Abstract
In the fatal degenerative Duchenne muscular dystrophy (DMD), skeletal muscle is progressively replaced by fibrotic tissue. Here, we show that fibrinogen accumulates in dystrophic muscles of DMD patients and mdx mice. Genetic loss or pharmacological depletion of fibrinogen in these mice reduced fibrosis and dystrophy progression. Our results demonstrate that fibrinogen–Mac-1 receptor binding, through induction of IL-1β, drives the synthesis of transforming growth factor-β (TGFβ) by mdx macrophages, which in turn induces collagen production in mdx fibroblasts. Fibrinogen-produced TGFβ further amplifies collagen accumulation through activation of profibrotic alternatively activated macrophages. Fibrinogen, by engaging its αvβ3 receptor on fibroblasts, also directly promotes collagen synthesis. These data unveil a profibrotic role of fibrinogen deposition in muscle dystrophy.
Keywords: Fibrinogen, DMD, mdx, muscular dystrophy, fibrosis, inflammation
Duchenne muscular dystrophy (DMD) results from mutations in the gene coding for the protein dystrophin, which localizes at the inner face of the sarcolemma (Campbell 1995). Besides progressive muscle degeneration and inflammation, fibrotic transition of muscle tissue is critical in DMD as it progressively deteriorates locomotor capacity, posture maintenance, and the vital function of cardiac and respiratory muscles. Indeed, DMD individuals have a high degree of fibrosis increasing with age, which is reproduced in the diaphragm muscle of mdx mice (the mouse model of DMD) (Stedman et al. 1991). Importantly, the underlying mechanisms of fibrosis development within dystrophic muscle remain largely unknown.
Fibrinogen is a soluble acute phase protein, which is released into the blood in response to stress. Apart from its key role in controlling blood loss following vascular injury, fibrinogen also extravasates at sites of inflammation or increased vascular permeability where it is immobilized and/or converted to fibrin (Rybarczyk et al. 2003) (from hereon we refer to both by the term “fibrin/ogen”). We showed previously that mice with defective fibrinolysis exhibited impaired muscle regeneration after experimental injury (Suelves et al. 2002). In this study, we investigated the role of fibrin/ogen deposition in the development of fibrosis in dystrophic muscle.
Results and Discussion
We first analyzed fibrin/ogen deposition in muscles of DMD patients and its correlation with disease course. Compared with muscles of healthy individuals or of fibromyalgia patients, DMD muscles showed significant fibrin/ogen accumulation (Fig. 1A). Similarly, in mdx mice muscles, fibrin/ogen deposits were readily detectable after disease onset, while absent before disease onset (Fig. 1B,C). Thus, fibrin/ogen deposition is associated with muscle dystrophinopathy.
Figure 1.
Fibrin/ogen accumulates in muscles of DMD patients and mdx mice. (A) Staining for fibrin/ogen (brown) in muscle biopsies of DMD and fibromyalgia patients, and healthy subjects. (B) Western blotting of wild-type and mdx muscle extracts before and after disease onset (14 and 30 d of age, respectively), with an anti-fibrin/ogen antibody. (C) Fibrin/ogen immunostaining in muscles of wild-type and mdx mice of 30 d of age. (D) Representative fibrin/ogen and Sirius Red staining in DMD muscle sections. Bars: A,C,D, 50 μm. (E) Integrated representation of fibrin/ogen accumulation (red line) and fibrosis (black line) in DMD patients, based on curve fitting of three data points, corresponding to quantitative morphometric results in the early, mid-, and late group (see the Materials and Methods). (F) Correlation between fibrin/ogen accumulation and fibrosis in DMD patients (multiple range tests after a significant Kruskal-Wallis test). Samples were divided in three groups: negative/low (n = 8), intermediate (n = 7), and strong (n = 11) immunostaining for fibrin/ogen. (G) Fibrin/ogen and Sirius Red (collagen) staining were quantified as percentage of total area in mdx diaphragm sections (at the indicated age in months). Mdx values were normalized with age-matched wild-type mice values. Data are presented as fold increase with respect to 0.5-mo-old mdx mice.
