Abstract
Our laboratory has pioneered long-term microdialysis to monitor pineal melatonin secretion in living animals across multiple circadian cycles. There are numerous advantages of this approach for rhythm analysis: (1) we can precisely define melatonin onset and offset phases; (2) melatonin is a reliable and stable neuroendocrine output of the circadian clock (versus behavioral output which is sensitive to stress or other factors); (3) melatonin measurements can be performed extremely frequently, permitting high temporal resolution (10 min sampling intervals), which allows detection of slight changes in phase; (4) the measurements can be performed for more than four weeks, allowing perturbations of the circadian clock to be followed long-term in the same animals; (5) this is an automated process (microdialysis coupled with on-line HPLC analysis), which increases accuracy and bypasses the labor-intensive and error-prone manual handling of dialysis samples; and (6) our approach allows real-time investigation of circadian rhythm function and permits appropriate timely adjustments of experimental conditions. The longevity of microdialysis probes, the key to the success of this approach, depends at least in part on the methods of the construction and implantation of dialysis probes. In this article, we have detailed the procedures of construction and surgical implantation of microdialysis probes used currently in our laboratory, which are significantly improved from our previous methods.
1. Introduction
Circadian rhythms of behavior and physiology are controlled by a central pacemaker located in the suprachiasmatic nucleus (SCN) in mammals (Moore-Ede, 1980). Unlike many physiological events that remain more or less constant during the course of a day, rhythmic events such as hormone secretion and sleep-wake cycles display dynamic changes across the diurnal cycle. These dynamic changes can be accurately followed in vivo most effectively via continuous and high resolution monitoring of individual animals. Because it is extremely difficult to directly investigate the pacemaker properties of the SCN in vivo, studies of the circadian pacemaker in freely moving animals have relied largely on the monitoring of locomotor activity rhythms under various experimental conditions (Aschoff et al., 1975; Pittendrigh and Daan, 1976). Studies that utilize behavior rhythms as the clock marker are beyond the scope of this chapter.
Properties of the circadian pacemaker have also been studied by monitoring in vivo secretion of circadian hormones such as melatonin using a microdialysis probe inserted into the pineal gland (Azekawa et al., 1990, 1991; Drifhgout et al., 1993; Barassin et al., 1999; Kalsbeek et al., 2000), the site of melatonin production (Borjigin et al., 1999). Melatonin production in the pineal gland is controlled by the circadian pacemaker in the SCN via a multi-synaptic neuronal pathway (Figure 1A; Kennaway, 1997). Among the common circadian markers used in rhythm studies, including sleep-wake, activity-rest, body temperature, melatonin and cortisol secretion rhythms, melatonin is consistently recognized as the best marker of the circadian pacemaker (Klerman et al, 2002; Arendt, 2005).
Figure 1. Pineal melatonin synthesis.
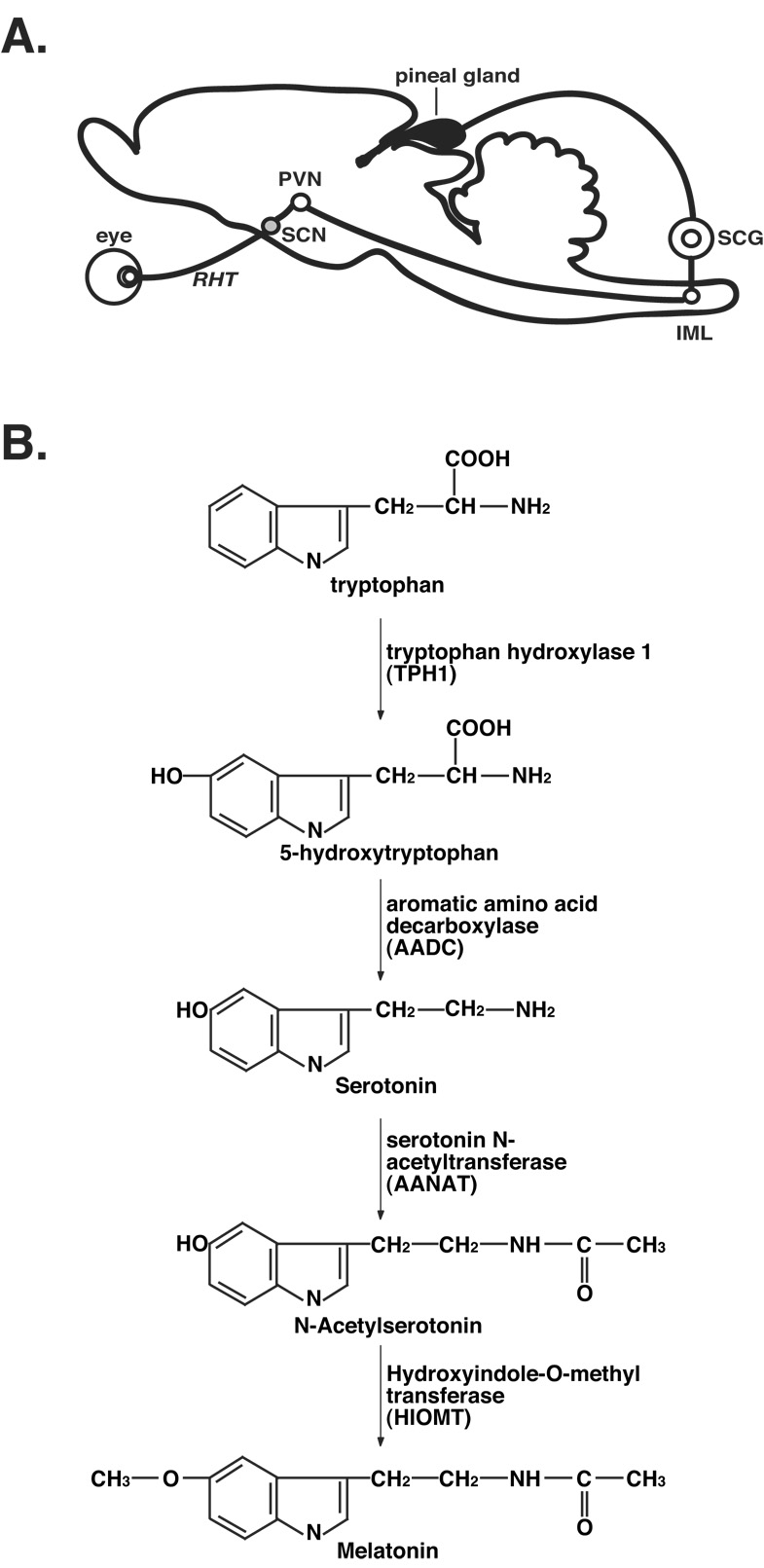
