Abstract
In tip-growing cells, the tip-high Ca2+ gradient is thought to regulate the activity of components of the growth machinery, including the cytoskeleton, Ca2+-dependent regulatory proteins, and the secretory apparatus. In pollen tubes, both the Ca2+ gradient and cell elongation show oscillatory behavior, reinforcing the link between the two. We report that in growing root hairs of Arabidopsis (Arabidopsis thaliana), an oscillating tip-focused Ca2+ gradient can be resolved through imaging of a cytosolically expressed Yellow Cameleon 3.6 fluorescence resonance energy transfer-based Ca2+ sensor. Both elongation of the root hairs and the associated tip-focused Ca2+ gradient show a similar dynamic character, oscillating with a frequency of 2 to 4 min−1. Cross-correlation analysis indicates that the Ca2+ oscillations lag the growth oscillations by 5.3 ± 0.3 s. However, growth never completely stops, even during the slow cycle of an oscillation, and the concomitant tip Ca2+ level is always slightly elevated compared with the resting Ca2+ concentration along the distal shaft, behind the growing tip. Artificially increasing Ca2+ using the Ca2+ ionophore A23187 leads to immediate cessation of elongation and thickening of the apical cell wall. In contrast, dissipating the Ca2+ gradient using either the Ca2+ channel blocker La3+ or the Ca2+ chelator EGTA is accompanied by an increase in the rate of cell expansion and eventual bursting of the root hair tip. These observations are consistent with a model in which the maximal oscillatory increase in cytosolic Ca2+ is triggered by cell expansion associated with tip growth and plays a role in the subsequent restriction of growth.
Tip growth of cells such as fungal hyphae, algal rhizoids, pollen tubes, and root hairs is sustained by targeted secretion of new membrane and wall material to the apical few micrometers of their elongating tips. Turgor is then thought to drive expansion at the cell apex, with the subapical wall resisting these expansive forces. The combination of localized secretion coupled to regulation of wall properties would then lead to the elongated cylindrical morphology of these cells (for review, see Gilroy and Jones, 2000).
The tip-focused Ca2+ gradient, characteristic of tip-growing cells, seems to play an important role in the spatial control of these systems. The cytosolic free Ca2+ concentration is approximately 100 nm at the base of the polarized cell but rises up to micromolar levels over the apical few micrometers of the expanding tip (for review, see Bibikova and Gilroy, 2002). This elevated apical Ca2+ is proposed to provide a spatial determinant for growth by facilitating membrane fusion at the tip and regulating a host of Ca2+-dependent proteins required for tip growth. Dissipating the Ca2+ gradient in pollen tubes, fungal hyphae, and root hairs has been shown to disrupt growth (Clarkson et al., 1988; Miller et al., 1992; Herrmann and Felle, 1995; Wymer et al., 1997), whereas experimentally altering the direction of the gradient leads to redirected growth, with the site of new expansion following where the new Ca2+ gradient is imposed (Malho and Trewavas, 1996; Bibikova et al., 1997). Thus, there is strong evidence supporting a regulatory role for the Ca2+ gradient through imposing spatial control on the site of cell expansion in these tip-growing systems. Indeed, the structures of the apical actin cytoskeleton and its regulatory proteins (villins, gelsolins, and actin-depolymerizing factors; Smertenko et al., 1998; Tominaga et al., 2000; Allwood et al., 2001; Ketelaar et al., 2003; Fan et al., 2004) are thought to be regulated by the tip-high Ca2+ (Yokota et al., 2005). Similarly, there is evidence that annexins (Blackbourn et al., 1992; Clark et al., 1992; Carroll et al., 1998), phosphoinositide metabolism (Preuss et al., 2006), and calmodulin and protein kinases (Moutinho et al., 1998; Yoon et al., 2006) also play roles in sustaining tip growth and are regulated via the cytosolic Ca2+ gradient.
Tip growth in some systems has been shown to oscillate, with periods of rapid expansion alternating with slower growth rates. While this has been confirmed for different species of pollen tubes by independent groups (Pierson et al., 1996; Messerli and Robinson, 1997; Watahiki et al., 2004; Hwang et al., 2005), it has not been repeated for tip growth in fungal hyphae (Lopez-Franco et al., 1994; Sampson et al., 2003). Oscillating growth in root hairs has only recently been reported (Monshausen et al., 2007) and is under further investigation in this report.
One of the most extensively studied tip-growing systems has been lily (Lilium longiflorum) pollen tubes. In this system, growth oscillations were accompanied by oscillations in the tip-focused Ca2+ gradient (Holdaway-Clarke et al., 1997; Messerli and Robinson, 1997; Messerli et al., 2000; Watahiki et al., 2004). Interestingly, the periodic increases in the Ca2+ gradient actually lagged the periodic increases in growth by about 4 s (Messerli et al., 2000). These observations led to a model for pollen tubes in which a mechanically sensitive Ca2+ channel may be gated via membrane tension during elongation growth. This stretch-activated channel would then support a Ca2+ increase that follows rather than coincides with maximal cell elongation (Messerli and Robinson, 2007). It has even been suggested that a burst of secretion may precede elongation in Agapanthus pollen (Coelho and Malhó, 2006). Lily pollen tubes in culture (for review, see Messerli et al., 2000) grow five to seven times faster than Arabidopsis (Arabidopsis thaliana) root hairs in culture (Monshausen et al., 2007), and it is unclear whether an equivalent relationship between oscillatory growth and Ca2+ changes exists for the root hair. Although a stretch-activated Ca2+ influx channel has been identified in pollen tubes (Dutta and Robinson, 2004), the Ca2+ channel sustaining the tip-focused Ca2+ gradient in root hairs is thought to be gated by membrane voltage and reactive oxygen species (ROS; Foreman et al., 2003). Thus, whether fluctuations in the root hair apical Ca2+ gradient occur and are functionally important for regulating cell elongation remains unknown.
