Abstract
The size and shape of the plant leaf is an important agronomic trait. To understand the molecular mechanism governing plant leaf shape, we characterized a classic rice (Oryza sativa) dwarf mutant named narrow leaf1 (nal1), which exhibits a characteristic phenotype of narrow leaves. In accordance with reduced leaf blade width, leaves of nal1 contain a decreased number of longitudinal veins. Anatomical investigations revealed that the culms of nal1 also show a defective vascular system, in which the number and distribution pattern of vascular bundles are altered. Map-based cloning and genetic complementation analyses demonstrated that Nal1 encodes a plant-specific protein with unknown biochemical function. We provide evidence showing that Nal1 is richly expressed in vascular tissues and that mutation of this gene leads to significantly reduced polar auxin transport capacity. These results indicate that Nal1 affects polar auxin transport as well as the vascular patterns of rice plants and plays an important role in the control of lateral leaf growth.
The plant vascular system, a continuous network of vascular bundles that interconnects all major plant organs, enables long-distance transport of photoassimilates and soil nutrients and provides mechanical support to the plant body (Esau, 1965; Dengler, 2001; Aloni, 2004; Scarpella and Meijer, 2004). Anatomical studies revealed that all vascular cells are differentiated from a vascular meristematic tissue, the procambium (Foster, 1952; Esau, 1965). Procambial cells are characteristically arranged in continuous strands and acquire their narrow shape through coordinated, oriented divisions, parallel to the axis of the emerging strand (Nelson and Dengler, 1997; Dengler, 2001; Ye, 2002; Scarpella and Meijer, 2004). Despite extensive descriptions of different aspects of vascular development and patterning, the molecular mechanisms governing these strictly regulated processes remain to be elucidated.
Among the various plant hormones that influence vascular development and patterning, the role of auxin is unique (Dengler, 2001; Aloni, 2004; Fukuda, 2004; Scarpella and Meijer, 2004; Teale et al., 2006). A long history of elegant physiological experiments established that the polar cell-to-cell transport of auxin plays a crucial role in vascular tissue differentiation and vascular patterning (Sachs, 1989; Aloni, 2004; Fukuda, 2004; Blakeslee et al., 2005; Sauer et al., 2006; Teale et al., 2006). The classical auxin application studies and polar auxin transport (PAT) inhibition studies also led to the formulation of the “auxin-flow canalization hypothesis,” which suggests that auxin flow, starting initially by diffusion, induces the formation of a polar auxin transport system and, as a consequence, promotes auxin transport and leads to canalization of the auxin flow along a narrow file of cells. This continuous polar transport of auxin through cells finally results in the differentiation of vascular strands (Sachs, 1981, 2000; Aloni, 2004; Fukuda, 2004).
Molecular genetic studies, primarily using the dicotyledon Arabidopsis (Arabidopsis thaliana) as a model system, have boosted our understanding of the molecular basis of PAT, PAT-mediated auxin distribution, and their association with vascular differentiation and patterning (Palme and Gälweiler, 1999; Dengler, 2001; Friml and Palme, 2002; Ye, 2002; Aloni, 2004; Fukuda, 2004; Blakeslee et al., 2005; Paponov et al., 2005; Teale et al., 2006). In-depth characterization of Arabidopsis mutants defective in PAT had enabled the identification of the putative auxin influx as well as efflux carriers, two of the major components of the PAT system. For example, the pin-formed1 (pin1) mutant showed irregular vascular patterns and strongly reduced basipetal auxin transport, which resembled plants treated with inhibitors of auxin efflux (Okada et al., 1991; Gälweiler et al., 1998). Gene cloning and protein localization analyses demonstrated that AtPIN1 was a member of a family of transmembrane proteins that asymmetrically distributed at the basal end of PAT-competent cells, supporting the hypothesis that AtPIN1 as well as other PIN proteins were components of the auxin efflux machinery (Gälweiler et al., 1998; Palme and Gälweiler, 1999; Friml, 2003; Aloni, 2004; Blakeslee et al., 2005; Leyser, 2005; Paponov et al., 2005; Petrasek et al., 2006). In addition, mutations in AtPGP1 and AtPGP19, two genes encoding putative P-glycoprotein ABC transporters, resulted in the disruption of PAT and pleiotropic growth phenotypes, including stunted stature and curled leaves (Noh et al., 2001, 2003; Luschnig, 2002). Recently, heterologous expression studies demonstrated that both AtPGP1 and AtPGP19 exhibited auxin efflux transport activity (Geisler et al., 2005).
Through monitoring the dynamic patterns of the polar AtPIN1 expression during critical stages of the formation of various defined veins, recent studies revealed a directing influence of auxin transport in the epidermis on vein patterning (Scarpella et al., 2003; Sauer et al., 2006). These works refined the overall picture of the canalization hypothesis for PAT-mediated vascular patterning (Scheres and Xu, 2006).
Although a large number of studies have established an instrumental role of PAT and PAT-mediated auxin distribution for vascular patterning in Arabidopsis, much less is known about whether the same paradigm also underlies vascular patterning in rice (Oryza sativa), a model system of monocot plants. While dicot plants show a reticulate pattern of highly branched veins, most monocot plants, such as rice, show parallel (striate) vascular patterns (Nelson and Dengler, 1997; Scarpella et al., 2003; Scarpella and Meijer, 2004). The auxin-inducible homeobox gene Oshox1 in rice was shown recently to be a molecular marker of a stage in vascular tissue development at which procambial cell fate is specified but not yet stably determined (Scarpella et al., 2000, 2005). Overexpression of Oshox1 led to reduced sensitivity to PAT inhibitors, indicating a role of PAT in vascular tissue differentiation in monocot species (Scarpella et al., 2002). An association between PAT and venation was demonstrated by characterization of the maize (Zea mays) rough shealth2 mutant, which shows aberrant leaf development (Tsiantis et al., 1999; Scanlon et al., 2002). Stronger evidence supporting the notion that PAT is involved in vascular patterning in monocot plants came from the characterization of the classic brachytic2 (br2) mutant in maize, which shows irregular vascular patterns in stalks (Multani et al., 2003). br2 carried mutation in a gene that is homologous to the above-mentioned Arabidopsis P-glycoproteins AtPGP1 and AtPGP19. Similar to the Arabidopsis case, PAT was significantly reduced in br2 mutants. The radicleless1 (ral1) mutant of rice showed distinctive embryonic and postembryonic vascular defects, including altered number and spacing of parallel veins, and was defective in auxin response (Scarpella et al., 2003). However, the gene responsible for the ral1 phenotype had yet to be identified.
Here, we report the in-depth characterization of a previously described rice mutant named narrow leaf1 (nal1; Dong et al., 1994; Zeng et al., 2003). The nal1 mutant exhibits altered vascular patterns in leaves and culms. Nal1 encodes a plant-specific protein with unknown biochemical function. We provide evidence showing that Nal1 is expressed preferentially in vascular tissues and that mutation of its function leads to reduced PAT capacity. These results support the hypothesis that Nal1 plays important roles in regulating PAT as well as vascular patterning of rice.