A. Melatonin production in the pineal gland is controlled by the circadian pacemaker in the SCN via a multi-synaptic pathway that includes the suprachiasmatic nucleus (SCN), the paraventricular nucleus of the hypothalamus (PVN), intermediolateral nucleus of the spinal cord (IML), and the superior cervical ganglion (SCG). Light signals from the eye, transmitted to the SCN via the retinohypothalamic pathway (RHT), entrain the circadian clock in the SCN, which generates the circadian rhythms of melatonin production in the pineal gland. B. Melatonin is synthesized from tryptophan by four enzymes: tryptophan hydroxylase 1 (TPH1), aromatic amino acid decarboxylase (AADC), arylalkylamine N-acetyltransferase (AANAT), and hydroxyindole-O-methyltransferase (HIOMT). In our studies, we routinely collect data for serotonin, N-acetylserotonin, and melatonin in a single HPLC chromatogram.
Melatonin is synthesized from tryptophan via four enzymatic steps (Figure 1B; Klein et al., 1992). Tryptophan hydroxylase 1 (TPH1) catalyzes the first reaction that converts tryptophan to 5-hydroxytrypophan, which is subsequently converted to 5-hydroxytryptamine (5-HT or serotonin), by aromatic amino acid decarboxylase (AADC). Both reactions occur in vivo during the daytime as well as at night. The last two reactions, which are active at night, are mediated by arylalkylamine N-acetyltransferase (AANAT) that converts serotonin to N-acetylserotonin (NAS) and by hydroxyindole-O-methyltransferase (HIOMT) that forms melatonin (Figure 1B).
In earlier experiments, microdialysis sampling and analyses of melatonin were carried out in vivo for 1–4 days in individual animals, and the results were averaged for a group of animals (Azekawa et al., 1990, 1991; Drifhgout et al., 1993; Kalsbeek et al., 2000). Inter-individual variations of the circadian pacemaker activity were difficult to define. We have pioneered the long-term pineal microdialysis technique that allows continuous on-line sampling and analysis of melatonin for up to four weeks in individual animals (Sun et al., 2002, 2003). This powerful technique enabled us to closely follow minute changes of the circadian status of individual animals for prolonged periods under various experimental conditions (Sun et al., 2003), allowed us to identify inter-individual differences in circadian chronotypes in laboratory animals (Liu and Borjigin, 2006), and permitted an in vivo investigation of the circadian pacemaker in a manner that is difficult using conventional approaches (Liu and Borjigin, 2005a, 2005b). We believe the success of our approach depends largely on the design and surgical implantation of the pineal microdialysis probe, which will be detailed in this chapter.
2. Experimental procedures
In our earlier publications (Sun et al., 2002, 2003; Liu and Borjigin, 2005a, 2005b, 2005c, 2006), a concentric probe from commercial sources was used. Unlike transverse probes that can cause tissue damage along their path into the brain (Drijfhout et a., 1993), the concentric probes inserted using our technique caused very little damage to the brain tissue surrounding the pineal gland. This unique feature of our previous method of surgical implantation was thought to be responsible for the in vivo longevity of the microdialysis probes in our studies (Sun et al., 2003). Because the pineal gland is located directly below the confluence of the superior sagittal sinus and the transverse sinus, the surgical implantation of the concentric probe was technically challenging (Sun et al., 2003). Recently, we have significantly improved the construction and surgical implantation of transverse microdialysis probes and have successfully used this approach for long-term microdialysis of the pineal. Compared to our previous method, this latest approach is far less costly and greatly simplifies the method of surgical implantation of the probe. The methods presented in this chapter are those currently used in our laboratory.
