also in contrast to gly1 plants, spray-inoculated g3pdhcyt plants were no more susceptible to C. higginsianum than the wild type (Fig. 7C). Together, these results suggest that the observed decrease in pathogen-induced G3P levels and associated enhanced susceptibility were specific to a mutation in the GLY1 isoform of G3Pdh.
Figure 7.
Molecular analysis, pathogen response, and G3P levels of g3pdhcyt plants. A, RT-PCR analysis of RNA extracted from Col-0 and g3pdhcyt using gene-specific primers for G3Pdhcyt. The level of β-tubulin was used as an internal control to normalize the amount of cDNA template. B, G3P levels in Col-0 and g3pdhcyt plants at 0 and 72 hpi. C, Disease symptoms on Col-0 or g3pdhcyt plants inoculated with C. higginsianum. Plants were spray inoculated with 106 spores/mL and the leaves were photographed at 4 dpi.
Table I.
FA composition of total leaf lipids from GLY1, gly1, and g3pdhcyt plants
| Genotype | Fatty Acida
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 16:2 | 16:3 | 18:0 | 18:1b | 18:2 | 18:3 | |
| GLY1 | 15.8 ± 1.2 | 3.0 ± 0.3 | 0.6 ± 0.1 | 14.2 ± 0.9 | 0.8 ± 0.1 | 1.9 ± 0.5 | 13.2 ± 2.0 | 50.5 ± 2.9 |
| gly1 | 18.1 ± 1.3 | 3.5 ± 0.4 | 0.6 ± 0.1 | 5.3 ± 0.6 | 0.8 ± 0 | 4.0 ± 0.5 | 16.7 ± 1.4 | 51.0 ± 1.7 |
| g3pdhcyt | 14.6 ± 1.0 | 3.9 ± 0.1 | 0.8 ± 0.2 | 14.2 ± 0.8 | 0.8 ± 0.1 | 1.9 ± 0.4 | 13.6 ± 1.4 | 50.2 ± 0.6 |
All measurements were made on plants grown at 22°C. Data are described as mol% ± sd (n = 6).
18:1 Δ9.
The act1 Mutation Overcomes Enhanced Susceptibility Conferred by a Deficiency in Camalexin
To determine whether G3P-associated resistance in act1 plants overlapped with known defense pathways, we assessed the effect of the act1 mutation in pad3 plants, which are deficient in camalexin and show enhanced susceptibility to C. higginsianum (Narusaka et al., 2004). Inoculation of pad3 plants with C. higginsianum resulted in much larger lesions, on average, as compared with wild type. In contrast, the average lesion size on act1 pad3 leaves was similar to that on wild-type leaves (Fig. 8, A and B). Thus, the act1 mutation can compensate for the pad3 mutation and a deficiency in camalexin. However, act1 pad3 plants were more susceptible than act1, suggesting that the compensatory effect mediated by the act1 mutation was partial. We next determined whether act1-mediated enhancement of resistance in the pad3 background was associated with an increase in camalexin levels (Fig. 8C). The camalexin levels increased approximately 5-fold in pathogen-inoculated wild-type plants, approximately 7-fold in act1 plants, approximately 2-fold in pad3 plants, and only marginally in act1 pad3 plants. These data confirm that act1-conferred enhanced resistance in the pad3 background was not associated with an increase in camalexin levels. Taken together, these data suggest that G3P-mediated basal resistance to C. higginsianum operates independent of, or downstream from, PAD3-mediated basal resistance.
Figure 8.
Pathogen response and camalexin levels in water- and C. higginsianum-inoculated wild-type (Col-0), pad3, and act1 pad3 plants. A, Disease symptoms on Col-0, act1, pad3, or act1 pad3 plants spot inoculated with 106 spores/mL of C. higginsianum. The leaves were photographed at 7 dpi. B, Lesion size in spot-inoculated Col-0, act1, pad3, or act1 pad3 plants. The lesion size was measured from 20 to 30 independent leaves at 7 dpi. Asterisks indicate data that are statistically significant from that of control (Col-0; P < 0.05). Error bars indicate sd. C, Camalexin levels in water- or C. higginsianum-inoculated Col-0, act1, pad3, or act1 pad3 plants determined at 3 dpi.