Collagen deposition (fibrosis) was prominent in DMD muscles and particularly found in the same areas occupied by fibrin/ogen (Fig. 1D). To investigate the relationship between the extent of fibrin/ogen deposition and fibrosis in DMD, a total of 39 cases were collected, and the patients were divided into three nonoverlapping groups according to their age: the early group (2–5 yr; n = 13), the mid group (6–7 yr; n = 14), and the late group (8–11 yr; n = 12). Quantitative analysis on muscle biopsies revealed that fibrin/ogen accumulation was already significant at 6–7 yr (mid group) remaining high thereafter. In contrast, collagen deposition increased only at older ages (8–11 yr; late group) (Fig. 1E). Furthermore, a strong correlation was found between fibrin/ogen accumulation and fibrosis in DMD muscles (P < 0.0007; Fig. 1F). In mdx diaphragm, both fibrin/ogen deposition and fibrosis were consistently increased with age (Fig. 1G). While fibrin/ogen deposition was already considerably elevated at 1 mo of age, collagen accumulation showed a clear increase only from 2.5 mo onward, indicating that deposition of fibrin/ogen precedes that of collagens in mdx muscle (Fig. 1G), similar to what we found in DMD muscles. These observations suggest an association between fibrin/ogen accumulation and deposition of collagen in dystrophic muscle.
To further analyze the role of fibrin/ogen deposition in fibrosis development during muscle dystrophy progression, we bred the mdx mice with fibrinogen-deficient mice (Suh et al. 1995) to generate the Fib−/−mdx double-mutant mice. As expected, after disease onset (at 1 mo of age), muscles from Fib−/−mdx mice exhibited no fibrin/ogen deposition (Supplemental Fig. 1a). Notably, collagen content in the diaphragm of Fib−/−mdx mice was found to be significantly reduced compared with Fib+/+ mdx mice (Fig. 2A). To corroborate our genetic experiments using a pharmacological approach, we treated 12-d-old mdx mice with saline or with ancrod, an established defibrinogenating agent (Lluis et al. 2001), until the age of 2.5 or 6.5 mo. Compared with saline, ancrod treatment resulted in a significant reduction of fibrin/ogen accumulation in mdx muscles (Supplemental Fig. 1b). Consistent with our findings in Fib−/−mdx mice, ancrod significantly decreased the degree of fibrosis in mdx diaphragms of 2.5- and 6.5-mo-old mice (Fig. 2B). These data indicate that fibrin/ogen, although not strictly required, does accelerate fibrosis development in mdx muscle. Of note, fibrin/ogen depletion also reduced muscle degeneration (Supplemental Fig. 1c,d) and protected mdx mice from functional deterioration (Supplemental Fig. 2).
Figure 2.
Fibrin/ogen regulates TGFβ expression and fibrosis in mdx diaphragm. (A) Fibrosis quantification in diaphragm sections of Fib+/+mdx mice, Fib−/−mdx mice, and wild-type control mice (3.5 mo of age). (B) Fibrosis quantification in diaphragms of saline- or ancrod-treated mdx mice of 2.5 mo of age (left panel) and 6.5 mo of age (right panel). (*) P < 0.05 versus mdx-saline. (C) qRT–PCR analysis of diaphragms of mdx and wild-type mice at indicated ages. Fold induction over wild-type mice. (*) P < 0.05 versus wild-type. (D) qRT–PCR analysis in 6.5-mo-old diaphragms of saline- or ancrod-treated mdx mice was performed as in C. (*) P < 0.05 versus wild-type mice; (#) P < 0.05 versus mdx-saline. (E) Anti-p-Smad2 and Smad2 immunostaining in diaphragm sections after 6 mo of treatment with saline or ancrod. Bar, 50 μm. (F) Mdx macrophages were treated with fibrinogen (Fg) ± actinomycin D or cycloheximide for 48 h. TGFβ expression was analyzed by qRT–PCR. Values are fold induction with respect to control conditions. (*) P < 0.05 versus control; (#) P < 0.05 versus Fg. (G) Mdx macrophages were treated with Fg ± an IL-1β-neutralizing antibody, control IgG antibody, or recombinant IL-1β for 48 h, and TGFβ expression was analyzed and represented as in F. (H) Primary fibroblasts from mdx diaphragms were incubated with conditioned medium (CM) from mdx macrophages treated or not with Fg (as in Fig. 2G) for 24 h, with or without a TGFβ -neutralizing antibody or control IgG. Gene expression was analyzed by qRT–PCR. Results are fold induction with respect to control-conditioned medium. (*) P < 0.05 versus control CM; (#) P < 0.05 versus CM from Fg-treated macrophages. Data are mean ± SEM. (A) n = 4 animals per group. (B) n = 6 animals per group. (C,D) n = 3 animals per group. (F–H) n = 3 experiments performed in triplicate.