Here, we report that root hairs of Arabidopsis exhibit both oscillatory growth and oscillations in the tip-focused Ca2+ gradient. In addition, we show that the maximum of the Ca2+ gradient lags the growth maximum by approximately 5 s. Treatments that dissipate the Ca2+ gradient promote tip expansion and eventual bursting, whereas artificially elevating cytosolic Ca2+ leads to rapid growth arrest. These results indicate that one role for the maximal Ca2+ levels attained during the oscillatory increase in cytosolic Ca2+ that accompany root hair growth is actually to limit turgor-driven expansion after each burst of elongation.
RESULTS
Oscillations in Cytosolic Ca2+ Accompany Root Hair Tip Growth
To determine the kinetics of the tip-focused Ca2+ gradient in growing root hairs, we used Arabidopsis plants stably transformed with a soluble version of the GFP-based Ca2+ sensor Yellow Cameleon (YC) 3.6 (Nagai et al., 2004), driven by the cauliflower mosaic virus 35S promoter. Expression of this protein had no detectable effect on root hair growth rates, density, or general morphology (Fig. 1; data not shown), indicating that it provided an appropriate approach for the analysis of root hair growth. We imaged the YC3.6 signal using a Zeiss LSM 510 confocal microscope and found that root hair elongation was sensitive to laser irradiation intensity, with higher levels of irradiation leading to a minor but significant inhibition of elongation rates (reduction from 2.01 ± 0.29 to 1.34 ± 0.26 μm min−1 at 60% laser attenuation [n = 6], Student's t test, P < 0.001; see “Materials and Methods” for specifics of the imaging protocol). However, using lower laser power (90% attenuation), we were able to monitor root hairs for extended periods (>10 min) with frequent sampling (images every 2 s) without significant alteration of root hair growth rate (1.85 ± 0.33 μm min−1 [n = 6], Student's t test, P = 0.38) or morphology (Fig. 1), allowing us to make measurements of root hair Ca2+ dynamics during growth.
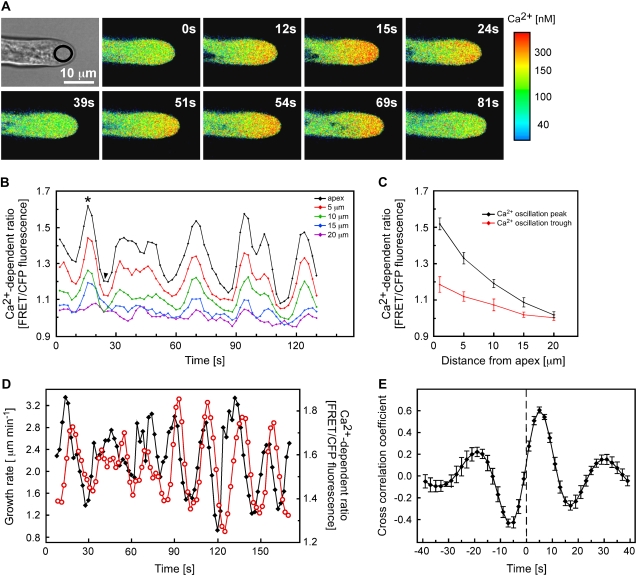
Figure 1.
Arabidopsis root hairs show an oscillating tip-focused Ca2+ gradient that peaks after maximal growth. A, Root hairs undergoing tip growth in Arabidopsis plants expressing the Ca2+ sensor YC3.6 targeted to the cytosol were imaged every 3 s. Cytosolic Ca2+ levels were calibrated as described in “Materials and Methods” and pseudocolor coded according to the scale at right. Numbers represent time in seconds. Representative results of more than 40 measurements are shown. Bar = 10 μm. B, Quantitative analysis of cytosolic Ca2+ oscillations in a representative growing root hair. Ca2+ levels were measured in 5-μm2 regions of interest (ROI) along the root hair length, as indicated in the inset. Increase in the FRET/CFP ratio reflects an increase in cytoplasmic Ca2+ level. C, Average Ca2+ levels during peaks and troughs of Ca2+ oscillations. Values selected for calculation of averages are depicted with the asterisk (peak) and arrowhead (trough) in B. D, Quantitative analysis of root hair growth rates and cytosolic Ca2+ levels at the root hair apex. Ca2+ was measured in the approximately 30-μm2 ROI indicated in A. Representative results of more than 10 measurements are shown. E, Cross-correlation analysis of Ca2+ oscillations with growth oscillations indicates that the increases in cytosolic Ca2+ lag the increases in growth rate by approximately 5 s. Cross-correlation was performed on data from eight separate root hairs.
As reported previously (for review, see Bibikova and Gilroy, 2002), growing root hairs were characterized by a tip-focused Ca2+ gradient. Invariably, Ca2+ levels were highest within 1 to 2 μm of the extreme apex and then rapidly declined with increasing distance from the tip, until reaching resting Ca2+ concentrations at approximately 20 μm behind the apex (Fig. 1, A–C). While this gradient persisted as long as root hairs continued to grow, our high temporal resolution measurements showed that the magnitude of the gradient oscillated with a frequency of approximately two to four peaks per minute (Fig. 1, B and C). The largest changes in cytoplasmic Ca2+ occurred at the extreme tip of the root hair, whereas Ca2+ levels in more subapical regions oscillated in phase but with smaller amplitudes (Fig. 1, A and B).
We were interested in whether these changes in Ca2+ were associated with alterations in growth rate. Applying high-resolution tip-tracking software previously used to measure growth of Arabidopsis root hairs (Messerli et al., 1999; Monshausen et al., 2007), we were able to confirm that growth rates of YC3.6-expressing root hairs oscillated at the same frequency of two to four peaks per minute as apical Ca2+ levels (Fig. 1D) and as in untransformed wild-type root hairs (Monshausen et al., 2007). The magnitude of both the oscillations in Ca2+ and growth rate were variable even within a single root hair (Fig. 1B; Supplemental Fig. S1). While we could not observe a clear relationship between the amplitude of growth peaks versus the amplitude of Ca2+ peaks using linear regression analysis (Supplemental Fig. S1), our measurements indicated a close temporal relationship between cytoplasmic Ca2+ and growth, where each burst of growth appeared to be followed by a rapid elevation in Ca2+ (Fig. 1D). Cross-correlation analysis comparing the temporal kinetics of Ca2+ and growth oscillations (Messerli et al., 2000) confirmed that growth peaks most likely preceded Ca2+ increases by 5.3 ± 0.3 s (Fig. 1E).