Enhanced Susceptibility of gly1 Plants Is Not Due to a Defect in SA or Ethylene Pathways
Because a mutation in sid2 results in enhanced susceptibility to C. higginsianum (Liu et al., 2007), we next evaluated the roles of SA and PR-1 in the interaction and the relationship between pathogen-triggered accumulation of SA/PR-1 and G3P levels in various host genotypes. The wild-type Col-0 plants inoculated with C. higginsianum induced high levels of PR-1 gene expression (Fig. 9A). The PR-1 gene expression correlated with accumulation of free and bound forms of SA; the inoculated leaves accumulated approximately 60-fold higher levels of SA and approximately 42-fold higher levels of SA glucoside (SAG) than mock-inoculated leaves (Fig. 9, B and C). To determine whether expression of PR-1 was associated with the G3P-associated resistance of act1 plants, we compared PR-1 expression levels in Col-0, gly1, and act1 plants before and after inoculation with the fungus. The inoculated leaves of all three genotypes showed a similar induction of PR-1 gene expression (Fig. 9A), suggesting that C. higginsianum was capable of eliciting a normal SA-dependent defense response in gly1 and act1 plants and that resistance of act1 plants was not associated with increased accumulation of PR-1 transcript. In contrast to PR-1 transcript, the pathogen-induced free SA levels in gly1 and act1 plants correlated with their infection phenotypes; pathogen-induced SA levels in gly1 and act1 plants were higher (approximately 186-fold) and lower (approximately 28-fold), respectively, than the wild-type plants (Fig. 9B). However, in comparison to mock-inoculated plants, pathogen-induced SAG levels were highest in wild type (approximately 42-fold), followed by act1 (approximately 30-fold) and gly1 (approximately 26-fold) plants (Fig. 9C). Together, these results suggest that the gly1 plants are able to accumulate high SA in response to C. higginsianum and that their enhanced susceptibility phenotype is not due to a defect in the SA pathway.
Figure 9.
PR-1 and PDF1.2 gene expression and SA/SAG levels. A, Northern-blot analysis of PR-1 gene expression in Col-0, gly1, and act1 plants spray inoculated with water or 106 spores/mL of C. higginsianum. The samples were collected at 3 dpi. RNA gel-blot analysis was performed on approximately 7 μg of total RNA extracted and ethidium bromide staining of rRNA was used as a loading control. B, SA levels in Col-0, gly1, and act1 plants inoculated with water or 106 spores of C. higginsianum. The plants were spray inoculated and the samples were collected at 3 dpi. C, SAG levels in Col-0, gly1, and act1 plants inoculated with 106 spores of C. higginsianum. The plants were spray inoculated and the samples were collected at 3 dpi. D, Northern-blot analysis of PDF1.2 gene expression in Col-0 plants spray inoculated with water (mock) or 106 spores/mL of C. higginsianum. The samples were collected at 3 dpi. RNA gel-blot analysis was performed on approximately 7 μg of total RNA extracted and ethidium bromide staining of rRNA was used as a loading control.
Because a mutation in the ethylene defense signaling pathway also leads to enhanced susceptibility of C. higginsianum (Liu et al., 2007), we next evaluated the relationship between pathogen-triggered accumulation of PDF1.2 and G3P levels in various host genotypes. The inoculated leaves of wild-type, gly1, and act1 genotypes showed a similar induction of PDF1.2 gene expression (Fig. 9D). Because both ethylene and JA pathways are required for induction of PDF1.2 (Penninckx et al., 1998), this result suggests that gly1 and act1 plants are not impaired in either ethylene or JA pathways. This was further corroborated by analyzing expression of PDF1.2 in response to exogenous application of ethylene or JA; both gly1 and act1 plants showed similar induction of PDF1.2 in response to ethylene or JA (data not shown). Together, these results suggest that increased susceptibility of gly1 plants is not due to impaired ethylene or JA pathways.