2.1. Probe construction
A transverse microdialysis probe depicted in Figure 2 is used in our laboratory. The support portion of the probe is constructed from blunt tip needles of two different configurations. The outer support is provided by a 21-gauge blunt tip needle with a shaft length of 0.5”. This added support, one of our latest improvements in probe construction, makes the probe virtually impossible to bend during dialysis and greatly enhances the longevity of the probe. The inner tubing in direct contact with the dialysis samples is constructed from a 25-gauge blunt tip needle with a shaft length of 1”. The plastic luer from the 25-gauge needle is removed first using a pair of pliers leaving the white glue still attached to the stainless steel tubing. The tip of the 25-gauge needle is then inserted into the 21-gauge needle and glued to the base of the plastic luer of the 21-gauge needle with a small amount of epoxy (Figure 2A). Care must be taken to keep epoxy below the opening of the 25-gauge needle so that it does not block the tubing opening or obstruct the later connection of the probe with the tubing. The needle shafts are then bent into a hook-like shape forming the TC4-left probe (Figure 2B). The dimensions of the TC4-left are determined by the size and shape of the animals’ skulls. A 1–1.5” microdialysis hollow fiber is then inserted into the end of the TC4-left and pulled into the 25-gauge stainless tubing by about 1/16”. A 1.5–2” tungsten rod with a sharpened tip is inserted, with the aid of a dissecting microscope, into the hollow fiber. Epoxy glue is used to fix the hollow fiber to the 25-gauge tip and to fix the tungsten rod to the hollow fiber. The epoxy used between the tungsten rod and the dialysis fiber should be carefully applied such that very little excess epoxy is visible (Figure 2C) to prevent tearing the brain tissue along its path during insertion. On the other hand, enough epoxy should be applied between the hollow fiber and the 25-gauge needle tip so that it forms a round ball with a diameter of more than 1/16”. This prevents the 25-gauge tip from penetrating into the brain tissue. The entire procedure for construction of TC4-right takes about 2 min. We have listed the configurations and suppliers of all materials used for our probe construction in the appendix.
Figure 2. Pineal microdialysis probe construction.
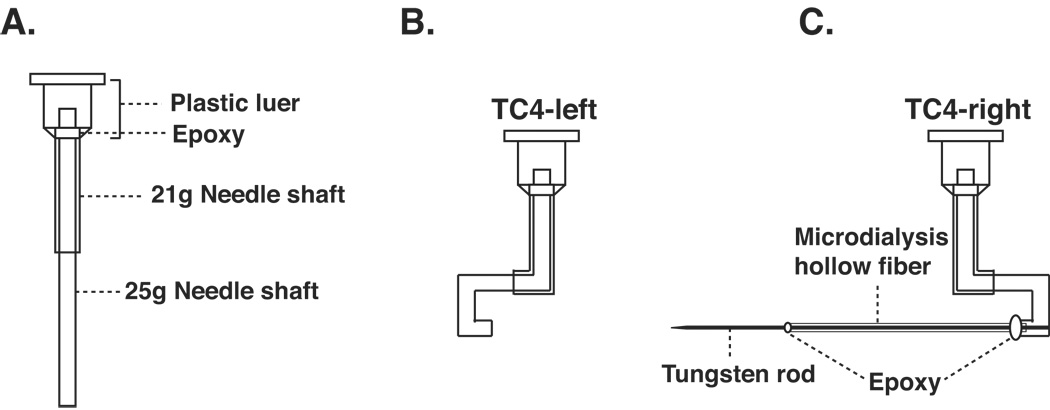
A. Probe base designs. B. TC4-left. C. TC4-right. Details of the dialysis probe construction are provided in the text.
One notable difference between the TC4-right and the device used by Drijfhout et al. (1993) is the lack of the epoxy glue along the length of the dialysis fiber in our design. Since the pineal gland is the only source of melatonin in the brain, melatonin can be collected from this type of probe only when the dialysis fiber transverses the pineal gland. We have adopted this method to prevent damage to the brain tissue during implantation resulting from unevenly applied epoxy. The completed dialysis probes are allowed to dry for at least 16h before use.
2.2. Dialysis probe implantation
The major advantage of the transverse dialysis probe is the ease of the probe implantation. Our previous method of probe implantation (Sun et al., 2003) required surgical opening of the skull and exposure of the pineal gland, which can be difficult to perform. The procedure of implanting the TC4-right is illustrated in Figure 3.
Figure 3. Dialysis probe implantation.
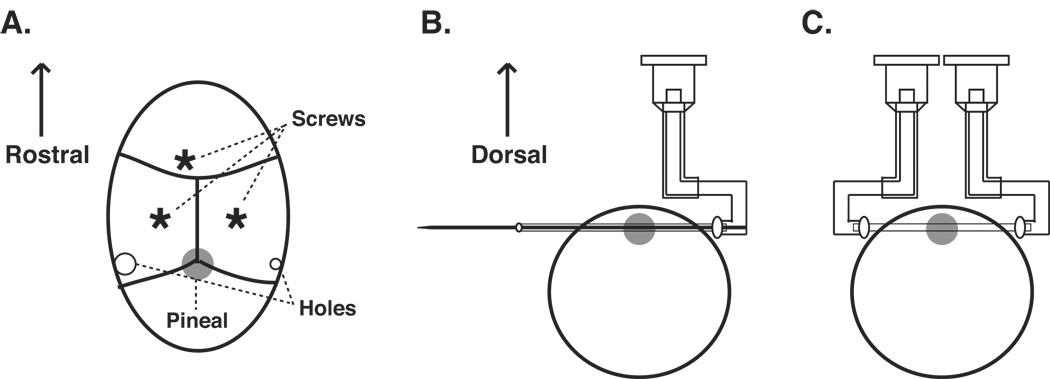
A. Localization of the probe insertion points on the skull. Schematics of the exposed skull surface are shown. B. Implantation of the TC4-right probe. C. Attachment of the TC4-left to the probe. The procedures of dialysis probe implantation are detailed in the text.