In agreement with previous observations (Wymer et al., 1997), nongrowing root hairs showed no sustained tip-focused Ca2+ gradient and no oscillations in Ca2+ levels could be detected (Supplemental Fig. 2).
Blocking Ca2+ Entry Leads to Uncontrolled Expansion
Our observation that Ca2+ concentrations increased after growth parallels results obtained with pollen tubes, in which it has even been suggested that pulsatile expansion may actually be divorced from the oscillating Ca2+ gradient (Messerli et al., 2000). To further assess the relationship between Ca2+ and tip growth, we therefore attempted to manipulate cytoplasmic Ca2+ levels while simultaneously monitoring cell expansion.
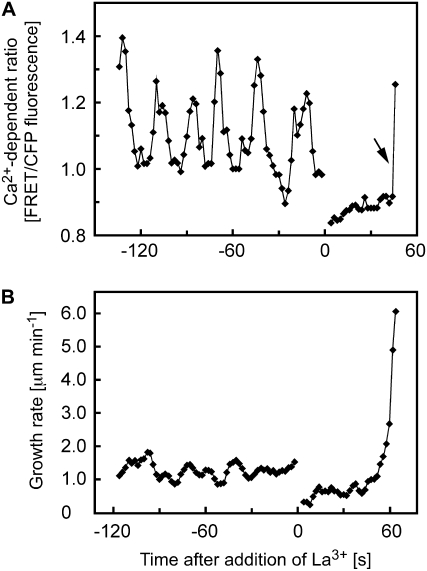
Published data indicate that Ca2+ enters the cytoplasm of tip-growing root hairs from the extracellular environment (Herrmann and Felle, 1995; Wymer et al., 1997). To attenuate this influx, we incubated roots with La3+, a blocker of Ca2+-permeable channels. Monitoring Ca2+ levels during La3+ treatment showed that 200 μm La3+ rapidly caused the dissipation of the tip-focused Ca2+ gradient. Thus, within less than 10 s of treatment, either no difference between apical Ca2+ levels and those 20 μm from the tip could be observed, or a slight decline to below these subapical Ca2+ levels immediately after La3+ treatment was observed (Fig. 2A). These observations suggest that the tip-focused gradient is largely supported by influx into the cytosol by La3+-sensitive channels and collapses very rapidly upon their inhibition, whereas basal Ca2+ levels are largely maintained under these conditions. Interestingly, however, although inhibited relative to its rate before the addition of La3+, expansion of the cell apex continued for a few minutes following La3+ treatment. This expansion accelerated until the root hairs eventually burst at their tips (Table I, Fig. 2B). At higher concentrations of La3+ (1 mm), almost all growing root hairs ruptured within 10 min of treatment, whereas at lower concentrations of the inhibitor (200 μm), only the vigorously growing root hairs closer to the apex of the root consistently burst; older, more basal root hairs ceased to elongate but continued to swell at the apex for some time (Table I; data not shown). To ascertain that the growth effects of La3+ were indeed due to inhibition of Ca2+ influx rather than to unspecific effects, we sought to attenuate Ca2+ influx by the alternative means of chelating extracellular Ca2+. Treatment with 4 mm EGTA also led to rapid bursting of almost all growing root hairs within 10 min of treatment (Table I).
Figure 2.
Effects of La3+ on cytosolic Ca2+ and growth of Arabidopsis root hairs. A, Treatment with 200 μm La3+ triggers rapid dissipation of the tip-focused Ca2+ gradient in a growing root hair. Ca2+ levels were measured in the approximately 30-μm2 ROI at the root hair apex indicated in Figure 1A. The rapid increase in Ca2+ at the end of this recording is due to Ca2+ entry during bursting of the root hair. The arrow denotes this Ca2+ increase due to bursting. B, Treatment with 200 μm La3+ causes acceleration of elongation and eventual bursting of growing root hairs. Representative results of seven (Ca2+) and 10 (growth) measurements are shown.
Table I.
Frequency of root hair bursting in response to EGTA and La3+
Root hairs were treated as indicated, and percentages of burst hairs in the apical 400-μm region of the root hair zone were scored after 10 min. Data are from at least six different roots for each treatment.
| Treatment | No. of Root Hairs Analyzed | Percentage of Burst Root Hairs |
|---|---|---|
| 4 mm EGTA | 119 | 98.5 |
| 1 mm La3+ | 87 | 96.0 |
| 0.2 mm La3+, apical 200 μma | 54 | 86.2 |
| 0.2 mm La3+, apical 400 μma | 117 | 65.4 |
Root hairs were monitored in either the apical 200- or 400-μm region of the root hair zone.
These results indicate that expansion of the root hair tip can be sustained by processes that are not strictly dependent on the high cytoplasmic Ca2+ concentrations normally found in the root hair apex. It is important here to distinguish between such experimentally induced expansion/swelling that leads to eventual bursting and the highly controlled cell elongation that is sustained to allow normal growth to occur. The expansion observed in the absence of a clear tip-focused Ca2+ gradient seemed to occur in an uncontrolled manner, leading to cell rupture. Endogenous oscillatory increases in Ca2+ levels at the growing root hair apex may thus play a role in restricting expansion after each burst of growth to maintain control of the sustained elongation characteristic of tip growth.