Enhanced Susceptibility of gly1 Plants Is Not Due to Increased Sensitivity to Reactive Oxygen Species
Increased sensitivity to reactive oxygen species (ROS) has been associated with enhanced susceptibility to some necrotrophic pathogens (Mengiste et al., 2003). To test whether increased levels of ROS in gly1 might be responsible for the increased susceptibility of this genotype to the hemibiotroph C. higginsianum, we quantified and compared basal and pathogen-induced H2O2 levels in wild-type and gly1 plants. Both genotypes had similar basal levels of H2O2 (Fig. 10A). This was further investigated by histochemical staining of the leaves using diaminobenzidine or fluorescent staining using 2′,7′-dichlorofluorescein (data not shown). Furthermore, the inoculated leaves of wild-type and gly1 plants showed a similar increase (approximately 4-fold) in H2O2 levels. The wild-type and gly1 plants also were tested for sensitivity to paraquat (methyl viologen), an agent that promotes the formation of ROS by inhibiting electron transport during photosynthesis. The plants were treated by placing a 5-μL droplet of 5 to 100 μm paraquat on individual leaves and the lesion size was monitored after 12, 24, and 48 h posttreatment. The wild-type and gly1 plants showed similar size lesions (data shown for 48 h; Fig. 10, B and C), suggesting that gly1 plants are not more sensitive to ROS than wild-type plants.
Figure 10.
Basal and pathogen-induced H2O2 levels and sensitivity to paraquat. A, Basal and C. higginsianum-induced H2O2 levels in 4-week-old Col-0 and gly1 plants. The plants were spray inoculated with 106 spores/mL of C. higginsianum and the samples were collected at 3 dpi. B, Lesion size in Col-0 or gly1 leaves treated with 15 or 25 μm paraquat. The lesion size was measured from approximately 15 independent leaves at 2 d posttreatment. C, Typical morphological phenotype seen in leaves spot inoculated with 15 μm paraquat. The leaves were photographed 2 d posttreatment.
DISCUSSION
The pathosystem involving Arabidopsis and the hemibiotrophic pathogen C. higginsianum offers an exciting opportunity to investigate corresponding mechanisms of pathogenicity and defense in these two experimentally amenable organisms. Based on the evidence described here, we suggest that levels of host G3P, or of G3P-derived metabolites, are important for basal resistance to C. higginsianum. A mutation in GKGLI1 or G3PdhGLY1 conferred enhanced susceptibility to C. higginsianum, and overexpression of G3PdhGLY1 increased resistance to the pathogen. Resistance was correlated with the endogenous G3P levels in the host. Although inoculation with C. higginsianum also led to an increase in hexose sugars in the plant tissues, this increase did not correlate with the enhanced susceptibility or resistance phenotypes seen in the gly1 and act1 plants, respectively. An increase in hexose sugars also occurs in compatible interactions involving biotrophic pathogens and is believed to result from decreased photosynthesis and/or increased respiration and invertase activity (Berger et al., 2007).
In Arabidopsis, G3P can be synthesized via the GK-mediated phosphorylation of glycerol or the G3Pdh-mediated reduction of DHAP. The Arabidopsis genome encodes a single GK and multiple isoforms of G3Pdh. The relative contributions of these proteins, in generating the Arabidopsis G3P pool, probably differ in different cellular compartments and during various cellular processes. For example, even though GK is one of the key enzymes contributing to G3P biosynthesis, a mutation in GK (gli1) does not alter the plastidial 16:3 levels (Kachroo et al., 2005). This suggests that, rather than being directed toward chloroplastic lipid biosynthesis, a majority of the G3P generated via GK remains in the cytosol. In comparison, a mutation in G3PdhGLY1 impairs chloroplastic lipid synthesis, resulting in the reduction of 16:3 levels (Miquel et al., 1998; Kachroo et al., 2004). Our findings showed that GLY1 was the major player in generation of G3P relevant to basal resistance to C. higginsianum.
GLY1-overexpressing plants and act1 plants are both resistant to C. higginsianum in contrast with the wild type, which is susceptible to the pathogen. Both act1 and GLY1-overexpressing plants accumulated significantly higher G3P in response to pathogen infection. This suggests that an ability to accumulate G3P upon inoculation is important for expression of high levels of resistance. Although the basal G3P levels in act1 were marginally higher than wild type, GLY1-overexpressing plants had wild-type-like basal levels. One possibility is that substrate (DHAP) levels limit the accumulation of G3P in 35S-GLY1 plants in spite of increased availability of the enzyme and that pathogen inoculation may increase the levels of DHAP. Indeed, substrate limitations also affect activities of other enzymes participating in glycerol metabolism, including GK (S.C. Venugopal, A. Kachroo, and P. Kachroo, unpublished data) and ACT1 (Kachroo et al., 2004). For example, ACT1-overexpressing plants do not develop the constitutive defense phenotypes associated with reduced 18:1 levels unless treated with glycerol (Kachroo et al., 2004). Glycerol application serves to increase the substrates for ACT1-catalyzed reactions because ACT1 utilizes G3P as a substrate for acylation with 18:1 (Kunst et al., 1988).