RESULTS
The nal1 Mutant Shows Reduced Leaf Width
One of the most characteristic phenotypes of the rice nal1 mutant was reduced leaf width (Dong et al., 1994; Zeng et al., 2003). The leaves of nal1 plants appeared normal in shape but were generally smaller than those of wild-type plants (Table I; Fig. 1C). Measurement of leaf growth indicated that the nal1 mutation significantly affected the width of the leaf blade. Compared with width, the length of the mutant leaf blades was less affected. For example, the mature second leaves (from the top of the plant) of soil-grown nal1 plants usually attained approximately 63% of the width of the wild-type counterparts (Table I), while the length of the nal1 leaves was about 87% of that of wild-type plants (Table I).
Table I.
Morphometric analysis of wild-type and nal1 plants
Leaf morphometric analyses were done on mature leaves of 3-month-old plants. Blade width and vascular pattern parameters were measured through the middle region of the leaf blade. Vascular pattern parameters were measured in dark-field microscopic digital images of cleared leaf preparations. Numbers of stem vascular bundles in the outer and inner rings were determined in digital microscopic images of transverse sections through the middle part of the fourth internode of plants at the ripened inflorescence stage. The internode IV morphometric analyses and plant height measurement were done at the mature stage of 3-month-old plants. Results represent means ± sd of populations of the size indicated in parentheses. Asterisks indicate the significance of differences between wild-type and nal1 plants as determined by Student's t test: * 0.01 ≤ P < 0.05, ** 0.001 ≤ P < 0.01, *** P < 0.001.
| Organ | Wild Type | nal1 |
|---|---|---|
| Leaf | ||
| Blade width (cm) | 1.9 ± 0.2 (15) | 1.2 ± 0.1 (15)*** |
| Blade length (cm) | 32.1 ± 5.5 (15) | 27.7 ± 1.7 (15)** |
| Number of large veins | 8.1 ± 0.8 (33) | 7.2 ± 1.1 (42)*** |
| Total number of small veins | 39.4 ± 2.8 (30) | 22.2 ± 3.5 (30)*** |
| Number of small veins between two adjacent large veins | 4.9 ± 0.3 (30) | 3.0 ± 0.4 (30)*** |
| Stem | ||
| Mature plant height (cm) | 88.1 ± 2.9 (20) | 62.8 ± 1.9 (20)*** |
| Internode IV length (cm) | 4.5 ± 1.4 (20) | 1.2 ± 0.2 (20)*** |
| Number of vascular bundles in the outer ring | 23.6 ± 2.7 (23) | 25.2 ± 2.5 (21)* |
| Number of vascular bundles in the inner ring | 25.5 ± 1.99 (23) | 26.5 ± 4.36 (21) |
| Total cell number of internode IV in y axis | 530.3 ± 100.6 (16) | 159.1 ± 69.1 (16)*** |
Figure 1.
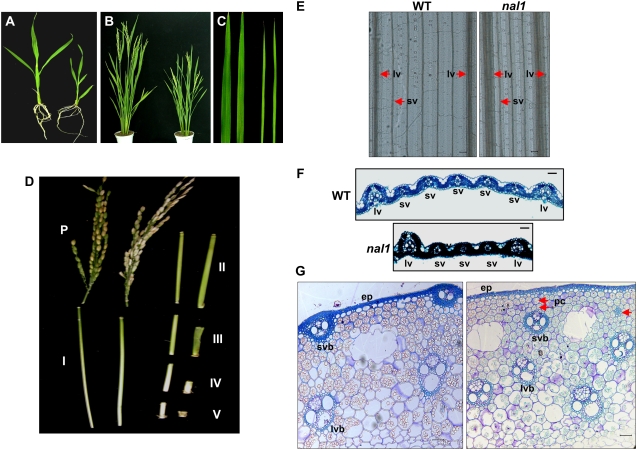
The nal1 mutant shows a defective vascular system in leaves and culms. A, Gross morphology of 1-month-old wild-type (left) and nal1 (right) plants. B, Gross morphology of wild-type (left) and nal1 (right) plants at the heading stage. C, Leaf morphology of wild-type (left) and nal1 (right) plants. Shown are the second leaf blades (from the top of the plant) of 3-month-old plants. D, Phenotypic exhibition of the components of rice plant height in representative wild-type (left) and nal1 (right) plants at the mature stage. P, Panicle. I to V indicate the corresponding internodes from top to bottom. E, Whole-mount clearing of the mature fifth leaves of wild-type (WT) and nal1 plants. Shown are regions between two large veins in the middle of mature leaf blades. lv, Large vein; sv, small vein. Bars = 150 μm. F, Transverse sections of the mature fifth leaves in the middle part of wild-type and nal1 plants. Bars = 20 μm. G, Transverse sections of the middle part of internode IV of wild-type (left) and nal1 (right) plants at the mature stage. ep, Epidermis; lvb, large vascular bundles; pc, parenchyma cells; svb, small vascular bundles. The single red arrow indicates an immature vascular bundle, and the double red arrows indicate parenchyma cell layers in the region between the epidermis and the outer ring of small vascular bundles. Bars = 20 μm.
The nal1 Mutant Exhibits an Altered Internode Elongation Pattern
In addition to narrow leaves, the nal1 mutant also showed an altered internode elongation pattern (Fig. 1, A, B, and D). At maturity, the height of the mutant reached approximately 71% of the wild-type height (Table I). We compared the internode elongation patterns between nal1 and wild-type plants. As shown in Figure 1D and Table I, while the nal1 mutation had little effect, if any, on the length of the panicle and the uppermost two internodes (designated internodes I and II, respectively), it significantly shortened the length of the third, the fourth, and the fifth internodes (designated internodes III, IV, and V, respectively).
To determine whether the dwarf phenotype of the nal1 culm results from defective cell division and/or cell elongation, longitudinal sections of the severely shortened internode IV of the mature mutant culm were compared with their wild-type counterparts. As shown in Figure 1D and Table I, the total cell number in y axes in internode IV of nal1 was approximately 60% less than that of wild-type plants, while no significant change in cell length was observed. These observations suggested that the Nal1 gene may play a role in cell division rather than cell elongation.
The nal1 Mutant Shows Defective Vascular Patterns in Leaves and Culms
Wild-type rice leaves show a typical parallel venation pattern, in which three orders of major longitudinal veins (i.e. the midveins, large veins, and small veins) lie parallel along the distal axis of the leaf (Nelson and Dengler, 1997; Scarpella et al., 2003). A wealth of anatomical studies revealed a constant relationship between leaf blade width and longitudinal vein quantity in monocots (Russell and Evert, 1985; Dannenhoffer et al., 1990; McHale, 1993; Nelson and Dengler, 1997). A comparison of mature leaves revealed that the total numbers of large veins and small veins were all reduced in the nal1 mutant (Table I). Notably, the average number of small veins between two large veins was reduced from approximately five in the wild type to approximately three in the mutant (Fig. 1, E and F; Table I). These observations indicated that the nal1 mutation affected the formation of leaf veins, which might account for the narrow leaf phenotype of the mutant.
The finding that the nal1 mutation affected leaf venation prompted us to examine the vascular system in culms. Transverse sections of internode IV, the length of which was significantly reduced in nal1 (Fig. 1D; Table I), were compared between wild-type and mutant plants. In cross sections of the wild-type internode IV, vascular bundles of inner ring and outer ring were uniformly arranged and formed a typical radial organization, with outer small vascular bundles arranged concentrically below the epidermis (Fig. 1G, left). In contrast, the distribution pattern of vascular bundles in the nal1 mutant culm was severely disrupted (Fig. 1G, right). In addition to the altered distribution pattern, the mutant culm also contained immature vascular bundles and an overall increased number of vascular bundles, especially in the outer ring (Table I; Fig. 1G, right). Furthermore, increased numbers of parenchyma cells in the region between the epidermis and the outer ring of small vascular bundles could be observed (Fig. 1G, right).