Increasing the Tip-High Ca2+ Gradient Arrests Growth
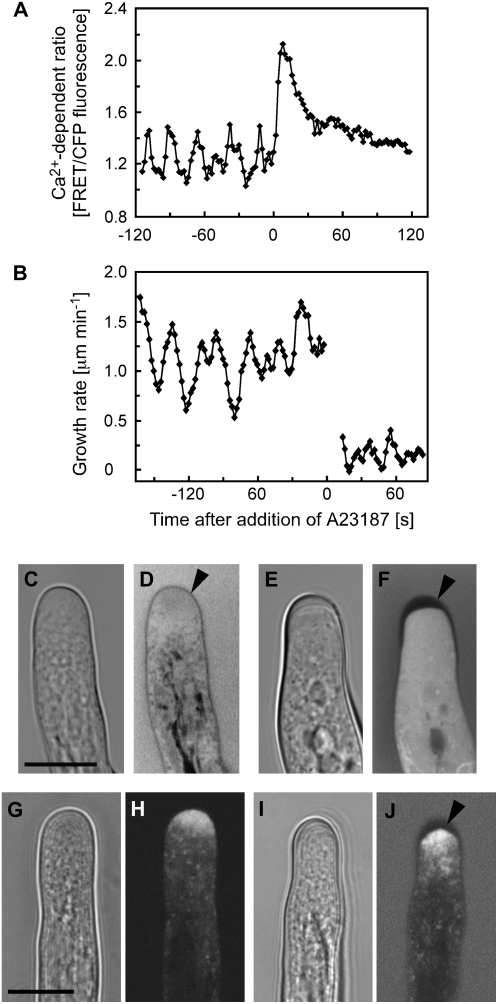
To investigate this potential regulatory role of Ca2+ in restricting growth, we artificially increased cytoplasmic Ca2+ by treating root hairs with the Ca2+ ionophore A23187. Figure 3 shows that prior to application of 10 μm A23187, both the tip-focused Ca2+ gradient and the growth rate oscillated as shown in Figure 1. Immediately after treatment, however, Ca2+ levels rapidly increased (Fig. 3A). The elevated Ca2+ levels subsequently declined over 30 to 60 s, likely due to the activation of Ca2+ homeostatic systems compensating for the increased influx elicited by the ionophore. Interestingly, upon A23187 treatment, elongation was arrested within a few seconds of treatment (Fig. 3B). In many root hairs, this high Ca2+-induced cessation of cell expansion was accompanied by a thickening of the apical cell wall. This thickening was visible in bright-field images as a cap-like structure with altered refractory properties (Fig. 3, compare C and E). To confirm the nature of this structure, we loaded root hairs with fluorescein diacetate, supplemented the external medium with fluorescein dextran (10 kD), and imaged the cell apex using confocal optical sections of less than 1 μm thickness. We found that if image acquisition was performed soon after the addition of fluorescein dextran to the medium, very little of the dye had yet permeated the cell wall. Thus, as both cytosolic fluorescein and extracellular fluorescein were excluded from the wall space, the thickness of the wall became apparent by the absence of fluorescence (Fig. 3F). Although the extent of wall thickening was variable in ionophore-treated cells, it was most evident in younger root hairs, and equivalent thickening was never found in untreated control root hairs (Fig. 3, C–F). These observations indicate that while Ca2+ ionophore treatment rapidly arrested cell expansion of root hairs, secretion of wall material was sustained, leading to a thickening in which tip growth-related secretion normally occurs. YFP∷RabA4b is thought to mark the apical secretory vesicle machinery in growing Arabidopsis root hairs, dissipating once growth ceases (Preuss et al., 2004, 2006). Monitoring the distribution of this yellow fluorescent protein (YFP) marker in root hairs revealed that, as reported previously, RabA4b decorated an apical accumulation of vesicles (Fig. 3H) and this accumulation was maintained despite A23187-arrested elongation (Fig. 3J), consistent with the idea that secretion is sustained in these ionophore-treated nongrowing cells.
Figure 3.
Effects of the Ca2+ ionophore A23187 on cytosolic Ca2+ and growth of Arabidopsis root hairs. A, Treatment with 10 μm A23187 triggers a rapid increase of cytosolic Ca2+ in a growing root hair. Ca2+ levels were measured in the approximately 30-μm2 ROI at the root hair apex indicated in Figure 1A. Representative results of 10 measurements are shown. B, Treatment with 10 μm A23187 arrests root hair tip growth. All detectable growth of the root hair had ceased by 14 s, when measurements resumed. The nongrowing root hair did not remain in the same plane as the root axis continued to shift after treatment, necessitating constant refocusing. At or below the limits of resolution for the tracker (0.2 μm min−1), this combination of changes in focal plane and root expansion appear as very slow growth for this example. Representative results of 10 measurements are shown. C to F, Bright-field and fluorescence images of root hairs loaded with fluorescein diacetate and immersed in medium containing fluorescein dextran. C and D, Untreated growing control root hairs. Cell wall (arrowhead) thickness is indicated by the exclusion of cytosolic and extracellular fluorescein fluorescence. E and F, A23187-induced Ca2+ increase and growth inhibition are accompanied by a thickening of the apical root hair cell wall (arrowhead). Images were acquired 1 h after the start of ionophore treatment. Representative results of 11 measurements are shown. G to J, Bright-field and fluorescence images of root hairs expressing YFP-RabA4b. G and H, Untreated growing control root hairs. Note that YFP-RabA4b accumulates at the apex of the growing root hair. I and J, A23187-induced apical cell wall thickening is accompanied by an accumulation of YFP-RabA4b at the cell apex. The root hair was immersed in medium containing fluorescein dextran to help visualize cell wall thickness (arrowhead). Representative results of 10 measurements are shown. Bars = 10 μm.
DISCUSSION
The oscillations in elongation of pollen tubes are thought to reflect either the rapid usage of growth components, which must be reaccumulated to support the next phase of growth, or feedback in either the regulatory or metabolic machinery supporting tip growth (Feijo et al., 2001). Until recently, whether such oscillatory patterns represent an element in the process of root hair tip growth has been unclear. Figure 1 shows that when made with sufficient resolution, oscillations in growth rate could be monitored in root hairs of Arabidopsis with a frequency of two to four peaks per minute (see also Monshausen et al., 2007). During these oscillations, elongation decelerated and accelerated, with growth rates rising to up to three times basal levels. It is important to note that elongation never completely paused during the slowest phase of the oscillation. Thus, the regulatory mechanisms behind the oscillatory growth most likely represent the effects of cellular machinery fine-tuning the rate of expansion. In lily pollen tubes, growth oscillates at approximately one to three peaks per minute, with peak growth rates reaching two to five times the basal level (Pierson et al., 1996; Messerli and Robinson, 1997). Thus, despite the slower growth rate of the root hair, the kinetics of its growth oscillations closely parallel those of pollen tubes, suggesting a possibly conserved oscillatory mechanism.