Because mutations in act1 and gly1 do not influence the resistance response to a nonfungal pathogen (Chandra-Shekara et al., 2007), G3P metabolism appears to play a specific role in the resistance response to C. higginsianum. G3P levels can affect several enzymatic processes involved in the synthesis of carbohydrates or amino acids (Aubert et al., 1994). For instance, high levels of G3P can act as a competitive inhibitor of Gl6P isomerase and prevent generation of Gl6P. Gl6P serves as a starting material for the pentose phosphate pathway and as a substrate for Gl6Pdh, which is required for monomerization of NPR1, a key regulator of the SA signaling pathway (Dong, 2004). Thus, it is conceivable that G3P levels modulate one or more primary or secondary metabolic pathways, which in turn are associated with plant defense signaling that occurs specifically in response to C. higginsianum infection.
Increased catalysis by ACT1 also results in a concomitant decrease in 18:1 levels, which in turn can induce a novel broad-spectrum resistance-conferring pathway in Arabidopsis (Kachroo et al., 2004, 2005; Chandra-Shekara et al., 2007). However, act1 mutant plants are unable to reduce 18:1 levels in response to glycerol application (Kachroo et al., 2004, 2005, 2008; Chandra-Shekara et al., 2007). Therefore, increased resistance to C. higginsianum in act1 plants is probably not derived from the 18:1-mediated defense pathway, but is likely to be associated with increases in the levels of G3P itself. Also, because both gly1 and act1 plants are affected in biosynthesis of plastidial lipids (Kunst et al., 1988; Miquel et al., 1998; Kachroo et al., 2003, 2004), the act1-conferred enhanced resistance to C. higginsianum may not involve plastidial lipid biosynthesis.
Recent evidence suggests that, in addition to synthesis of G3P, cytosolic G3Pdh participates in a mitochondrial G3P shuttle, impairment of which results in increased basal levels of ROS (Shen et al., 2006). ROS are known to play a role in plant defense against biotrophic pathogens and also contribute to cell death (Kotchoni and Gachomo, 2006). A positive correlation between cell death and increased necrotrophic growth (Gorvin and Levine, 2000), however, cannot be attributed as a cause for increased susceptibility in gly1 plants because they appear to accumulate basal levels of ROS similar to the wild type and are no more sensitive to ROS than the wild type. Because gly1 plants are also impaired in systemic acquired resistance to a bacterial pathogen (Nandi et al., 2004), it is likely that G3P levels contribute toward several physiological responses required for initiating or maintaining normal defense against a wide range of pathogens. Enhanced susceptibility of gli1 to the necrotrophic pathogen Botrytis cinerea (Kang et al., 2003) further supports this assertion.
Based on these data, we propose a novel role for glycerol metabolism in basal resistance. Future research will focus on understanding the flux of glycerol metabolic intermediates during pathogenesis in the host.
MATERIALS AND METHODS
Plant and Fungal Growth Conditions, Genetic Analyses, and Pathogen Inoculations
Plants were grown in MTPS 144 Conviron walk-in chambers at 22°C, 65% relative humidity, and a 14-h photoperiod. Crosses were performed by pollinating emasculated flowers of gly1 or gli1 plants with pollen from act1 or 35S-GLY1 plants, respectively. act1 pad3 plants were obtained from act1 × pad3 cross. All these genotypes were in the Col-0 ecotype background. Genotypes were determined by conducting cleaved amplified polymorphic sequence analysis. A KO mutation in G3Pdhcyt (At2g41540) was identified by screening SALK insertional line 020444. The KO plants were screened using gene-specific (GATGTGAAACTACCCCTTCCC, CTGTGGAGCTGCTAAATGGAG) and gene-specific and a T-DNA left-border primer (GCGTGGACCGCTTGCTGCAACT).
Colletotrichum higginsianum Sacc. (IMI 349063) was obtained from CABI Bioscience. The fungus was maintained on potato dextrose agar.