Positional Cloning of Nal1
The genetic locus defined by the nal1 mutant was previously located on the long arm of rice chromosome 4 (Dong et al., 1994; Zeng et al., 2003). For fine mapping, the nal1 mutant in the genetic background of Zhefu802 (an indica cultivar) was crossed to Nipponbare (a japonica cultivar) to generate a large F2 population. Genetic analysis with 2,000 segregants showing the nal1 mutant phenotype initially placed the Nal1 gene between simple sequence repeat markers RM5503 and RM3836 (Fig. 2A). Within this region, we developed four PCR-based molecular markers (Table II) and further delimited the target gene to a 29-kb region defined by markers M3 and M4 on a single bacterial artificial chromosome (BAC) clone, AL662950 (Fig. 2A). Within this 29-kb interval, four open reading frames (ORFs), which were designated ORF1 to ORF4, were predicted (Fig. 2A). Genomic DNAs corresponding to the four putative ORFs were PCR amplified from the nal1 mutant and sequenced. Sequence comparison revealed a 30-bp deletion in the nal1-derived ORF1 (Fig. 2B), while no sequence difference was found in ORF2 to ORF4. ORF1, therefore, was considered a strong candidate for the Nal1 gene.
Figure 2.
Nal1 encodes a plant-specific protein with unknown biochemical function. A, Fine genetic and physical mapping of Nal1. The target gene was initially mapped to a genetic interval between markers RM5503 and RM3836 on rice chromosome 4. Analysis of an F2 mapping population of 2,000 plants delimited the gene to a 29-kb region between markers M3 and M4 on BAC clone AL662950. Markers and the numbers of recombination events identified are indicated. B, Schematic representation of the Nal1 gene structure. The nal1 allele contains a 30-bp deletion in the fourth exon. Black boxes indicate the coding sequence, white boxes indicate the 5′ and 3′ untranslated regions, and lines between boxes indicate introns. The start codon (ATG) and the stop codon (TGA) are indicated. C, Genetic complementation of nal1 (top) and molecular identification of transgenic plants (bottom). The wild type (left), nal1 containing an empty vector (middle), and a transgenic plant containing the 12.4-kb DNA fragment of ORF1 in the genetic background of nal1 (right) are shown. The 30-bp deletion in the nal1 was used as a PCR-based marker to distinguish the three plants. D, Deduced amino acid sequence of Nal1. The deleted 10 amino acids are underlined, and the red letters indicate the putative nuclear localization signal. E, Phylogeny of the Nal1 protein family. A neighbor-joining tree was built by MEGA3 using a Poisson correction model with gaps to complete deletion. Topological robustness was assessed by bootstrap analysis with 500 replicates. The bar is an indicator of genetic distance based on branch length. Nal1 and AK120320 are from rice; BT014552 and BT013672 are from tomato; AC191706, AC191543, AC205276, and AC208803 are from maize; and AK248497 and AK252329 are from barley. F, Confocal image (left), transmitted light image (middle), and merged image (right) of Arabidopsis cell culture protoplasts transfected with free GFP. Bar = 10 μm. G, Confocal image (left), transmitted light image (middle), and merged image (right) of Arabidopsis cell culture protoplasts transfected with Nal1-GFP fusion proteins. Bar = 10 μm. H, Steady-state nuclear protein body patterns of 35S:Nal1-GFP in root cells from a 5-d-old transgenic Arabidopsis seedling. Confocal image (left), transmitted light image (middle), and merged image (right) of root cortex cells. Bar = 20 μm.
Table II.
PCR-based molecular markers used for genetic mapping
| Marker | Primer Pairsa | Fragment Sizeb | Restriction Enzyme |
|---|---|---|---|
| M1 | F, 5′-ACAGTACCTTCATTCAGCCTCTTC-3′; R, 5′-GATGCGTTCGTCTCACCAGTT-3′ | 368 | FokI |
| M2 | F, 5′-CAACTAAGGCTGGGGATGAAAAG-3′; R, 5′-ACCGAGTCTGGCGTCTACACAA-3′ | 911 | PstI |
| M3(STS) | F, 5′-TGGGGGTGGAGGACAGGT-3′; R, 5′-TTTTTTGCGGGGAGATGGAG-3′ | 433 | |
| M4 | F, 5′-GGACAGCCAAAAGTAGTAGTAGTAG-3′; R, 5′-GGTAAATAATAGGGGGAAATA-3′ | 313 | NsiI |
F, Forward primer; R, reverse primer.
Numbers indicate the size (in base pairs) of amplified fragments.
To test the possibility that the 30-bp deletion in nal1-derived ORF1 is responsible for the mutant pheno-type, we performed genetic complementation analysis. A 12.4-kb genomic DNA fragment containing the entire Nal1 coding region, the 2,673-bp upstream sequence, and the 1,685-bp downstream sequence was introduced into the nal1 background by Agrobacterium tumefaciens-mediated transformation (Hiei et al., 1994). The 30-bp deletion in the nal1-derived ORF1 led to the development of a PCR-based marker to determine the mutant background in complementation tests (Fig. 2C, bottom). Among the 24 transgenic plants confirmed by genomic PCR, 17 primary lines (T0) showed complementation of the nal1 phenotype (Fig. 2C; data not shown). Further characterization of three of these lines showed that the complemented phenotype was inherited in the T1 generation (data not shown). These results demonstrated that ORF1 is equivalent to the Nal1 gene.
Nal1 Encodes a Plant-Specific Protein with Unknown Biochemical Function
Sequence comparison between genomic DNA and cDNA revealed that the Nal1 gene was composed of five exons and four introns (Fig. 2B) that encoded a protein with 582 amino acids (Fig. 2D). In nal1, the 30-bp deletion, which existed in exon 4 of the mutated Nal1 gene, resulted in the in-frame absence of 10 amino acids (Fig. 2, B and D).
A survey of the published rice genome database (http://www.gramene.org/) identified the existence of another gene named Nal1-like (AK120820), which was predicted to encode a protein that shares greater than 70% amino acid sequence identity with Nal1 (Fig. 2E). BLAST searches of the DNA and protein databases also identified Nal1-like genes from other plant species, including monocots and dicots (Fig. 2E). Notably, three putative homologous genes, At2g35155 (AY059774), At5g45030 (AY099637), and At3g12950 (BT012224), which shared around 60% amino acid sequence identity with the Nal1 protein, were identified in the Arabidopsis genome (Fig. 2E). Putative Nal1 homologous genes were also identified from the genomes of tomato (Solanum lycopersicum), maize, and barley (Hordeum vulgare; Fig. 2E). Database searches failed to identify yeast or animal proteins showing significant similarity to Nal1. The existence of Nal1-like genes in different plant species might suggest conserved biochemical function of the Nal1 protein family. However, the Nal1 sequence did not match any protein of known biochemical function in the public databases.