Although genetically encoded Ca2+ reporters such as YC2.1 have been used as a noninvasive method to monitor Ca2+ changes during pollen tube growth (Watahiki et al., 2004), until recently the small dynamic range of these probes had limited their usefulness in resolving the dynamics of the Ca2+ gradient in root hairs. However, the enhanced dynamic range of the YC3.6 reporter (Nagai et al., 2004) has allowed us to monitor oscillatory changes in the tip-focused Ca2+ gradient during oscillating growth (Fig. 1). Cross-correlation analysis indicates that the oscillations of the Ca2+ gradient lagged oscillations in growth by 5 s. A similar phenomenon has been observed in pollen tubes, in which the cytosolic Ca2+ increase also lagged growth by 4 s (Messerli et al., 2000; Messerli and Robinson, 2007).
It is important to note that while the Ca2+ gradient underwent regular oscillations, there was a statistically significantly elevated Ca2+ level at the tip throughout the oscillatory cycle (Fig. 1). This consistently elevated Ca2+ concentration is likely to support basal levels of exocytosis during apical growth. Indeed, Figure 3 shows that when Ca2+ levels were artificially elevated by ionophore treatment, there was an increase in apical wall thickness, consistent with a Ca2+-promoted fusion of secretory vesicles, leading to exocytosis of wall material. A similar phenomenon has been reported in pollen tubes, in which Reiss and Herth (1979) observed an apical thickening as growth was arrested. Importantly, our analysis of the organization of the apical secretory membrane system using YFP∷RabA4b indicates that even after growth arrest by application of Ca2+ ionophore and Ca2+ elevation, the apical secretory machinery remains in place, consistent with the idea that the elevated Ca2+ level could drive this apparatus to high levels of secretion and so to the wall thickening we observed.
However, during normal tip growth of the root hair, oscillatory Ca2+ increases are superimposed on the stable tip-focused Ca2+ gradient. The maximum of each Ca2+ oscillation occurred after an increase in growth, suggesting that while the basal gradient may be supporting sustained growth, the Ca2+ peaks may play additional role(s) in organizing spatial and temporal aspects of root hair elongation. One possibility is that the increase in Ca2+ is acting to “prime” the hair for the subsequent pulse of growth (i.e. acting to prepare the secretory apparatus for the next round of cell expansion). However, the strong cross-correlation of the Ca2+ increase to following the period of maximal growth rate, rather than preceding it, suggests a role in processes following the burst of increased growth. Alternatively, elevated Ca2+ levels could be acting on enzymes that regulate wall structure to rigidify the wall and so help limit turgor-driven expansion. Such dual Ca2+-dependent regulation is well characterized for many mammalian regulatory processes in which the spatial and temporal dynamics of a change in Ca2+ can trigger different responses in the same cell. For example, in B lymphocytes, the transcriptional regulators NFkB and c-Jun N-terminal kinase are selectively activated by a large transient increase in Ca2+, whereas, in the same cells, up-regulation of the nuclear factor of activated T-cell transcription factor requires a sustained, low-level increase in the same ion (Dolmetsch et al., 1997). It is also important to note that our cytosolic Ca2+ measurements are monitoring bulk cytosolic Ca2+ levels and could reflect Ca2+ influx both from across the plasma membrane and from internal sites that may help support different aspects of the tip-focused Ca2+ gradient (i.e. maintaining the basal gradient versus generating the oscillatory component) and have specific targets and actions within the cell. Our observations that (1) blocking Ca2+ influx into the root hair leads to uncontrolled expansion/bursting, (2) artificially increasing Ca2+ causes growth arrest, and (3) the peak of the Ca2+ oscillation occurs after a burst in growth are all consistent with a role for the maximal component of the oscillatory cytosolic Ca2+ increase in limiting rather than in facilitating expansion. Similar effects have been seen in fungi, in which the hyphae of Saprolegnia ferax show abnormal growth with enlarged hyphal diameter when they are transferred to nominally zero-Ca2+ medium (Jackson and Heath, 1989), suggesting that the restraints on growth may also have changed. Previous analyses of treatments that alter the Ca2+ gradient in the root hair often reported a cessation of root hair growth (Clarkson et al., 1988; Miller et al., 1992; Herrmann and Felle, 1995; Wymer et al., 1997). For the channel blockers used in these previous experiments, the cessation of growth likely reflects growth conditions, such as much higher Ca2+ levels in the medium, whereas the bursting we observe is suppressed.
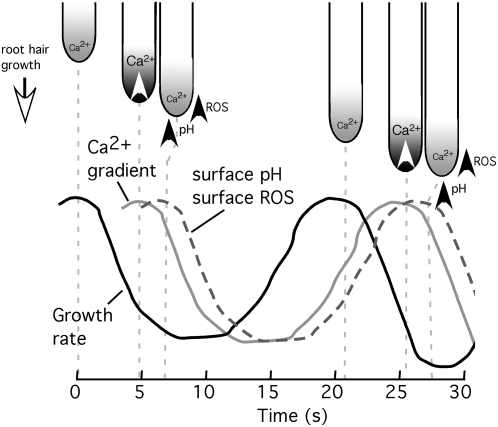
During oscillating growth, we have also measured dynamic increases in extracellular ROS and pH that oscillate with a similar frequency as growth but lag growth oscillations by 7 to 8 s (Monshausen et al., 2007). These extracellular changes are thought to play a role in restricting growth at the tip (pH) and along the shank immediately behind the tip (ROS). Intriguingly, these oscillations in extracellular ROS and pH lag the oscillating increases in the intracellular Ca2+ gradient we show here. Therefore, it is possible that the oscillatory nature of the cytosolic Ca2+ gradient, and of the extracellular ROS and pH changes, may be linked as part of a system to limit growth once an initial burst of elongation has occurred, with elevation in Ca2+ being driven by each growth pulse and itself triggering subsequent ROS and pH response systems to limit further expansion. Such a model fits well with the likely Ca2+ dependence of the NADPH oxidases, which contain an EF hand-like Ca2+ binding domain that appears critical for supporting tip growth, and with recent data suggesting that ROS and Ca2+ regulation of growth form a feedback loop to sustain tip growth (Takeda et al., 2008). The spatial and temporal aspects of these three oscillatory parameters in relation to growth are depicted in the model shown in Figure 4.