The animals (3 weeks to 12 weeks of age) are deeply anesthetized with the combination of ketamine (10 mg/mL, 0.5 mL/100 g weight, i.p.) and xylazine (2 mg/mL, 0.5 mL/100 g weight, i.p.). The animal’s head is shaved and positioned in a stereotaxic instrument with the head flat. The skull is exposed by a 2 cm coronal incision between the two ears along the interaural line using a pair of scissors and by reflecting the underlying muscle with a cotton tip. Three stainless steel screws are first placed as shown in Figure 3A.
Two small burr holes are created on both sides of the skull by sharp dental drill bits. In general, it is easier to locate the horizontal positions for the probe once the skull is exposed, since the pineal gland is right beneath lambda (the intersection of the lambdoidal and saggital sutures). The depth of the holes should be examined carefully, and will depend on the species, strain, and age of the animals, and need to be evaluated each time when different types of animals are used. For 8-week old male Sprague Dawley rats, the position should be AP of +0.8 mm and V of −2.5 mm. The hole on the animal’s right side is about 0.5 mm in diameter, whereas the one on the left side is about 1 mm in diameter (Figure 3A). The smaller size hole on the right side prevents the tip of the 25-gauge needle from penetrating the skull. The larger hole on the left side allows the probe to easily exit the brain during implantation.
Next, the TC4-right probe is pushed into the brain tissue very carefully from the right side of the skull leaving the epoxy ball outside of the skull (Figure 3B). Following the complete probe insertion, the epoxy on the left side is removed using a cautery; the tungsten rod is then carefully pulled out of the probe. The excess dialysis fiber is cut using a pair of scissors and the hollow fiber tip is then secured to the tip of the TC4-left using epoxy (Figure 3C). The complete TC4 probe (including both TC4-left and TC4-right) is fixed to the anchor screws on top of the skull with dental cement (not shown). Finally the muscles and skin are sutured. The entire procedure of the probe implantation takes no more than 30 min for each animal and is considerably easier to perform than our previous method (Sun et al., 2003). Following the surgery, the animal is returned to its cage and housed individually; the animal is allowed to recover from the surgery for at least 16 hrs before microdialysis. Materials used for the surgical implantation of the probe are all commonly available and are therefore not listed in the Appendix. Researchers are encouraged to consult Cooley and Vanderwolf (1990) for details of surgical instruments and basic methods.
2. 3. On line analysis
Various investigators have employed an on-line connection between the outflow of the dialysis tube and the analytical system (Johnson and Justice, 1983; Drijfhout et al., 1993). Aided by a computer-controlled sample injector, this method allows the automation of sample collection, sample injection, and sample analysis, which bypasses the labor intensive and tedious manual procedures. This is an important feature of our system since more than 576 samples are generated daily from a cohort of 8 animals. More importantly, such an approach permits a near real-time investigation of the dynamics of the circadian clock activities and allows timely adjustment of experimental protocols. In addition, the automated on-line analysis increases analytical reproducibility and obviates the need for preservatives in the samples.
2.3.1. Perfusion
Microdialysis experiment begins by connecting the animal’s dialysis probe inlet (see Figure 3C) with a peristaltic perfusion pump using tubing and tube connectors (Figure 4). We perfuse with artificial cerebral spinal fluid (aCSF), containing NaCl (148 mM), KCl (3 mM), CaCl2.2H2O (1.4 mM), MgCl2.6H2O (0.8 mM), Na2HPO4.7H2O (0.8 mM), and NaH2PO4.H2O (0.2 mM). Bicarbonate, used by some formulations of aCSF solution, is not added in our preparation, since it can generate CO2 bubbles within the HPLC pump and affect pump performance. The solution should be filtered immediately before use to sterilize and remove any insoluble materials. We routinely use pre-made aCSF solution stored at room temperature for up to 30 days.
Figure 4. Configuration of automated microdialysis sample collection, injection, and on-line analysis.