Subcellular Localization of Nal1
Using the WoLF PSORT programs (http://wolfpsort.org/), it was predicted that the Nal1 protein contains a putative nuclear localization signal (PKRLRSD, amino acid residues 567–573; Fig. 2D). To determine the subcellular localization of Nal1, GFP was fused to the C terminus of Nal1 under the control of the 35S promoter and the fusion gene was transformed into Arabidopsis protoplast (Meskiene et al., 2003). The Nal1-GFP chimeric protein was localized to the nucleoplasm and cytoplasm and concentrated in discrete structures (Fig. 2G), which appeared similar to nuclear protein bodies. However, nuclear protein body formation was not detected in control cells, which expressed GFP alone under the control of the 35S promoter (Fig. 2F). Similarly, our parallel experiment indicated that At3g12950, one of the Arabidopsis homologs of Nal1, also can form nuclear protein bodies in Arabidopsis protoplast (data not shown). To substantiate these observations, we generated transgenic Arabidopsis plants containing the 35S:Nal1-GFP fusion gene. Confocal observation of the root segments of the transgenic seedlings indicated that the Nal1-GFP fusion protein form nuclear protein bodies and that the number, size, and brightness of the nuclear protein bodies show high variation in different cells (Fig. 2H; data not shown).
Expression Pattern of Nal1
Reverse transcription (RT)-PCR analysis indicated that Nal1 was expressed in organs such as leaf sheaths, leaves, culms, and young panicles (Fig. 3A). The nal1 mutation led to irregular venation patterns in leaves and culms, suggesting that the Nal1 gene might act in vascular tissues. To test this, we generated transgenic rice plants expressing a 1,918-bp fragment of the Nal1 promoter fused with the GUS reporter gene. GUS staining of transgenic plants indicated that Nal1 was preferentially expressed in vascular tissues of etiolated coleoptiles (Fig. 3, B and C). In a cross section of culm at heading stage, strong GUS staining was found in the phloem of vascular bundles (Fig. 3D). RNA in situ hybridization with cross sections of 1-month-old seedlings provided further evidence that Nal1 was richly expressed in vascular tissues (Fig. 3, E–I). The finding that Nal1 was preferentially expressed in vascular tissues together with the fact that the nal1 mutant showed defective vascular patterns in leaves and culms implied that Nal1 might be involved in rice vascular patterning.
Figure 3.
Nal1 expression pattern. A, RT-PCR analysis of Nal1 expression. Total RNA was isolated from leaf sheaths (S), leaves (L), culms (C), and panicles (P) of wild-type plants. Amplification of the rice ACTIN1 gene was used as a control. B to D, Nal1 expression revealed by GUS staining in Nal1 promoter-GUS transgenic rice plants. B, Nal1 promoter-GUS expression patterns in 5-d-old dark-grown coleoptiles showing intense GUS staining in vascular tissues. C, Transverse section of the middle part of the coleoptile in B showing intense expression of GUS in the phloem. D, Transverse section of a fourth internode at the heading stage showing the expression of Nal1 promoter-GUS in the phloem of vascular bundles. ph, Phloem; v, vascular bundle. Bars = 400 μm in B, 20 μm in C, and 100 μm in D. E to I, Nal1 expression patterns revealed by RNA in situ hybridization. E, Transverse section of a 1-month-old seedling showing intense expression of Nal1 in vascular tissues of leaves and leaf sheaths. F, Magnified image of the boxed region in E showing the strong expression of Nal1 in developing vascular bundles of leaf sheath. G, Transverse section of a young culm. H, Magnified image of the boxed region in G showing the strong expression of Nal1 in vascular bundles. I, Background control. No hybridization signal was observed with the sense probe. X, Xylem. Bars = 200 μm in B, 130 μm in E, G, and I, 70 μm in F and H, 50 μm in C, and 20 μm in D.
The nal1 Mutant Shows Reduced Polar Auxin Transport Activity
The phytohormone auxin plays a pivotal role in different aspects of vascular tissue development and patterning (Ye, 2002; Aloni, 2004; Fukuda, 2004; Scarpella and Meijer, 2004; Teale et al., 2006). Our finding that the nal1 mutation led to defective vascular development in leaves and culms raised the interesting possibility that the polar auxin flow in the nal1 mutant may be altered. Given that the etiolated cereal coleoptile is used as a model system to study auxin-mediated growth and development (Haga et al., 2005; Li et al., 2007), together with our anatomical investigations showing that the overall structures and vasculatures of etiolated coleoptiles between the wild type and nal1 were similar (Fig. 4, D–G), we compared the PAT activities in etiolated coleoptiles of wild-type and mutant plants. We first examined the uptake of tritium-labeled indole-3-acetic acid ([3H]IAA) using 2-mm coleoptile fragments and found that the uptake capacity of [3H]IAA in nal1 was comparable to that of the wild type (Fig. 4A). To measure basipetal transport of IAA, [3H]IAA was applied to the apical tips of dark-grown coleoptile fragments, and the basipetal auxin transport activity in nal1 coleoptiles was reduced to approximately 33% of that of wild-type plants (Fig. 4B). As a control, acropetal transport was also measured through feeding [3H]IAA to the basal tips of coleoptile fragments and found to be much lower than basipetal transport, but it was similar between nal1 and the wild type (Fig. 4B). The PAT activities of nal1 and wild-type plants were also examined in the presence of N-1-naphthylphthalamic acid (NPA), an inhibitor of PAT (Morris et al., 2004). As shown in Figure 4B, in the presence of 20 μm NPA, the PAT activity in wild-type coleoptiles was reduced to approximately 36% of that of buffer control. In the case of nal1, the PAT activity was already low and application of NPA led to only a little reduction in PAT. In order to compare the efflux rates of auxin transport between the wild type and mutant, coleoptile segments were incubated sequentially in the [3H]IAA-containing solution and in auxin-free buffer, and then the amount of auxin retained in the coleopitle segments was measured. As shown in Figure 4C, in the absence of NPA, the [3H]IAA retained in nal1 coleoptiles was approximately 32% higher than that of the wild type. In the presence of 2 μm NPA, the amount of auxin retained in wild-type coleoptiles was increased by 140%, whereas that in nal1 coleoptiles was only increased by 24%. Together, these results indicated that mutation of Nal1 led to defective basipetal PAT activity and rendered the nal1 mutant less sensitive than the wild type to NPA-mediated inhibition of polar auxin flow.
Figure 4.