Four-week-old Arabidopsis (Arabidopsis thaliana) plants were used for both spray and spot inoculations. Spore suspensions at concentrations of 104 to 106 spores/mL were used for various experiments. For spot inoculations, 10 μL of spore suspension was used to inoculate Arabidopsis leaves. After inoculations, the plants were transferred to a PGV36 Conviron walk-in chamber and covered with a plastic dome to maintain high humidity. Disease symptoms were scored between 4 to 11 dpi. A digital Vernier caliper was used to measure lesion size in spot-inoculated leaves. Each experiment was repeated at least twice and each included 30 to 50 individual plants. Statistical significance was determined using Student's t test.
RNA Extraction, RT-PCR, and Northern Analyses
Small-scale extraction of RNA was performed using the TRIzol reagent (Invitrogen) following the manufacturer's instructions. RNA quality and concentration were determined by gel electrophoresis and determination of A260. Northern-blot analysis and synthesis of random-primed probes was carried out as described earlier (Kachroo et al., 2003). RT was carried out using SuperScript II (Invitrogen). Two to three independent RNA preparations were used for northern-blot analysis and RT-PCR and each of these was analyzed at least twice by northern-blot analysis and RT-PCR. The RT-PCR was carried out for 35 cycles to determine absolute levels of transcripts. The number of amplification cycles was reduced to 23 to 30 to evaluate and quantify differences among transcript levels before they reached saturation. Gene-specific primers used for RT-PCR analysis were G3Pdhcyt ATGGTGGGAAGCATTGAGGC and TCATGTTCTCTTGTGATGAGTATC primers, β-tubulinA.thaliana CGTGGATCACAGCAATACAGAGCC and CCTCCTGCACTTCCACTTCGTCTTC, and β-tubulinC.hig GTTCACYTSCAGACCGGCCAGT and GCAGTCGCAGCCCTCAGCCT.
Plant Transformation and Generation of Transgenic Lines Overexpressing GLY1
Plant transformation was carried out using the floral-dip method (Clough and Bent, 1998). Full-length GLY1 cDNA was amplified as NcoI/XbaI-linked PCR products using forward (ATTACCATGGCGGCTTCGGTGCAACC) and reverse (CGGGATCCTCATACTTCTTCAATCTGA) primers and cloned downstream of double 35S promoters in a pRTL2.GUS vector. For Arabidopsis transformation, the fragment containing the promoter, GLY1 cDNA, and the terminator was removed from pRTL2-GLY1 and cloned into the HindIII site of the binary vector pBAR1. Transgenic seeds were selected on soil sprayed with the herbicide BASTA.
FA, Glycerol, G3P, and Neutral Sugar Quantifications
FA analysis was done by placing leaf tissue in 2 mL of 3% H2SO4 in methanol. After a 30-min incubation at 80°C, 1 mL of hexane with 0.001% butylated hydroxytoluene was added. The hexane phase was then transferred to vials for gas chromatography (GC). One-microliter samples were analyzed by GC on a Varian FAME 0.25 mm × 50 m column and quantified with flame ionization detection. Ion chromatography (BioLC or ICS3000; Dionex Inc.) was used to quantify glycerol, G3P, and neutral sugars levels from plants as described (Downie, 1994). The samples were run on MA1 and PA1 columns. Approximately 1 g of leaf tissue was extracted and 2-deoxy-Glc was used as an internal standard.
Glycerol, G3P, and Paraquat Treatments
Glycerol (Invitrogen) treatments were carried out by spraying 50 mm solution of glycerol prepared in sterile water. Treatments with G3P were carried out by spraying or injecting 25 or 50 mm solution of G3P (Sigma-Aldrich) prepared in sterile water. Paraquat was prepared in sterile water and 5-μL droplets from 5, 15, 25, 50, and 100 μm solutions were spot inoculated on the leaves.