To investigate the mechanisms by which fibrin/ogen might be influencing fibrosis development in mdx diaphragm, we first examined the levels of the profibrotic cytokine transforming growth factor-β (TGFβ), which is known to promote fibrosis in mdx diaphragm (Andreetta et al. 2006). TGFβ expression increased with age in mdx diaphragm, both at the mRNA (Fig. 2C) and protein levels (mean ± SD: 110 ± 39, 210 ± 50, and 745 ± 183 pg/mg protein at 2.5, 6, and 12 mo, respectively), correlating with the age-induced fibrosis in mdx diaphragm. Importantly, consistent with the observed attenuated fibrosis, fibrin/ogen depletion for 6 mo in mdx mice reduced the expression of TGFβ and of its target genes collagen I and tissue inhibitor of metalloproteinases-1 (TIMP-1) (Fig. 2D). Moreover, phospho-Smad2 staining was drastically reduced in diaphragms of ancrod-treated (Fig. 2E) and fibrinogen-deficient mdx mice (data not shown), indicating that interfering with fibrinogen deposition impaired functional TGFβ signaling. These results thus suggest that fibrin/ogen promotes fibrosis by increasing TGFβ expression and signaling in mdx diaphragm.
Macrophage depletion experiments demonstrated their deleterious role in mdx muscular dystrophy (Wehling et al. 2001). Since inflammatory cells are a critical source of TGFβ in mdx diaphragm (Zhou et al. 2006), the influence of fibrin/ogen on the local inflammatory response was next analyzed. We found that the number of macrophages was reduced in fibrin/ogen depleted mdx diaphragm (Supplemental Fig. 3a). Compared with wild-type controls, the levels of proinflammatory cytokines such as TNFα, IL-6, MIP-2 (the functional mouse homolog of human IL-8), and IL-1β were greatly increased in muscle extracts of mdx mice, and significantly reduced when mdx mice were treated with ancrod (Supplemental Fig. 3b). We next investigated whether fibrin/ogen had a direct effect on the cytokine production capacity of primary macrophages derived from mdx mice. Fibrinogen induced the rapid expression of TNFα, IL-6, MIP-2, and IL-1β in these cells, as in a classical proinflammatory macrophage response (Supplemental Fig. 3d). In agreement with the capacity of fibrinogen to activate the NF-κB pathway via engaging its receptor Mac-1 (Supplemental Fig. 3c; Sitrin et al. 1998; Rubel et al. 2003), treatment of primary macrophages with the NF-κB inhibitor BAY11-7085 abrogated fibrinogen-induced proinflammatory cytokine production (Supplemental Fig. 3d). This induction was also impaired in the presence of a Mac-1-blocking antibody (Supplemental Fig. 3d). Furthermore, mdx macrophages responded to fibrin/ogen in a transwell migration assay in a Mac-1-dependent manner (data not shown), confirming the involvement of Mac-1/fibrin/ogen binding in these inflammatory effects (Flick et al. 2004). These results indicate that, by engaging Mac-1 and activating NF-κB, fibrin/ogen up-regulates the expression of proinflammatory cytokines including IL-1β.