The nal1 mutant shows defective PAT activity. A, Comparison of auxin uptake between wild-type (WT) and nal1 plants in dark-grown coleoptiles. Values represent means ± sd of six independent assays. B, Comparison of auxin transport between wild-type and nal1 plants in dark-grown coleoptiles. Values represent means ± sd of six independent assays. The asterisk indicates that the difference between the wild type and nal1 is significant (Student's t test, P < 0.001). C, IAA efflux of dark-grown coleoptile segments. Five coleoptile segments were used in each assay, and values are given as means ± sd of three independent assays. The difference between the wild type and nal1 is significant (Student's t test, P < 0.05). D and E, Cross sections of dark-grown wild-type (D) and nal1 (E) shoots showing that the vasculature of nal1 coleoptile is similar to that of the wild type. Bars = 50 μm. F, Magnified image of the boxed region in D. Bar = 50 μm. G, Magnified image of the boxed region in E. Bar = 50 μm. H to K, Immunohistochemical analysis of etiolated wild-type and nal1 seedlings using the anti-AtPIN1 antibodies as probe. H, Transverse section through the middle part of a 5-d-old dark-grown wild-type seedling. I, Bright-field image of K. J, Transverse section through the middle part of a 5-d-old dark-grown nal1 seedling. K, Bright-field image of J. Bars = 50 μm. L, Protein gel-blot analysis using the anti-AtPIN1 antibodies as probe. Membrane protein extracts from wild-type and nal1 coleoptiles were probed with anti-AtPIN1 antibodies. The signal (arrowhead) abundance detected in nal1 was significantly reduced from that in the wild type. The numbers at left denote the molecular masses of marker proteins in kilodaltons. Coomassie blue staining of the protein samples was used as a loading control (bottom gel). M, Quantitative real-time RT-PCR analysis comparing OsPIN1 relative expression levels in 5-d-old coleoptiles of the wild type and nal1. Values represent means ± sd of three independent assays. The difference between the wild type and nal1 is significant (Student's t test, P < 0.05).
The nal1 Mutation Might Affect the Abundance of a Deduced Rice Homolog of AtPIN1, an Auxin Efflux Facilitator in Arabidopsis
The findings that nal1 shows reduced PAT activity in the shoot and decreased sensitivity to NPA-mediated PAT inhibition raised the interesting possibility that the nal1 mutation might affect auxin efflux. Considering the established roles of the Arabidopsis PIN family proteins in the regulation of auxin efflux, together with the existence of highly conserved PIN proteins in rice (Gälweiler et al., 1998; Palme and Gälweiler, 1999; Paponov et al., 2005; Xu et al., 2005), we conducted immunohistochemical analysis in 5-d-old dark-grown seedlings using antibodies raised against the AtPIN1 protein, a well-characterized auxin efflux carrier in Arabidopsis. As shown in Figure 4, H to K, the AtPIN1 antibodies detected signals in the vascular tissues of both mutant and wild-type seedlings and, interestingly, the signals detected in nal1 was weaker than those in wild-type plants. To validate this observation, membrane proteins were extracted from both nal1 and wild-type coleoptiles and subjected to protein gel-blot analysis using the same AtPIN1 antibodies as probe. As shown in Figure 4L, a specific band with comparable size could be detected in both nal1 and the wild type. However, the abundance of the signal in nal1 was significantly reduced over that in the wild type (Fig. 4L). These results suggested that the nal1 mutation affected the abundance of a deduced rice homolog of AtPIN1, an auxin efflux facilitator in Arabidopsis. Among the three PIN1 family genes in rice, OsPIN1 (AF056027) shows the highest sequence similarity to AtPIN1 (Xu et al., 2005). Our quantitative real-time RT-PCR assays indicated that the OsPIN1 expression levels in dark-grown coleoptiles of nal1 were significantly reduced compared with those in the wild type (Fig. 4M), suggesting that the nal1 mutation affects the OsPIN1 abundance and activities at the transcription level.
DISCUSSION
Our results demonstrated that the nal1 mutation affects vascular patterns in leaves and culms of rice. Compared with their wild-type counterparts, mature leaves of the nal1 mutants showed distinctive vascular defects, including a reduced number of parallel veins, especially small veins (Fig. 1, E and F). On the other hand, transverse sections of the shortened internodes in the nal1 mutant also exhibited disturbed vascular patterns, in which the number and arrangement of the vascular bundles were altered (Fig. 1G). Gene cloning studies indicated that Nal1 encodes a novel protein that was preferentially expressed in vascular tissues (Fig. 3, B–I).
It had been established that auxin, especially its characteristic polar transport, plays a key role during pattern formation of the vascular system in both dicot and monocot plants (Tsiantis et al., 1999; Dengler, 2001; Scanlon et al., 2002; Aloni, 2004; Fukuda, 2004; Scarpella and Meijer, 2004; Teale et al., 2006). Our findings that the nal1 mutant showed abnormal vascular patterns in leaves and culms suggested that the nal1 phenotypes might be associated with auxin-dependent deficiencies. A series of analyses indicated that the nal1 mutant is defective in polar auxin transport. Supporting evidence for this notion came from measurements of [3H]IAA in etiolated coleoptiles, showing that the basipetal PAT activity in nal1 was significantly decreased (Fig. 4B) whereas the auxin retention in nal1 was significantly increased (Fig. 4C). Given that nal1 plants show abnormal vascular patterns in leaves and culms, the reduced PAT activity in nal1 could be due to defective vascular development in coleoptiles. Our investigation of 5-d-old dark-grown coleoptiles indicated that the morphology, vascular bundle number, and vascular structure between nal1 and the wild type were similar (Fig. 4, D–G). These observations rule out the possibility that the observed difference in IAA transport arises from structure differences between nal1 and the wild type.
Our auxin transport assay also indicated that the nal1 mutant was less sensitive than the wild type to the inhibitory effect of NPA on basipetal PAT (Fig. 4, B and C). It was proposed that the action of NPA to inhibit auxin transport was achieved through interacting with the so-called NPA-binding protein, which has long been considered part of the auxin efflux carrier complex (Dixon et al., 1996; Ruegger et al., 1997; Blakeslee et al., 2005). On the other hand, a characteristic feature of NPA action is that auxin accumulates in treated cells, and the effect of NPA on PAT was generally interpreted as an inhibition of auxin efflux (Morris et al., 2004). In this scenario, the findings that the nal1 mutant exhibited decreased PAT activity and altered responses to NPA led us to the hypothesis that the nal1 mutation might affect, directly or indirectly, auxin efflux. To test this, we looked at Arabidopsis, a model system in which the auxin efflux apparatus is relatively well understood. Among the molecular components involved in polar auxin transport in Arabidopsis identified to date, the best characterized AtPIN1 protein was believed to play an important role in the control of auxin efflux (Palme and Gälweiler, 1999). The existence of a family of highly conserved PIN proteins in the rice genome (Paponov et al., 2005), together with recent experimental evidence showing that one member of the rice PIN protein family, named OsPIN1, plays a role in auxin-dependent development (Xu et al., 2005), suggested that the function of the PIN proteins in regulating auxin efflux is conserved between these two species (Paponov et al., 2005; Xu et al., 2005). Therefore, available antibodies raised against the auxin efflux carrier AtPIN1 were used as probes to conduct immunohistochemical and protein gel-blot analyses in nal1 and wild-type plants. In both assays, the AtPIN1 antibodies could detect specific signals in vascular tissues (Fig. 4, H–K). Furthermore, signals detected by the AtPIN1 antibodies in the nal1 mutant were shown to be significantly weaker than those in wild-type plants (Fig. 4L). Gene expression analysis suggested that the nal1 mutation might affect the abundance and activities of OsPIN1 (Fig. 4M). Together, these results led us to the following speculations that need to be explored in our ongoing and future studies. First, the signals detected by the AtPIN1 antibodies might represent the rice homolog(s) of the AtPIN1 protein, which presumably acts as an auxin efflux carrier in rice. Second, reduced abundance of the rice homolog(s) of AtPIN1 might account for the PAT deficiency in the nal1 mutant. The results presented here demonstrated that mutation of the Nal1 function affects both auxin transport and vascular patterns; however, further studies are required to address whether the nal1 aberrations in vascular patterns are the result of its deficient auxin transport.