Figure 4.
Temporal and spatial relationships between growth, the tip-focused Ca2+ gradient, surface (wall) pH, and surface (wall) ROS. Relative timings of growth, pH, and ROS were taken from Monshausen et al. (2007).
Our observation that La3+ prevents the formation of the oscillations in tip-focused Ca2+ (Fig. 2) supports the idea that influx across the plasma membrane is a key element regulating the dynamics of the gradient, possibly acting as a primer to trigger Ca2+ release from internal sites, as proposed for pollen (Messerli and Robinson, 1997, 2003; Messerli et al., 1999). One possible influx mechanism is through Ca2+-permeable channels directly gated by tension in the plasma membrane, as seen in pollen tubes (Dutta and Robinson, 2004). These pollen tube channels are known to be Gd3+ sensitive (Dutta and Robinson, 2004), and the Ca2+ influx into root hairs is likewise Gd3+ sensitive (Supplemental Fig. S3). Alternatively, cytoskeletal elements may play a role in regulating mechanosensitive channel activity, as suggested for pollen tubes (Wang et al., 2004). The relationship between the ROS/hyperpolarization-activated Ca2+ channel thought to support the gradient in root hairs (Foreman et al., 2003) and such a mechanical response remains to be defined. It is probable that more than one Ca2+-permeable channel exists at the tips of root hairs, similar to the tip-growing rhizoids of Fucus, which contain two Ca2+-permeable channels, one with and one without mechanosensitivity (Taylor et al., 1996). Integration of the activity of these channels may well lie at the heart of the system that must precisely balance the promotion and restriction of turgor-driven expansion to permit root hair elongation without runaway expansion and the associated catastrophic failure of the apical wall. The molecular identity of these channels, how they relate to enhanced exocytosis of wall material, and their relationships to the extracellular ROS production and proton transport systems linked to growth restriction (Monshausen et al., 2007) are major challenges for future research.
MATERIALS AND METHODS
Plant Material
Seeds of Arabidopsis (Arabidopsis thaliana) Columbia were surface sterilized and germinated on Murashige and Skoog medium (Sigma) supplemented with 1% (w/v) Suc and 1% (w/v) agar at 21°C under continuous light conditions. Four-day-old seedlings were chosen for experiments.
Imaging of Cytosolic Ca2+ Levels
Arabidopsis seedlings expressing the fluorescence resonance energy transfer (FRET)-based Ca2+ sensor YC3.6 (Nagai et al., 2004) were transferred to purpose-built cuvettes and mounted as described previously (Monshausen et al., 2007). After several hours of growth in agar containing 0.1 mm KCl, 1 mm NaCl, and 0.1 mm CaCl2, pH approximately 6, supplemented with 1% (w/v) Suc, root hairs were ratio imaged with the Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss) using a 40× water-immersion, 1.2 numerical aperture, C-Apochromat objective. The YC3.6 Ca2+ sensor was excited with the 458-nm line of the argon laser. The cyan fluorescent protein (CFP; 473–505 nm) and FRET-dependent Venus (526–536 nm) emission were collected using a 458-nm primary dichroic mirror and the Meta detector of the microscope. Bright-field images were acquired simultaneously using the transmission detector of the microscope. For time-lapse analysis, images were collected every 2 or 3 s, with each individual image scan lasting 1.57 s.
In situ calibration was performed by raising Ca2+ to saturating levels for YC3.6. This was attempted by treatment with 1 m CaCl2 or 50% ethanol, or by mechanical perturbation just below the threshold for causing cell rupture. The maximum FRET/CFP ratio was attained in response to mechanical perturbation (Rmax = 2.5). The minimum FRET/CFP ratio (Rmin = 0.65) was recorded by treating the plants with 1 mm BAPTA-AM (Molecular Probes). Ca2+ levels were then calculated according to the equation Ca2+ = Kd (R − Rmin)/(Rmax − R)1/n, where R represents the FRET/CFP ratio measured during the experiment (Miyawaki et al., 1997), n represents the Hill coefficient that has been determined as 1 for YC3.6, and the Kd for Ca2+ = 250 nm (Nagai et al., 2004). Due to the inherent uncertainties of the precise in vivo Kd in such in situ calibrations, the raw FRET/CFP ratio data is included in each figure.
We used two different lines of transgenic Arabidopsis Columbia expressing 35S-driven YC3.6: line 1 was transformed with YC3.6 in the binary vector pGreenII (a generous gift of Jeffrey Harper, University of Nevada, Reno). To generate line 2, pGreenII was restriction digested with NcoI and EcoRI to obtain YC3.6 with the NOS terminator. This fragment was ligated into the Gateway entry vector pENTR11 (Invitrogen) and subsequently recombined into the binary Gateway-compatible destination vector pEarleyGate100 (Earley et al., 2006) according to a published protocol (Invitrogen). Because pENTR11 and pEarleyGate100 both contain kanamycin resistance as a selection marker, the backbone of the entry clone was cleaved with PvuII and SspI prior to recombination. Recombined plasmids were transformed into Escherichia coli Mach1 cells (Invitrogen), and clones were selected on Luria-Bertani/kanamycin plates. Recombinant plasmids were then transformed into Agrobacterium tumefaciens via electroporation, followed by transformation of Arabidopsis by floral dip (Clough and Bent, 1998). No differences in the Ca2+ oscillations were observed between these two independent Arabidopsis lines, and neither showed discernible alterations in root hair morphology or growth rate relative to wild-type plants.