SA and Camalexin Quantifications
SA and SAG were extracted and measured from approximately 0.3 g of fresh-weight leaf tissue, as described previously (Chandra-Shekara et al., 2004). Camalexin was quantified as described before (Zhou et al., 1998). In brief, 100 mg of leaf tissue was incubated in 400 μL of 80% methanol at 80°C for 20 min. The extract was concentrated to 75 μL under a gentle stream of nitrogen gas followed by addition of 75 μL of chloroform. The samples were vortexed, centrifuged at high speed, and dried under a gentle stream of nitrogen gas. The dried samples were redissolved in chloroform and spotted on a silica gel-thin-layer chromatography (TLC) plate (Whatman; 60 A, 20 × 20 cm, 250 μm thickness). The chromatogram was developed using an ethyl acetate:hexane (100:15) solvent system and the camalexin was visualized as blue spots under UV light. The camalexin spots were removed from the TLC plate, extracted in methanol, and the fluorescence was measured using a fluorimeter (315- and 385-nm wavelengths). The concentration of camalexin was determined as μg/g fresh weight by extrapolating from the standard curve.
H2O2 Quantification
For H2O2 determination, leaves were homogenized in 40 mm Tris-HCl, pH 7.0, and to this 20 μm 2′,7′-dichlorofluorescein was added. The samples were incubated for 1 h in dark and the H2O2 levels were measured using a spectrofluorimeter. The concentration of H2O2 was determined as μmol/mg protein by extrapolating from the standard H2O2 curve.
In Planta Infection Assays
In planta infection assays were carried out on Arabidopsis petioles. Attached petioles were inoculated with 10-μL droplets of spore suspensions (5 × 104 spores/mL) and, after incubating for 48 h, the inoculated leaves were detached and the petioles were gently shaved from the back (abaxial) surface with a single-edged razor blade until only a thin layer of the adaxial epidermis remained. After shaving, each section was placed on a microscope slide in water and the number of primary hyphae was counted as a percentage of the total number of appressoria formed.
Trypan Blue Staining
Leaf samples were taken from mock- and pathogen-inoculated plants and stained as described before (Chandra-Shekara et al., 2006).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Levels of Fru and Gal (A) or Glc, Suc, and sorbitol (B) in Col-0, gly1, or act1 leaves inoculated with water or C. higginsianum.
Supplementary Material
Acknowledgments
We would like to thank Ludmila Lapchyk and Etta Nuckles for excellent technical help, Amy Crume for managing the plant growth facility, John Johnson for help with gas chromatography, as well as Thomas Muse and Lev Orlov for help with genotyping. We thank Jane Glazebrook for providing the camalexin standard and John Browse and Jin-Ma Zhou for providing gly1 and gli1 (nho1) seeds, respectively. We thank David Smith for critical comments on this manuscript.
This work was supported by the National Science Foundation (grant nos. MCB 0421914 and IOS 0749731), the U.S. Department of Agriculture National Research Initiative (2004–03287), and the Kentucky Science and Engineering Foundation (820–RDE–005). This study is publication 08–12–082 of the Kentucky Agricultural Experiment Station.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Pradeep Kachroo (pk62@uky.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- AbuQamar S, Chen X, Dhawan R, Bluhm B, Salmeron J, Lam S, Dietrich RA, Mengiste T (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J 48 28–44 [DOI] [PubMed] [Google Scholar]
- Ahn IP, Kim S, Lee YH (2005) Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol 138 1505–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi T, Kojima M, Kosuge T (1979) The energetics of parasitism, pathogenism, and resistance in plant disease. In JG Horsfall, EB Cowling, eds, Plant Disease: An Advanced Treatise, Vol 4. Academic Press, New York, pp 47–74
- Aubert S, Gout E, Bligny R, Douce R (1994) Multiple effects of glycerol on plant cell metabolism. J Biol Chem 269 21420–21427 [PubMed] [Google Scholar]
- Berger S, Sinha AK, Roitsch T (2007) Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot 58 4019–4026 [DOI] [PubMed] [Google Scholar]
- Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, Petétot JM, Métraux JP, Nawrath C (2007) A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J 26 2158–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Vohl M-C, St-Pierre J, Hudson T, Gaudet D (2001) Glycerol: a neglected variable in metabolic processes? Bioessays 23 534–542 [DOI] [PubMed] [Google Scholar]