Another significant aspect of the nal1 phenotype is defective cell division. For example, the total cell number of the longitudinal sections of internode IV in nal1 mutants decreased to around one-third of that of wild-type plants (Fig. 1D; Table I). Given the established role of auxin in the control of cell division (Meijer and Murray, 2001; Morris et al., 2004; Woodward and Bartel, 2005), it is reasonable to generally associate the reduced cell number of the nal1 internode IV with its defective PAT-mediated auxin distribution. However, the detailed molecular mechanism by which Nal1 exerts its action on the cell cycle needs to be explored in future studies.
Subcellular localization studies using Arabidopsis protoplast or transgenic plants indicated that the Nal1-GFP fusion protein appears to form discrete nuclear protein bodies (Fig. 2, G and H). Accumulating evidence in Arabidopsis indicated that nuclear protein bodies are the sites of regulated protein degradation mediated by the ubiquitin/26S proteasome. For example, a Rac GTPase stimulates the recruitment of GFP-labeled AUXIN/IAAs from the nucleoplasm into nuclear protein bodies to be degraded by the ubiquitin/26S proteasome apparatus (Tao et al., 2005). AFP, a negative regulator of abscisic acid signaling, colocalizes with ABI5, a positive regulator of abscisic acid signaling, in nuclear protein bodies (Lopez-Molina et al., 2003). Abscisic acid also induces a heterogeneous nuclear ribonucleoprotein-type protein named AKIP1 to form nuclear protein bodies (Ng et al., 2004). Phytochrome B moves to the nucleus and forms nuclear protein bodies in response to different light conditions (Chen et al., 2003). Similarly, the E3 ligase COP1 has been observed to form nuclear protein bodies with several regulators of light signaling, including HY5 (Saijo et al., 2003), LAF1 (Seo et al., 2003), and HFR1 (Jang et al., 2005; Yang et al., 2005). In this context, our results showing that Nal1-GFP forms nuclear protein bodies link the Nal1 protein to the proteolysis process. As a first step to elucidate the biochemical and molecular mechanisms underlying the Nal1 actions, we are conducting experiments to determine whether Nal1 is involved in the degradation of other proteins or Nal1 itself is subjected to degradation by the 26S proteasome.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
A rice (Oryza sativa) mutant line showing a characteristic phenotype of narrow leaves was kindly provided by Dr. G.S. Khush at the International Rice Research Institute in the Philippines. The original mutant was back-crossed to the indica cultivar Zhefu802 for 10 successive generations, and the resulting mutant line was renamed nal1 (Dong et al., 1994; Zeng et al., 2003). nal1 and Zhefu802 were therefore considered to be a pair of nearly isogenic lines, and Zhefu802 was used as the wild-type line for all experiments involving comparisons of mutant and wild-type plants in this study. Rice plants were cultivated in the experimental field at the China National Rice Research Institute in the natural growing season.
Phenotypic Characterization of the nal1 Mutant
To investigate the morphology of the vascular bundles of the leaf blade and the internode, tissues at indicated stages were fixed in 5% formaldehyde, 5% glacial acetic acid, and 63% ethanol and dehydrated in a graded ethanol series. The samples were embedded in Paraplast Plus chips (Sigma). Microtome sections (8 μm thick) were affixed to poly-Lys-coated slides (Sigma), dewaxed in xylene, rehydrated through a graded ethanol series, and dried overnight before staining with toluidine blue for light microscopy.
Map-Based Cloning of Nal1
To identify the Nal1 gene using a positional cloning approach, nal1 was crossed to Nipponbare, a japonica cultivar, to generate a large F2 mapping population. For fine mapping, PCR-based markers were developed based on the sequence difference between japonica variety Nipponbare and indica variety 9311 (http://www.gramene.org/resources/). The primer sequences of the molecular markers used are listed in Table II. For RT-PCR, we used total RNA isolated from young seedlings as template. The full-length cDNA of Nal1 was amplified using the primer pair 5′-CGCTTTCGGCATTCGTTATCTACC-3′ and 5′-GGGATCCGGCAATGGTGTATATCA-3′, sequenced, and cloned into pBluescript SK+ vector (Stratagene).
Complementation Test
A 12.4-kb genomic DNA fragment containing the entire Nal1 coding region, the 2,673-bp upstream sequence, and the 1,685-bp downstream sequence was cut from BAC AL662950 with the restriction enzyme XbaI. This fragment was cloned into the XbaI sites of the binary vector pCAMBIA2300 to generate the transformation plasmid for the complementation test. The resulting transformation plasmid, as well as the empty pCAMBIA2300 vector as control, was introduced into the nal1 mutant by Agrobacterium tumefaciens-mediated transformation according to a published protocol (Hiei et al., 1994). The 30-bp deletion of the putative Nal1 allele in the mutant, which was confirmed in both cDNA and genomic sequences between nal1 and wild-type plants, could be detected with PCR using the primer pair 5′-GATGACTTTGACATTTCCACCGT-3′ and 5′-GAGTGATTCATTGGTAATGATAA-3′.
Phylogenetic Analysis
Homologous sequences of Nal1 were obtained through National Center for Biotechnology Information BLAST search. Maize (Zea mays) coding sequences were predicted by Fgenesh using homologous BAC clone sequences as input. Others were directly from a homologous cDNA clone. The collected protein sequences were then aligned by DIALIGN 2. Note that the program DIALIGN 2 employs the BLOSUM62 amino acid substitution matrix but does not use gap penalty. Instead, it assembles global alignments from gap-free local pair-wise alignments. The alignment was revised critically and used as input for MEGA3 to construct a phylogenetic tree. A neighbor-joining tree was built by MEGA3 using a Poisson correction model with gaps to complete deletions. Topological robustness was assessed by bootstrap analysis with 500 replicates.
Gene Expression Analysis
For RT-PCR analysis, total RNA was extracted from various tissues using TRIZOL reagent (Invitrogen). After RNase-free DNase (Takara) treatment, 2.5 μg of RNA was reverse transcribed using oligo(dT) primer and SuperScript III (Invitrogen). An appropriate amount of the products was further applied for a 20-μL PCR amplification using the following gene-specific primer pairs: 5′-GATGACTTTGACATTTCCACCGT-3′ and 5′-GAGTGATTCATTGGTAATGATAA-3′ for Nal1 (40 cycles of 15 s at 94°C, 15 s at 60°C, and 30 s at 72°C) and 5′-CATCTTGGCATCTCTCAGCAC-3′ and 5′-AACTTTGTCCACGCTAATGAA-3′ for ACTIN1 (25 cycles of 15 s at 94°C, 15 s at 60°C, and 30 s at 72°C).
For quantitative real-time RT-PCR, first-strand cDNAs were synthesized by reverse transcription from 3 μg of total RNA. The cDNAs were then used as templates in real-time PCR using the SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions. The amplification of the target genes was analyzed using the ABI Prism 7000 sequence detection system and software (PE Applied Biosystems). The relative expression levels of each transcript were obtained by normalization to the UBIQUITIN5 (UBQ5) gene. The following gene-specific primers were used for real-time PCR analysis: 5′-CGCCGTGCCGCTGCTGTCGTTCC-3′ and 5′-TCCCCCGGCGGCTGAGGTG-3′ for OsPIN1 and 5′-ACCACTTCGACCGCCACTACT-3′ and 5′-ACGCCTAAGCCTGCTGGTT-3′ for UBQ5. Three independent RNA isolations were used for cDNA synthesis, and each cDNA sample was subjected to real-time PCR analysis in triplicate.