Measurement of Root Hair Growth
Bright-field images were collected every 2 s simultaneously with fluorescence images using 458-nm excitation. High-resolution growth measurements were made using the computer vision tracking software as described previously (Messerli et al., 1999) providing 1:10 pixel resolution. For high-resolution analysis of root hair tip growth, plants were normally imaged with the root growing through a gel matrix, because this approach restricted the movement of the main root axis and allowed us to determine minute changes in root hair tip position caused by apical growth. To observe the effect of the Ca2+-modulating reagents EGTA, Ca2+ channel blocker La3+, and Ca2+ ionophore A23187 on root hair growth, growth measurements were performed on root hairs immersed in liquid medium. After observing the growth of a root hair for several minutes before treatment, the reagent (at 2× concentration) was gently mixed into the medium and growth measurements were continued on the same cell. The use of liquid medium allowed noticeable shifting of the root axis and made measurements of root hair tip growth more difficult. However, this approach afforded the necessary rapid access of the reagents to the root hairs without delays due to diffusion through an agar medium.
To study the effect of irradiation intensity on root hair growth, elongation rates were monitored under three different imaging conditions: (1) using the 458-nm line of the argon laser (at 4.7-A tube current, 90% attenuation), a root hair was imaged once at time zero and again after 5 min; (2) a root hair was imaged every 2 s for 5 min (458 nm at 4.7 A, 90% attenuation); and (3) a root hair was imaged every 2 s for 5 min (458 nm at 4.7 A, 60% attenuation). The average growth rate was calculated on the basis of the increase in root hair length during these 5 min.
Cross-correlation analysis was performed to determine the temporal relationship between tip-restricted Ca2+ oscillations and growth oscillations. The correlation coefficient 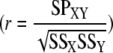 was determined as the measurements of the growth oscillations were shifted in time with respect to the Ca2+ oscillations. SSX and SSY are the sum of the squares for corresponding Ca2+ and growth recordings, while SPXY is the sum of the products of the two corresponding recordings. A perfect sine wave produces a correlation coefficient of 1 at time zero when compared with itself and a value of −1 at time zero when compared with itself 180° out of phase. As data sets were shifted in time with respect to each other, the data points at the tail ends no longer overlapped. These points were removed from the analysis. The temporal resolution of the analysis is the same as that used to acquire the images, but there is an offset of one-half of the temporal resolution due to the fact that the growth rate measurements were plotted at the halfway point between the corresponding images used to determine the growth rate.
was determined as the measurements of the growth oscillations were shifted in time with respect to the Ca2+ oscillations. SSX and SSY are the sum of the squares for corresponding Ca2+ and growth recordings, while SPXY is the sum of the products of the two corresponding recordings. A perfect sine wave produces a correlation coefficient of 1 at time zero when compared with itself and a value of −1 at time zero when compared with itself 180° out of phase. As data sets were shifted in time with respect to each other, the data points at the tail ends no longer overlapped. These points were removed from the analysis. The temporal resolution of the analysis is the same as that used to acquire the images, but there is an offset of one-half of the temporal resolution due to the fact that the growth rate measurements were plotted at the halfway point between the corresponding images used to determine the growth rate.
Monitoring Root Hair Cell Wall Thickening
Arabidopsis roots were treated with 5 μm fluorescein diacetate for 5 min. After washing, fluorescein dextran (10 kD) was added to the medium until intracellular and extracellular fluorescein fluorescence intensities were approximately equal. Root hair apices were imaged with the Zeiss LSM 510 microscope using the 40× water-immersion objective described above. Fluorescein was excited with the 488-nm line of the argon laser. Emission was collected using a 488-nm primary dichroic mirror and a 505-nm long-pass filter. Optical sections of less than 1 μm thickness were acquired. Bright-field images were acquired simultaneously using the transmission detector of the microscope.
Monitoring YFP∷RabA4b Localization in Arabidopsis Root Hairs
Root hairs of Arabidopsis expressing YFP-RabA4b (Preuss et al., 2004) were imaged using the Zeiss LSM 510 microscope and the same imaging parameters described above for fluorescein.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Regression analysis of the relationship between peak Ca2+ levels during each oscillation and the preceding or following peak in growth rate.
Supplemental Figure S2. Ca2+ levels at the apex of a nongrowing root hair. Root hairs that had ceased undergoing tip growth in Arabidopsis plants expressing the Ca2+ sensor YC3.6 targeted to the cytosol were imaged every 3 s.
Supplemental Figure S3. Effect of Gd3+ treatment on cytosolic Ca2+. Root hairs undergoing tip growth in Arabidopsis plants expressing the Ca2+ sensor YC3.6 targeted to the cytosol were imaged every 3 s.
Supplemental Movie S1. Cytosolic Ca2+ oscillations during tip growth of an Arabidopsis root hair. Cytosolic Ca2+ was monitored in plants expressing the soluble Ca2+ sensor YC3.6 as described in “Materials and Methods.” Images were taken every 3 s. Movie duration is 3.5 min. Ca2+ levels were pseudocolor coded according to the scale in Figure 1.
Supplementary Material
Acknowledgments
We are grateful to Dr. Sona Pandey (Donald Danforth Plant Science Center) for her expert introduction to Gateway technology. We thank Tom Slewinski and Dr. David Braun (Pennsylvania State University) for the generous gift of the pEarleyGate100 vector and Dr. Marisa Otegui for the YFP-RabA4b plants.