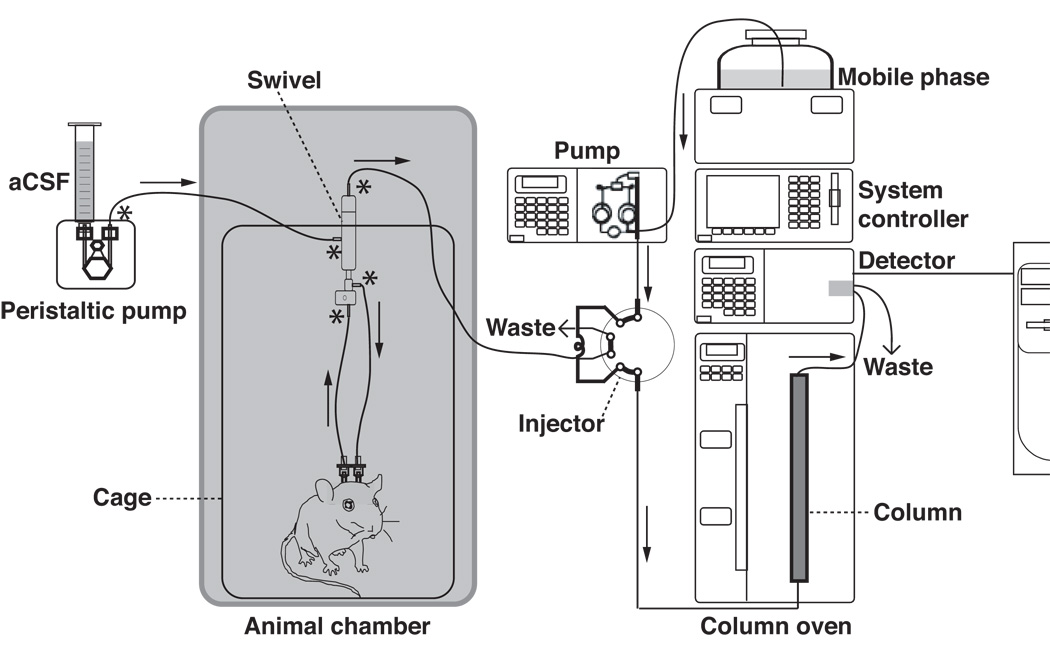
Arrows indicate the direction of the liquid flow, whereas the stars indicate the locations where tubing adaptors are used. See text for details.
The aCSF solution is delivered by a peristaltic pump and a 1–10 ml disposable syringe (see Figure 4). The fluid is delivered at a flow rate of 2 ul/min. The connection of the animal to the perfusion pump is achieved by PEEK tubing (0.12 mm inner diameter) and short pieces of tube connectors. The tip of the PEEK tubing to be connected with the plastic luer ends of the TC4 probe is glued (using glue stick and glue gun) inside a 1 ml disposable syringe tip so that the tubing is joined with the dialysis probe seamlessly. To prevent the PEEK tubing from entanglement, a dual channel swivel is used between the perfusion pump and microdialysis probe, and between the probe and sample injector (Figure 4). The swivel is mounted on a counterbalanced arm (not shown) that allows free movement of animals both laterally and vertically.
2.3.2. Collection of dialysis samples
Sampling of the pineal dialysates is begun 16–24 hrs after probe implantation. The dialysates are collected on-line in the loop (20ul) of an HPLC valve over the course of 10 min, and are injected by means of a 2-position/10-port valve with fast microelectric actuator at the end of the 10 min sample collection (only a 1-position/6-port valve is shown in Figure 4 for simplicity). The use of the 2-position/10-port valve allows samples from two animals to be collected and injected alternately. This arrangement allows three 10-min samples to be collected and analyzed over the course of one hour for each animal and permits sufficiently high-resolution sampling of in vivo melatonin secretion. Moreover, since two animals linked to the same valve actuator can be analyzed by the same HPLC instrument (see below), this arrangement saves on reagents and other HPLC consumables. Duration of the sample collection and timing of sample injection are controlled automatically by a digital sequence programmer.
2.3.3. Analysis of dialysis samples
All indoles in the melatonin synthetic pathway shown in Figure 1 are naturally fluorescent, thus enabling a direct quantification of pineal dialysates by a fluorescence detector following the HPLC column separation of the pineal microdialysis samples (Chin, 1990). Dialysis samples collected in one of the loops of the actuator are rapidly injected into the HPLC column by an isocratic pump. Separation of the dialysis samples is conducted by a reversed phase C18 column, 250 × 4.6 mm with 5 um packing, which is maintained at 30°C using a column oven (Drijfhout et al., 1993). When used 24/7 (24 hours a day and 7 days a week), these columns last about 3 months, which translates to 12,960 injections per column. As shown in Figure 4, the entire analysis system consists of an isocratic pump, a column and column oven, a fluorescence detector, and a system controller. In addition to columns, other consumables of the HPLC system include xenon lamps for the fluorescence detector, which last about 3 months when used uninterrupted, various parts for HPLC pumps (out check valve, in check valve, suction filter, plunger, diaphragm, line filter, plunger seal), and the mobile phase reagents. The system is controlled by a computer with HPLC software, which displays chromatograms of each animal in real-time.
The mobile phase used for column separation of the pineal dialysates consists of 22% (v/v) acetonitrile, 0.5 g/l heptane-sulfonic acid, 10 mM sodium acetate (pH 4.5, adjusted with concentrated acetic acid), and Na2-EDTA (0.1 mM) (Drijfhout et al., 1993). The flow rate of the pump is set at a constant rate of 1.5 ml/min. The detection limit of the assay is 2–6 fmol/sample for 5-HT, NAS, and melatonin (Figure 1).