For promoter-GUS assay, a 1,918-bp genomic fragment upstream of the Nal1 gene translation start codon was PCR amplified with the primers 5′-AAGTCGACTCCGAACCAAACACCAACACAC-3′ and 5′-AAGAATTCACAGTTTGCGAACCTATTATA-3′. The DNA fragment was cloned into the SalI and EcoRI sites of the vector pCAMBIA1391Z. The resulting plasmid was transformed into rice, and the resulted transgenic plants were analyzed by GUS staining assay as described (Scarpella et al., 2003).
RNA in situ hybridization was performed as described by Schwarzacher and Heslop-Harrison (2000). The 3′ end of Nal1 was amplified from wild-type plants with the following primers: 5′-CACCAACCTGAACAACCCCT-3′ and 5′-GGTCTTTTGATCTACTACTA-3′. The PCR product was subcloned into pGEM-T Easy vector (Promega) and used as a template to generate RNA probes. Transverse sections (8 μm thick) were probed with digoxigenin-labeled antisense probes (DIG Northern Starter Kit; Roche). The slides were observed with a microscope (Leica) and photographed using a 3CCD color video camera (Apogee Instruments).
Subcellular Localization of Nal1
The Nal1 cDNA was amplified from wild-type rice plants with the primers 5′-AAGTCGACATGAAGCCTTCGGACGATAA-3′ and 5′-AGCCATGGTCTCCAGGTCAAGGCTTGAT-3′ and cloned into the SalI/NcoI sites of the CaMV35S:GFP (S65T)-NOS-30 cassette vector (Niwa et al., 1999; Li et al., 2003). The resulting 35S:Nal1-GFP construct was introduced into Arabidopsis (Arabidopsis thaliana) protoplast as described (Meskiene et al., 2003).
The 35S:Nal1-GFP construct was digested with HindIII/EcoRI and cloned into the binary vector pCAMBIA1300 (http://www.cambia.org.au) to transform Arabidopsis plants using the floral dip method (Clough and Bent, 1998). Progeny of an identified T3 transgenic plant homozygous for the transgene were used to investigate the Nal1-GFP localization. The root cells of the 5-d-old transgenic seedlings grown on Murashige and Skoog (1962) agar plates were analyzed. Confocal images were collected using a Zeiss LSM 510 Meta confocal laser scanning microscope. GFP fluorescence was excited with a 488-nm argon laser, and images were collected in the 500- to 530-nm range. In all cases, transmissible light images were collected simultaneously. At least 20 seedlings were observed, and a representative image is presented.
Auxin Influx and Efflux Assays
Auxin uptake assays were conducted according to a method described previously (Dai et al., 2006) with some modifications. The upper ends (0.5 cm away from the tip) of 3-d-old dark-grown coleoptiles were harvested and allowed to equilibrate in a 1.5-mL microcentrifuge tubes containing 1/2 MS buffer (5 mm MES and 1% [w/v] Suc, pH 5.8) for 2 h. The coleoptile fragments (0.2 cm in length) were then incubated in 1/2 MS buffer containing 100 nm [3H]IAA (25 Ci/mmol; Amersham) for 10 min. Samples were then transferred into a new 1.5-mL microcentrifuge tube and rinsed in 1 mL of ice-cold 1/2 MS buffer three times. After rinsing, the samples were incubated in scintillation liquid for 18 h and the radioactivity was counted with a liquid scintillation counter (1450 MicroBeta TriLux; Perkin-Elmer). For each assay, five coleoptiles were measured.
The polar auxin transport assays were performed using coleoptiles of 5-d-old dark-grown rice seedlings as described previously (Okada et al., 1991; Dai et al., 2006; Li et al., 2007) with minor modifications. In each assay, five apical coleoptile segments each (0.2 cm away from the tip) of 2.0-cm length were preincubated in 1/2 MS medium with shaking at 100 rpm for 2 h. The coleoptile segments were then placed in a 1.5-mL microcentrifuge tube with one end submerged in 10 μL of 1/2 MS liquid medium containing 500 nm [3H]IAA plus 500 nm free IAA in the dark at room temperature for 2 h. NPA (Chem Service) was added to the medium as indicated. The unsubmerged ends of the segments (0.5 cm in length) were excised and washed three times in 1/2 MS liquid medium. After rinsing, the segments were incubated in 2.0 mL of universal scintillation fluid for 18 h and the radioactivity was counted with a liquid scintillation counter (1450 MicroBeta TriLux; Perkin-Elmer). Based on the orientation of the coleoptile segments submerged in the buffer, basipetal or acropetal auxin transport was measured, with the acropetal auxin transport as control.
IAA efflux of dark-grown coleoptile segments was assayed as described previously (Dai et al., 2006). In each assay, five apical dark-grown coleoptile segments (2.0 mm in length) were put into 50 μL of 1/2 MS medium buffer containing 500 nm [3H]IAA plus 500 nm free IAA and incubated at room temperature for 2 h with shaking at 100 rpm. NPA (2 μm) was added to the medium as indicated. The segments were then rinsed and incubated for another 2 h in the same buffer without IAA to allow the IAA to be transported out. After washing twice, the segments were then incubated in 2.0 mL of universal scintillation fluid for 18 h, and the radioactivity was counted with a liquid scintillation counter (1450 MicroBeta TriLux; Perkin-Elmer).
Immunohistochemical Localization and Protein Gel-Blot Analysis
Immunohistochemical localizations and microscopy were conducted as described previously (Noh et al., 2003). The antibody used was anti-AtPIN1 (Gälweiler et al., 1998) at a final dilution of 1:200. Fluorescein isothiocyanate-conjugated antibody was used as a secondary antibody. A confocal laser scanning microscope (Zeiss LSM 510 Meta) was used for immunofluorescence imaging.
One gram of coleoptiles from 5-d-old dark-grown seedlings was used for membrane protein extraction using the ProteoExtract kit (M-PEK; Calbiochem). Membrane proteins were separated by 10% SDS-PAGE and probed with anti-AtPIN1 (1:200) antibodies.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: Nal1 (EU093963), putative OsPIN1 (AF056027).
Acknowledgments
We thank Dr. Jiayang Li (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for critical reading of the manuscript. We thank Dr. Gurdev Singh Khush (International Rice Research Institute) for providing the original nal1 mutant, Dr. Bin Han (National Center for Gene Research, China) for providing the BAC clone AL662950, and Dr. Kang Chong (Institute of Botany, Chinese Academy of Sciences) for assistance in RNA in situ hybridization.
This work was supported by grants from the Ministry of Science and Technology of China (grant nos. 2005CB120801 and 2006AA10A101) and the National Natural Science Foundation of China (grant nos. 30700054 and 90717007).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Chuanyou Li (cyli@genetics.ac.cn).
Open Access articles can be viewed online without a subscription.