- Chandra-Shekara AC, Gupte M, Navarre DA, Raina S, Raina R, Klessig D, Kachroo P (2006) Light-dependent hypersensitive response and resistance signaling to turnip crinkle virus in Arabidopsis. Plant J 45 320–335 [DOI] [PubMed] [Google Scholar]
- Chandra-Shekara AC, Navarre D, Kachroo A, Kang H-G, Klessig DF, Kachroo P (2004) Signaling requirements and role of salicylic acid in HRT- and rrt-mediated resistance to turnip crinkle virus in Arabidopsis. Plant J 40 647–659 [DOI] [PubMed] [Google Scholar]
- Chandra-Shekara AC, Venugopal SC, Barman SR, Kachroo A, Kachroo P (2007) Plastidial fatty acid levels regulate resistance gene-dependent defense signaling in Arabidopsis. Proc Natl Acad Sci USA 104 7277–7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dong X (2004) NPR1, all things considered. Curr Opin Plant Biol 7 547–552 [DOI] [PubMed] [Google Scholar]
- Downie B (1994) Sugar content and endo-beta-mannanase activity in white spruce (Picea glauca [Moench.] Voss.) seeds during germination. PhD thesis. University of Guelph, Guelph, Ontario, Canada
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ (2004) Glycerol-insensitive Arabidopsis mutants: gli1 seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress. Plant J 37 617–625 [DOI] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch T (1997) Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9 1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16 449–455 [DOI] [PubMed] [Google Scholar]
- Flor H (1971) Current status of gene-for-gene concept. Annu Rev Phytopathol 9 275–296 [Google Scholar]
- Glazebrook J (2001) Genes controlling expression of defense responses in Arabidopsis—2001 status. Curr Opin Plant Biol 4 301–308 [DOI] [PubMed] [Google Scholar]
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205–227 [DOI] [PubMed] [Google Scholar]
- Gomez-Ariza J, Campo S, Rufat M, Estopa M, Messequer J, San Sequndo B, Coca M (2007) Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms proteins in rice plant. Mol Plant Microbe Interact 20 832–842 [DOI] [PubMed] [Google Scholar]
- Gorvin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10 751–757 [DOI] [PubMed] [Google Scholar]
- Hancock JG, Huisman OC (1981) Nutrient movement in host-pathogen systems. Annu Rev Phytopathol 19 309–331 [Google Scholar]
- Huckelhoven R (2007) Cell wall-associated mechanisms of disease resistance and susceptibility. Annu Rev Phytopathol 45 2.1–2.27 [DOI] [PubMed] [Google Scholar]
- Kachroo A, Daqi F, Havens W, Navarre D, Kachroo P, Ghabrial S (2008) An oleic acid–mediated pathway induces constitutive defense signaling and enhanced resistance to multiple pathogens in soybean. Mol Plant Microbe Interact 21 564–575 [DOI] [PubMed] [Google Scholar]
- Kachroo A, Kachroo P (2007) Salicylic acid-, jasmonic acid- and ethylene-mediated regulation of plant defense signaling. In J Setlow, ed, Genetic Regulation of Plant Defense Mechanisms. Springer, New York, pp 55–83 [DOI] [PubMed]
- Kachroo A, Lapchyk L, Fukushigae H, Hildebrand D, Klessig D, Kachroo P (2003) Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell 15 2952–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Venugopal SC, Lapchyk L, Falcone D, Hildebrand D, Kachroo P (2004) Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis. Proc Natl Acad Sci USA 101 5152–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo P, Venugopal SC, Navarre DA, Lapchyk L, Kachroo A (2005) Role of salicylic acid and fatty acid desaturation pathways in ssi2-mediated signaling. Plant Physiol 139 1717–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Li J, Zhao T, Xiao F, Tang X, Thilmony R, He SY, Zhou J-M (2003) Interplay of the Arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc Natl Acad Sci USA 100 3915–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchoni SO, Gachomo EW (2006) The reactive oxygen species network pathways: an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J Biosci 31 389–404 [DOI] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C (1988) Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA 85 4143–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, et al (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310 1180–1183 [DOI] [PubMed] [Google Scholar]