This work was supported by grants to S.G. (National Science Foundation grant nos. MCB 0641288 and IBN 03–36738), G.B.M. (National Science Foundation grant no. MCB 0641288), and M.A.M. (National Institutes of Health grant no. NCRR P41 RR–001395).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Simon Gilroy (sgilroy@wisc.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Allwood EG, Smertenko AP, Hussey PJ (2001) Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett 499 97–100 [DOI] [PubMed] [Google Scholar]
- Bibikova T, Gilroy S (2002) Root hair development. J Plant Growth Regul 21 383–415 [Google Scholar]
- Bibikova TN, Zhigilei A, Gilroy S (1997) Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 203 495–505 [DOI] [PubMed] [Google Scholar]
- Blackbourn HD, Barker PJ, Huskisson NS, Battey NH (1992) Properties and partial protein sequence of plant annexins. Plant Physiol 99 864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AD, Moyen C, Van Kesteren P, Tooke F, Battey NH, Brownlee C (1998) Ca2+, annexins, and GTP modulate exocytosis from maize root cap protoplasts. Plant Cell 10 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G, Dauwalder M, Roux S (1992) Purification and immunolocalization of an annexin-like protein in pea seedlings. Planta 187 1–9 [DOI] [PubMed] [Google Scholar]
- Clarkson DT, Brownlee C, Ayling SM (1988) Cytoplasmic calcium measurements in intact higher-plant cells: results from fluorescence ratio imaging of fura-2. J Cell Sci 91 71–80 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Coelho P, Malhó R (2006) Correlative analysis of [Ca2+]c and apical secretion during pollen tube growth and reorientation. Plant Sig Behav 1 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386 855–858 [DOI] [PubMed] [Google Scholar]
- Dutta R, Robinson KR (2004) Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol 135 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45 616–629 [DOI] [PubMed] [Google Scholar]
- Fan XX, Hou J, Chen XL, Chaudhry F, Staiger CJ, Ren HY (2004) Identification and characterization of a Ca2+-dependent actin filament-severing protein from lily pollen. Plant Physiol 136 3979–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijo JA, Sainhas J, Holdaway-Clarke T, Cordeiro MS, Kunkel JG, Hepler PK (2001) Cellular oscillations and the regulation of growth: the pollen tube paradigm. Bioessays 23 86–94 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422 442–446 [DOI] [PubMed] [Google Scholar]
- Gilroy S, Jones DL (2000) Through form to function: root hair development and nutrient uptake. Trends Plant Sci 5 56–60 [DOI] [PubMed] [Google Scholar]
- Herrmann A, Felle HH (1995) Tip growth in root hair cells of Sinapis alba L: significance of internal and external Ca2+ and pH. New Phytol 129 523–533 [Google Scholar]
- Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK (1997) Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JU, Gu Y, Lee YJ, Yang Z (2005) Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol Biol Cell 16 5385–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SL, Heath IB (1989) Effects of exogenous calcium ions on tip growth, intracellular Ca2+ concentration, and actin arrays in hyphae of Saprolegnia ferax. Exp Mycol 13 1–12 [Google Scholar]
- Ketelaar T, de Ruijter NCA, Emons AMC (2003) Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell 15 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Franco R, Bartnicki-Garcia S, Bracker CE (1994) Pulsed growth of fungal hyphal tips. Proc Natl Acad Sci USA 91 12228–12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malho R, Trewavas AJ (1996) Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli M, Robinson KR (1997) Tip localized Ca2+ pulses are coincident with peak pulsatile growth rates in pollen tubes of Lilium longiflorum. J Cell Sci 110 1269–1278 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Creton R, Jaffe LF, Robinson KR (2000) Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol 222 84–98 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Danuser G, Robinson KP (1999) Pulsatile influxes of H+, K+ and Ca2+ tag growth pulses of Lilium longiflorum pollen tubes. J Cell Sci 112 1497–1509 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Robinson KR (2003) Ionic and osmotic disruptions of the lily pollen tube oscillator: testing proposed models. Planta 217 147–157 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Robinson KR (2007) MS channels in tip-growing systems: mechanosensitive ion channels. Curr Top Membranes 58 393–412 [Google Scholar]
- Miller DD, Callaham DA, Gross DJ, Hepler PK (1992) Free Ca2+ gradient in growing pollen tubes of Lilium. J Cell Sci 101 7–12 [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (1997) Fluorescent indicators for Ca2+ based on the green fluorescent proteins and calmodulin. Nature 388 882–887 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho A, Trewavas AJ, Malho R (1998) Relocation of a Ca2+-dependent protein kinase activity during pollen tube reorientation. Plant Cell 10 1499–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A (2004) Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101 10554–10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol 174 160–173 [DOI] [PubMed] [Google Scholar]
- Preuss ML, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E (2006) A role for the RabA4b effector protein PI-4K beta 1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol 172 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E (2004) The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16 1589–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss H-D, Herth W (1979) Calcium ionophore A23187 affects localized wall secretion in the tip region of pollen tubes of Lilium longiflorum. Planta 145 225–232 [DOI] [PubMed] [Google Scholar]
- Sampson K, Lew RR, Heath IB (2003) Time series analysis demonstrates the absence of pulsatile hyphal growth. Microbiology 149 3111–3119 [DOI] [PubMed] [Google Scholar]
- Smertenko AP, Jiang CJ, Simmons NJ, Weeds AG, Davies DR, Hussey PJ (1998) Ser6 in the maize actin-depolymerizing factor, ZmADF3, is phosphorylated by a calcium-stimulated protein kinase and is essential for the control of functional activity. Plant J 14 187–193 [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319 1241–1244 [DOI] [PubMed] [Google Scholar]
- Taylor AR, Manison NFH, Fernandez C, Wood J, Brownlee C (1996) Spatial organization of calcium signaling involved in cell volume control in the Fucus rhizoid. Plant Cell 8 2015–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Yokota E, Vidali L, Sonobe S, Hepler PK, Shimmen T (2000) The role of plant villin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 210 836–843 [DOI] [PubMed] [Google Scholar]
- Wang YF, Fan LM, Zhang WZ, Zhang W, Wu WH (2004) Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol 136 3892–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watahiki MK, Trewavas AJ, Parton RM (2004) Fluctuations in the pollen tube tip-focused calcium gradient are not reflected in nuclear calcium level: a comparative analysis using recombinant yellow cameleon calcium reporter. Sex Plant Reprod 17 125–130 [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S (1997) Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J 12 427–439 [DOI] [PubMed] [Google Scholar]
- Yokota E, Tominaga M, Mabuchi I, Tsuji Y, Staiger CJ, Oiwa K, Shimmen T (2005) Plant villin, lily P-135-ABP, possesses G-actin binding activity and accelerates the polymerization and depolymerization of actin in a Ca2+-sensitive manner. Plant Cell Physiol 46 1690–1703 [DOI] [PubMed] [Google Scholar]
- Yoon GM, Dowd PE, Gilroy S, McCubbin AG (2006) Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell 18 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.