3. Microdialysis-based circadian studies
One potential concern of the microdialysis approach lies in the fact that it is often difficult to accurately compare the amount of melatonin recovered from different individuals. This is in part due to the difficulties of targeting the microdialysis probe precisely and reproducibly at the same position within the pineal gland for all animals, and due to the inherent variation of the amplitudes of melatonin secretion in different individuals. It could also be difficult to compare melatonin peak values during different cycles in the same animals due to a slow decay of the probe performance in vivo over time. This issue was addressed in Sun et al. (2003) and is illustrated in Figure 5, where melatonin secretion is shown for the same animal over a 30-day period. During the first 4 days, the animal was housed in a 12h:12h light:dark cycle with lights-off at 18:00h and displayed comparable levels of melatonin peak values during this period. On day 5, the onset of darkness was delayed for 3 hrs (at 21:00h), which also resulted in a slight reduction of peak values of melatonin from day 5 to day 14. On day 15, the onset of dark was delayed further to 3:00h. An additional reduction in peak melatonin levels is apparent between day 17 and day 30. One might conclude from this data that the reduction of melatonin amplitude is a consequence of a change of the circadian pacemaker property in response to delays of the light:dark cycle. Comparison of multiple animals, however, did not support such conclusion, since this finding was not reproducible in other animals. These data suggest that the microdialysis-based estimation of circadian amplitudes in longitudinal studies requires analysis of multiple animals.
Figure 5. Long term microdialysis of a single rat, ID2176.
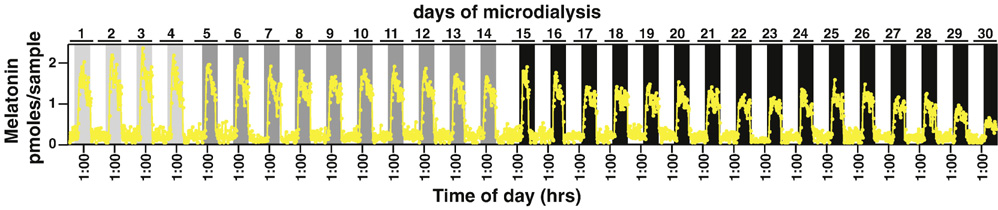
The rat was entrained in light:dark (LD) 12:12h (lights-off at 18:00 h) for more than 2 weeks prior to the pineal microdialysis. Melatonin profiles were shown for the last 4 days of entrainment with lights-off at 18:00 h (days 1–4; lighter gray shades), following an LD delay of 3 hrs (days 5–14; darker gray shades), and following an LD delay of 6 hrs (days 15–30; black shades). Detailed profiles of each shift have been published elsewhere (Liu and Borjigin, 2005b).
Although amplitudes of samples recovered from different days are difficult to compare precisely within the same individual animals, samples collected within a window of a few hours demonstrate remarkable consistencies across the diurnal cycle, especially when normalized to the daily maximum levels. This point is illustrated by our discovery that timing of melatonin secretion within the same animal is precisely controlled under entrained conditions (Figure 6). As shown in Figure 6A, 5-HT, NAS, and melatonin secretion all displays dramatic circadian patterns. Despite the difference in the amplitude of 5-HT, NAS, and melatonin secretion during the 5-day period, the timing of all three circadian products was remarkably consistent from day-to-day, when normalized to their nocturnal maximum levels (Figure 6B). No detectable differences were seen for 5-HT onset (assessed at 80% of the maximum levels), NAS onset (20%), and melatonin onset (20%) over the experimental period (Figure 6B). The same consistent pattern was obtained for NAS and melatonin offset (decline phase), except for the first day of analysis (day 1). We now routinely normalize daily melatonin secretion to its nocturnal maximum levels and discard the data from day 1, the day immediately following the surgery. These data demonstrate that pineal microdialysis technique offers a precise method to determine the timing of daily melatonin secretion and thus timing of the endogenous circadian pacemaker in freely moving animals.
Figure 6. Circadian rhythms of pineal 5-HT, N-acetylserotonin (NAS), and melatonin in a LEW rat over a 5-day period.