References
- Aloni R (2004) The induction of vascular tissues by auxin. In PJ Davies, ed, Plant Hormones: Biosynthesis, Signal Transduction, Action! Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 471–492
- Blakeslee JJ, Peer WA, Murphy AS (2005) Auxin transport. Curr Opin Plant Biol 8 494–500 [DOI] [PubMed] [Google Scholar]
- Chen M, Schwab R, Chory J (2003) Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc Natl Acad Sci USA 100 14493–14498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang H, Li B, Huang J, Liu X, Zhou Y, Mou Z, Li J (2006) Increased expression of MAP KINASE KINASE7 causes deficiency in polar auxin transport and leads to plant architectural abnormality in Arabidopsis. Plant Cell 18 308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenhoffer JM, Evert W, Evert RF (1990) Leaf vasculature in barley, Hordeum vulgare (Poaceae). Am J Bot 77 636–652 [Google Scholar]
- Dengler NG (2001) Regulation of vascular development. J Plant Growth Regul 20 1–13 [Google Scholar]
- Dixon MW, Jacobson JA, Cady CT, Muday GK (1996) Cytoplasmic orientation of the naphthylphthalamic acid-binding protein in zucchini plasma membrane vesicles. Plant Physiol 112 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Xiong Z, Qian Q, Zu X, Chen S (1994) Breeding near-isogenic lines of morphological markers in indica rice. Chin J Rice Sci 8 135–139 [Google Scholar]
- Esau K (1965) Vascular Differentiation in Plants. Holt, Rinehart and Winston, New York
- Foster AS (1952) Foliar venation in angiosperms from an ontogenetic standpoint. Am J Bot 39 752–766 [Google Scholar]
- Friml J (2003) Auxin transport: shaping the plant. Curr Opin Plant Biol 6 7–12 [DOI] [PubMed] [Google Scholar]
- Friml J, Palme K (2002) Polar auxin transport: old questions and new concepts? Plant Mol Biol 49 273–284 [PubMed] [Google Scholar]
- Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5 379–391 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan CH, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230 [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44 179–194 [DOI] [PubMed] [Google Scholar]
- Haga K, Takano M, Neumann R, Iino M (2005) The rice coleoptile phototropism1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin. Plant Cell 17 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6 271–282 [DOI] [PubMed] [Google Scholar]
- Jang IC, Yang JY, Seo HS, Chua NH (2005) HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev 19 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O (2005) Auxin distribution and plant pattern formation: how many angels can dance on the point of PIN? Cell 121 819–822 [DOI] [PubMed] [Google Scholar]
- Li P, Wang Y, Qian Q, Fu Z, Wang M, Zeng D, Li B, Wang X, Li J (2007) LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res 17 402–410 [DOI] [PubMed] [Google Scholar]
- Li X, Qian Q, Fu Z, Wang Y, Xiong G, Zeng D, Wang X, Liu X, Teng S, Hiroshi F, et al (2003) Control of tillering in rice. Nature 422 618–621 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Kinoshita N, Chua NH (2003) AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev 17 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C (2002) Auxin transport: ABC proteins join the club. Trends Plant Sci 7 329–332 [DOI] [PubMed] [Google Scholar]
- McHale NA (1993) UM7 and FAT genes control development of the leaf blade in Nicotiana sylvestris. Plant Cell 5 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M, Murray JAH (2001) Cell cycle controls and the development of plant form. Curr Opin Plant Biol 4 44–49 [DOI] [PubMed] [Google Scholar]
- Meskiene I, Baudouin E, Schweighofer A, Liwosz A, Jonak C, Rodriguez PL, Jelinek H, Hirt H (2003) Stress-induced protein phosphatase 2C is a negative regulator of a mitogen-activated protein kinase. J Biol Chem 278 18945–18952 [DOI] [PubMed] [Google Scholar]
- Morris AD, Friml J, Zazimalova E (2004) Auxin transport. In PJ Davies, ed, Plant Hormones: Biosynthesis, Signal Transduction, Action! Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 437–470
- Multani DS, Briggs SP, Chamberlin MA, Blakeslee JJ, Murphy AS, Johal GS (2003) Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science 302 81–84 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15 473–497 [Google Scholar]
- Nelson T, Dengler N (1997) Leaf vascular pattern formation. Plant Cell 9 1121–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CK, Kinoshita T, Pandey S, Shimazaki K, Assmann SM (2004) Abscisic acid induces rapid subnuclear reorganization in guard cells. Plant Physiol 134 1327–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H (1999) Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J 18 455–463 [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS (2003) Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423 999–1000 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K, Gälweiler L (1999) PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol 2 375–381 [DOI] [PubMed] [Google Scholar]
- Paponov IA, Teale WD, Trebar M, Blilou I, Palme K (2005) The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci 10 170–177 [DOI] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, et al (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312 914–918 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SH, Evert RF (1985) Leaf vasculature in Zea mays L. Planta 164 448–458 [DOI] [PubMed] [Google Scholar]
- Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9 151–262 [Google Scholar]
- Sachs T (1989) The development of vascular networks during leaf development. Curr Top Plant Biochem Physiol 8 168–183 [Google Scholar]
- Sachs T (2000) Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol 41 649–656 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW (2003) The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev 17 2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinohl V, Friml J, Benkova E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon MJ, Henderson DC, Bernstein B (2002) SEMAPHORE1 functions during the regulation of ancestrally duplicated knox genes and polar auxin transport in maize. Development 129 2663–2673 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Boot KJ, Rueb S, Meijer AH (2002) The procambium specification gene Oshox1 promotes polar auxin transport capacity and reduces its sensitivity toward inhibition. Plant Physiol 130 1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Meijer AH (2004) Pattern formation in the vascular system of monocot and dicot plant species. New Phytol 164 209–242 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Rueb S, Boot KJ, Hoge JH, Meijer AH (2000) A role for the rice homeobox gene Oshox1 in provascular cell fate commitment. Development 127 3655–3669 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Rueb S, Meijer AH (2003) The RADICLELESS1 gene is required for vascular pattern formation in rice. Development 130 645–658 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Simons EJ, Meijer AH (2005) Multiple regulatory elements contribute to the vascular-specific expression of the rice HD-Zip gene Oshox1 in Arabidopsis. Plant Cell Physiol 46 1400–1410 [DOI] [PubMed] [Google Scholar]
- Scheres B, Xu J (2006) Polar auxin transport and patterning: grow with the flow. Genes Dev 20 922–926 [DOI] [PubMed] [Google Scholar]
- Schwarzacher T, Heslop-Harrison P (2000) Practical in Situ Hybridization. BIOS Scientific Publishers, New York
- Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, Chua NH (2003) LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423 995–999 [DOI] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Nibau C, Wua HM (2005) RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell 17 2369–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7 847–859 [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Brown MIN, Skibinski G, Langdale JA (1999) Disruption of auxin transport is associated with aberrant leaf development in maize. Plant Physiol 121 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhu L, Shou H, Wu P (2005) A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol 46 1674–1681 [DOI] [PubMed] [Google Scholar]
- Yang J, Lin R, Sullivan J, Hoecker U, Liu B, Xu L, Deng XW, Wang H (2005) Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell 17 804–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZH (2002) Vascular tissue differentiation and pattern formation in plants. Annu Rev Plant Biol 53 183–202 [DOI] [PubMed] [Google Scholar]
- Zeng D, Qian Q, Dong G, Zhu X, Dong F, Teng S, Guo L, Cao L, Cheng S, Xiong Z (2003) Development of isogenic lines of morphological markers in indica rice. Acta Bot Sin 45 1116–1120 [Google Scholar]