- Liu G, Kennedy R, Greenshields DL, Peng G, Forseille L, Selvaraj G, Wei Y (2007) Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol Plant Microbe Interact 20 1308–1319 [DOI] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R (2003) The BOS1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15 2551–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Cassagne C, Browse J (1998) A new class of Arabidopsis mutants with reduced hexadecatrienoic acid fatty acid levels. Plant Physiol 117 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Welti R, Shah J (2004) The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell 16 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusaka Y, Narusaka M, Park P, Kubo Y, Hirayama T, Seki M, Shiraishi T, Ishida J, Nakashima M, Enju A, et al (2004) RCH1, a locus in Arabidopsis that confers resistance to the hemibiotrophic fungal pathogen Colletotrichum higginsianum. Mol Plant Microbe Interact 17 749–762 [DOI] [PubMed] [Google Scholar]
- O'Connell R, Herbert C, Sreenivasaprasad S, Khatib M, Esquerre-Tugaye M-T, Dumas B (2004) A novel Arabidopsis-Colletotrichum pathosystem for the molecular dissection of plant-fungal interactions. Mol Plant Microbe Interact 17 272–282 [DOI] [PubMed] [Google Scholar]
- Oliver RP, Ipcho SVS (2004) Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol Plant Pathol 5 347–352 [DOI] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker BW (2000) Differential impact of carbon assimilation on the expression of quantitative and qualitative resistance in alfalfa (Medicago sativa). Physiol Mol Plant Pathol 57 87–93 [Google Scholar]
- Perfect SE, Hughes HB, O'Connell RJ, Green JR (1999) Collectrichum: a model genus for studies on pathology, fungal-plant interactions. Fungal Genet Biol 27 186–198 [DOI] [PubMed] [Google Scholar]
- Price J, Li TC, Kang SG, Na JK, Jang JC (2003) Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol 132 1424–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell (Suppl) 14 S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf J, Walter MH, Hess D (1995) Primary metabolism in plant defense. Plant Physiol 108 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaarschmidt S, Kopka J, Ludwig-Muller J, Hause B (2007) Regulation of arbuscular mycorrhization by apoplastic invertases: enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J 51 390–405 [DOI] [PubMed] [Google Scholar]
- Scheideler M, Schlaich NL, Fellenberg K, Beissbarth T, Hauser NC, Vingron M, Slusarenko AJ, Hoheisel JD (2002) Monitoring the switch from housekeeping to pathogen defense metabolisms in Arabidopsis thaliana using cDNA arrays. J Biol Chem 277 10555–10561 [DOI] [PubMed] [Google Scholar]
- Schulze-Lefert P, Panstruga R (2003) Establishment of biotrophy by parasitic fungi and reprogramming of host cells for disease resistance. Annu Rev Phytopathol 41 641–667 [DOI] [PubMed] [Google Scholar]
- Shen W, Wei Y, Dauk M, Zheng Z, Zou J (2003) Identification of a mitochondrial glycerol-3-phosphate dehydrogenase from Arabidopsis thaliana: evidence for a mitochondrial glycerol-3-phosphate shuttle in plants. FEBS Lett 536 92–96 [DOI] [PubMed] [Google Scholar]
- Shen W, Wei Y, Dauk M, Zheng Z, Zou J (2006) Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis. Plant Cell 18 422–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PS, Tan KC, Oliver RP (2003) The nutrient supply of pathogenic fungi; a fertile field for study. Mol Plant Pathol 4 203–210 [DOI] [PubMed] [Google Scholar]
- Taiz L, Zeiger E (2006) Respiration and lipid metabolism. In L Taiz, E Zeiger, eds, Plant Physiology. Sinauer Associates, Sunderland, MA, pp 253–288
- Tang D, Simonich MT, Innes RW (2007) Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol 144 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Penninckx IA, Broekaert WF, Cammue BP (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13 63–68 [DOI] [PubMed] [Google Scholar]
- Wei Y, Periappuram C, Datla R, Selvaraj G, Zou J (2001) Molecular and biochemical characterization of a plastidic glycerol-3-phosphate dehydrogenase from Arabidopsis. Plant Physiol Biochem 39 841–848 [Google Scholar]
- Wei Y, Shen W, Dauk M, Wang F, Selvaraj G, Zou J (2004) Targeted gene disruption of glycerol-3-phosphate dehydrogenase in Colletotrichum gloeosporioides reveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen. J Biol Chem 279 429–435 [DOI] [PubMed] [Google Scholar]
- Williams PH (1979) How fungi induce disease. In JG Horsfall, EB Cowling, eds, Plant Disease: An Advanced Treatise, Vol 4. Academic Press, New York, pp 163–179
- Yoder OC, Turgeon BG (2001) Fungal genomics and pathogenicity. Curr Opin Plant Biol 4 315–321 [DOI] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J (1998) PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10 1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.