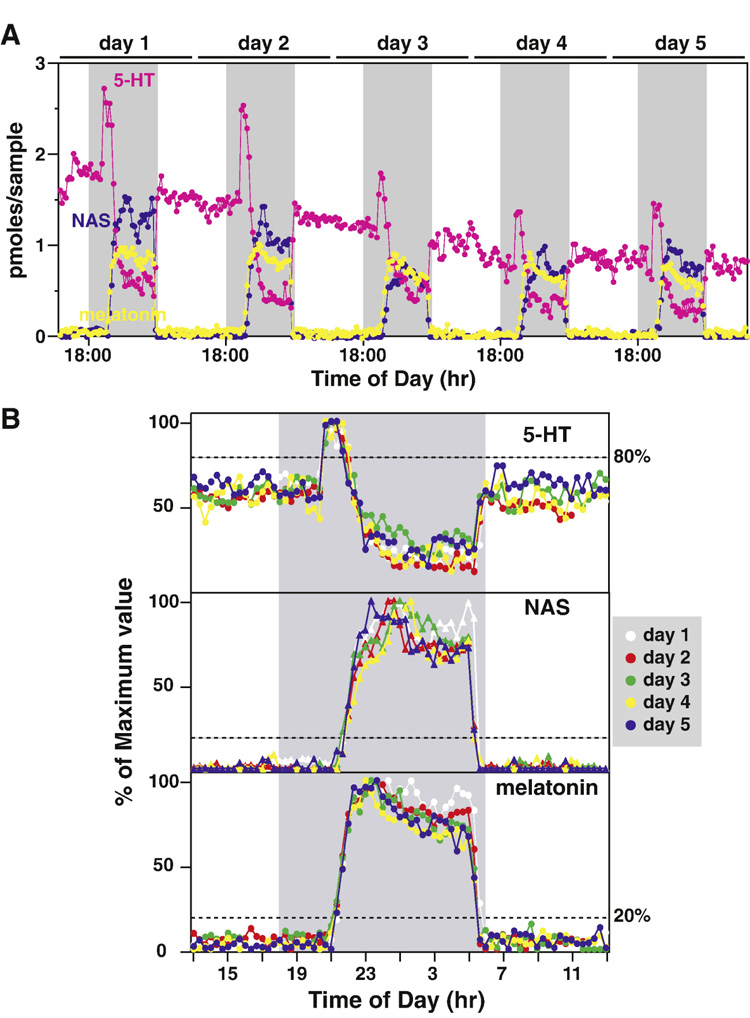
A. 5-HT (pink), NAS (blue), and melatonin (yellow) profiles of a single LEW rat are shown for 5 consecutive cycles. The shaded gray areas represent the dark period (18:00 h to 6:00 h). The daily boundaries were arbitrarily set at 13:00 h for ease of comparison. B. 5-HT (top panel), NAS (middle panel), and melatonin (bottom panel) data from each 24 h period (13:00 h to 13:00 h) were normalized to its nocturnal maximum values and superimposed together. Pineal secretory activities of 5-HT, NAS, and melatonin in different cycles were represented with different colors, and the values of 5-HT, NAS, and melatonin from the same cycle were represented with the same colors. The horizontal dashed lines represent onset phase of 5-HT (top panel, 80%), NAS (middle panel, 20%), and melatonin (bottom panel, 20%). Data are from Liu and Borjigin (2006).
Because of the high-resolution analysis of melatonin over many days, this technique is uniquely suited to determine minute changes of circadian pacemaker properties in the same animals in response to changes in environment. This is well illustrated by our recent discovery that melatonin onset free-runs at a different rate compared with melatonin offset when animals are released into constant darkness (Liu and Borjigin, 2005a), which is consistent with earlier studies (Illnerova and Vanecek, 1983; Illnerova et al., 1986), and by our finding that a complete reentrainment to new time zones requires stable reestablishment of both onset and offset of melatonin (Liu and Borjigin, 2005b). The need for the long-term and high-resolution investigation of circadian rhythms in behaving animals is highlighted by the fact that circadian features, such as timing of melatonin onset, display large inter-individual, inter-strain, and inter-species differences in laboratory animals (Liu and Borjigin, 2006). These studies demonstrate that microdialysis-basis melatonin analysis is a powerful tool for providing information on the endogenous timing of the circadian pacemaker. Future studies that utilize pineal microdialysis will incorporate simultaneous analyses of the circadian pacemaker using molecular and pharmacological tools in freely moving animals.
Acknowledgement
Mr. Phill Martin assisted in the schematic drawings of the HPLC components shown in Figure 4. This research is supported by NIH grants NS041971 and NS057583 (to JB). Authors would like to acknowledge the generous support of the Carnegie Institution of Washington during the early phase of our studies described in this chapter.
Appendix
Note: The following lists of materials are currently used in our laboratory and by no means represent the only sources from which these materials could be obtained.
(1). List of materials and suppliers for probe construction (see Figure 2 and Figure 3 and the text for their uses)
| DESCRIPTION | VENDOR | CAT. NUMBERS |
|---|---|---|
| Blunt tip needles, 21g×1/2” | Brico Medical Supplies | BN2105 |
| Blunt tip needles, 25g×1” | Brico Medical Supplies | BN2510 |
| Tungsten Rod, .004” diameter | ESPI metals | KNC7339 |
| Microdialysis Hollow Fiber, ID-0.2mm; OD-0.216mm, MWCO of 13 kD | Spectrum Labs | 132294 |
| Epoxy glue, fast drying | ITW performance polymers | 20845 |
(2) List of materials and suppliers for on-line analysis of melatonin (see Figure 4 from left to right for their arrangements)
| DESCRIPTION | VENDOR | CAT. NUMBERS |
|---|---|---|
| Peristaltic pump | Instech Laboratories, Inc | P720 |
| PEEK tubing | Bioanalytical Systems, Inc | MF-5366 |
| Tubing adaptors (connectors) | CMA microdialysis | 340 9500 |
| Dual channel swivel | Instech Laboratories, Inc | 375/D/22 |
| Sample injector (2-position/10-port actuator) | VICI | EMT2CSF10MWE |
| Digital sequence programmer (not shown in Figure 4, needed to control the injector) | VICI | DVSP4 |
| HPLC pump | Shimadzu | 228-45000-32 |
| System controller | Shimadzu | 220-91398-32 |
| Fluorescence detector | Shimadzu | 228-45096-32 |
| HPLC column oven | Shimadzu | 228-45010-32 |
| C18 reversed phase column (250 × 4.6 mm, 5 um) | Supelco | 58298 |
| HPLC software | Shimadzu | 220-91432-35 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendt J. Melatonin: characteristics, concerns, and prospects. J Biol Rhythms. 2005;20:291–303. doi: 10.1177/0748730405277492. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase-shifts of the Zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- Azekawa T, Sano A, Sei H, Yamamoto A, Aoi K, Morita Y. Pineal microdialysis in freely moving rats. Brain Res Bull. 1991;26:413–417. doi: 10.1016/0361-9230(91)90015-c. [DOI] [PubMed] [Google Scholar]
- Azekawa T, Sano A, Aoi K, Sei H, Morita Y. Concurrent on-line sampling of melatonin in pineal microdialysates from conscious rat and its analysis by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1990;530:47–55. doi: 10.1016/s0378-4347(00)82301-4. [DOI] [PubMed] [Google Scholar]
- Barassin S, Saboureau M, Kalsbeek A, Bothorel B, Vivien-Roels B, Malan A, Buijs RM, Guardiola-Lemaitre B, Pevet P. Interindividual differences in the pattern of melatonin secretion of the Wistar rat. J Pineal Res. 1999;27:193–201. doi: 10.1111/j.1600-079x.1999.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Borjigin J, Li X, Snyder SH. The pineal gland and melatonin: molecular and pharmacologic regulation. Annu Rev Pharmacol Toxicol. 1999;39:53–65. doi: 10.1146/annurev.pharmtox.39.1.53. [DOI] [PubMed] [Google Scholar]
- Chin JR. Determination of six indolic compounds, including melatonin, in rat pineal using high-performance liquid chromatography with serial fluorimetric-electrochemical detection. J Chromatogr. 1990;528:111–121. doi: 10.1016/s0378-4347(00)82367-1. [DOI] [PubMed] [Google Scholar]
- Cooley, Vanerwolf . Stereotaxic surgery in the rat: A photographic series. London Canada: A.J. Kirby Co.; 2001. [Google Scholar]
- Drijfhout WJ, Grol CJ, Westerink BH. Microdialysis of melatonin in the rat pineal gland: methodology and pharmacological applications. J Neurochem. 1993;61:936–942. doi: 10.1111/j.1471-4159.1993.tb03605.x. [DOI] [PubMed] [Google Scholar]
- Drijfhout WJ, Brons HF, Oakley N, Hagan RM, Grol CJ, Westerink BH. A microdialysis study on pineal melatonin rhythms in rats after an 8-h phase advance: new characteristics of the underlying pacemaker. Neuroscience. 1997;80:233–239. doi: 10.1016/s0306-4522(97)00080-8. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Vanecek J. Extension of the rat pineal N-acetyltransferase rhythm in continuous darkness and on short photoperiod. Brain Res. 1983;261:176–179. doi: 10.1016/0006-8993(83)91301-x. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Hoffman K, Vanecek J. Adjustment of the rat pineal N-acetyltransferase rhythm to change from long to short photoperiod depends on the direction of the extension of the dark period. Brain Res. 1986;362:403–408. doi: 10.1016/0006-8993(86)90473-7. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Justice JB. Model studies for brain dialysis. Brain Res Bull. 1983;10:567–571. doi: 10.1016/0361-9230(83)90156-9. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pevet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ. Light, neurotransmitters and the suprachiasmatic nucleus control of pineal melatonin production in the rat. Biol Signals. 1997;6:247–254. doi: 10.1159/000109135. [DOI] [PubMed] [Google Scholar]
- Klein DC, Schaad NL, Namboordiri MA, Yu L, Weller JL. Regulation of pineal serotonin N-acetyltransferase activity. Biochem Soc Trans. 1992;20:299–304. doi: 10.1042/bst0200299. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Free-running rhythms of pineal circadian output. J Biol Rhythms. 2005a;20:430–440. doi: 10.1177/0748730405277868. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Reentrainment of the circadian pacemaker through three distinct stages. J Biol Rhythms. 2005b;20:441–450. doi: 10.1177/0748730405279388. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J Pineal Res. 2005;39:91–96. doi: 10.1111/j.1600-079X.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Relationship between nocturnal serotonin surge and melatonin onset in rodent pineal gland. J Circadian Rhythms. 2006;4:12. doi: 10.1186/1740-3391-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Ede M, Sulzman FM, Fuller CA. The clocks that time us. Cambridge: Harvard University Press; 1982. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemaker in nocturnal rodents: I. The stability and lability of circadian frequency. J Comp Physiol A. 1976;106:223–252. [Google Scholar]
- Sun X, Deng J, Liu T, Borjigin J. Circadian 5-HT production regulated by adrenergic signaling. Proc Natl Acad Sci U S A. 2002;99:4686–4691. doi: 10.1073/pnas.062585499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Liu T, Deng J, Borjigin J. Long-term in vivo pineal microdialysis. J Pineal Res. 2003;35:118–124. doi: 10.1034/j.1600-079x.2003.00064.x. [DOI] [PubMed] [Google Scholar]