We next investigated whether fibrin/ogen regulates TGFβ production by mdx macrophages. TGFβ was induced in these cells 48 h after fibrin/ogen stimulation both at the RNA (Fig. 2F) and secreted protein levels (mean ± SD,picograms per milliliter: 1414 ± 270 in control versus 3102 ± 343 with fibrin/ogen; n = 3; P < 0.05), but not at an early time point (6 h) (data not shown). Consistent with this late induction pattern, the fibrin/ogen-stimulated TGFβ mRNA expression in mdx macrophages was blocked by actinomycin D and by cycloheximide (Fig. 2F), suggesting the involvement of a newly synthesized protein intermediate. IL-1β has been shown to induce TGFβ gene transcription (Lee et al. 2006), and it is an early Mac-1-fibrin/ogen-inducible gene product (Supplemental Fig. 3d; Perez et al. 1999). When the function of IL-1β was blocked with an IL-1β neutralizing antibody, the fibrin/ogen-dependent TGFβ expression at 48 h in macrophages was significantly reduced (Fig. 2G), indicating that, in our experimental system, fibrin/ogen induced TGFβ expression at least in part through the action of IL-1β. In addition, and consistent with the observed expression pattern of TGFβ, IL-1β expression was increased in 6.5-mo mdx diaphragms compared with wild type, while it was reduced in fibrin/ogen-depleted mdx mice (Fig. 2D). Confirming the profibrotic action of IL-1β via increasing the expression of TGFβ in vivo (Bonniaud et al. 2005), treatment of mdx mice with an IL-1 receptor antagonist (IL-1ra) for 1 mo reduced both TGFβ levels and the extent of collagen deposition in mdx diaphragm (data not shown). Taken together, our results suggest that fibrin/ogen induces an early proinflammatory macrophage response that includes synthesis of the intermediate IL-1β, which in turn will promote the synthesis and secretion of TGFβ by macrophages.
The profibrotic activity of fibrinogen-induced TGFβ was next examined. Conditioned medium from fibrin/ogen-stimulated mdx macrophages induced collagen I expression in mdx fibroblasts (Fig. 2H). This effect was specific for TGFβ, since collagen I induction was blunted in the presence of a neutralizing antibody against TGFβ (Fig. 2H). Similar data were obtained when analyzing the expression of the TGFβ target gene TIMP-1 (Fig. 2H). Hence, by promoting TGFβ secretion by mdx macrophages, fibrin/ogen stimulates the synthesis of collagen by mdx fibroblasts and its further accumulation, thereby likely promoting fibrosis development in mdx muscles.
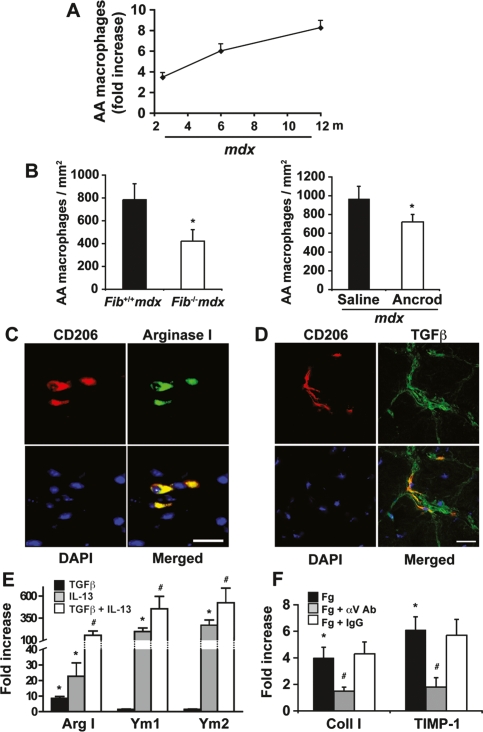
Alternatively activated macrophages (as opposed to classically activated, proinflammatory macrophages) have been associated with certain fibrotic pathologies (Wynn 2004). These macrophages are activated by Th2-derived cytokines, such as IL-13, and can be identified by specific cell surface markers such as CD206 (also known as mannose receptor) (Stein et al. 1992). They also express high levels of chitinase-like secretory lectins Ym1 and Ym2, and TIMP-1 (Wynn 2004). However, no link between alternatively activated macrophages and muscular dystrophy has been established. While unchanged in wild-type mice, we found that the number of CD206+ cells increased progressively with age in mdx diaphragms (Fig. 3A). In addition, quantitative RT–PCR (qRT–PCR) analysis showed an age-dependent increased expression of the macrophage alternative activation markers Ym1 and Ym2 in diaphragms of mdx mice (Fig. 2C), phenocopying the age-dependent deposition of fibrin/ogen and collagen in mdx diaphragm (Fig. 1G). Importantly, fibrin/ogen depletion in mdx mice reduced significantly the number of CD206+ alternatively activated macrophages (Fig. 3B) and the expression of Ym1 and Ym2 (Fig. 2D) in the diaphragm. By double-immunostaining, CD206+ macrophages coexpressed both arginase I (Fig. 3C) and TGFβ (Fig. 3D), thus suggesting that fibrin/ogen regulates the presence of alternatively activated profibrotic macrophages in mdx diaphragm.
Figure 3.
Fibrin/ogen enhances alternative macrophage activation in mdx diaphragm. (A) Immunohistochemical quantification of CD206+ cells in diaphragms of wild-type and mdx mice of different ages (fold increase with respect to wild-type mice). (B) Alternatively activated (AA) macrophages (F4/80+CD206+) in diaphragm muscle sections of Fib+/+mdx and Fib−/−mdx mice (left panel) and saline- or ancrod-treated mice of 6.5 mo of age (right panel). (C,D) Double-positive cells for CD206 (red) and Arginase I (green) (C), and for CD206 (red) and TGFβ (green) (D) in an 8-mo-old mdx diaphragm. Bars, 50 μm. (E) Mdx macrophages were treated with recombinant TGF, IL-13, or both for 24 h. Gene expression was analyzed by qRT–PCR. Results are fold induction with respect to control conditions. (*) P < 0.05 versus control conditions; (#) P < 0.05 versus TGFβ and IL-13 alone. (F) mdx fibroblasts were treated with Fg ± an αv-neutralizing antibody or control IgG for 5 h. Gene expression as analyzed by qRT–PCR. (*) P < 0.05 versus control conditions; (#) P < 0.05 versus Fg alone. Data are mean ± SEM. (A,B) n = 6 animals per group. (E,F) n = 3 experiments performed in triplicate.
TGFβ, in conjunction with IL-13, may amplify the expression of arginase I—a key enzyme in the initiation of collagen synthesis by fibroblasts (Wynn 2004; Bronte and Zanovello 2005)—in alternatively activated macrophages (Boutard et al. 1995). We found that simultaneous treatment of mdx macrophages with TGFβ and IL-13 resulted in the synergistic induction of the alternatively activated gene products arginase I, Ym1, and Ym2 (Fig. 3E), suggesting that fibrin/ogen, by inducing TGFβ (see above), might further stimulate collagen synthesis through amplifying the alternative activation of arginase-producing macrophages.
Additionally, fibrin/ogen also directly induced the expression of collagen by mdx fibroblasts (Fig. 3F), and this stimulation was independent of new protein synthesis (data not shown). Consistent with the reported binding of fibrin/ogen to fibroblasts via engaging αvβ3 integrins (Rybarczyk et al. 2003), we found that incubation of mdx fibroblasts with an anti-αv antibody abrogated the induction of collagen expression by fibrin/ogen (Fig. 3F). Similar effects were obtained for TIMP-1 (Fig. 3F). Thus, these results further support the idea that fibrin/ogen might promote fibrosis in mdx diaphragm by direct and indirect fibroblast activating mechanisms.
Muscle degeneration in DMD patients has been associated with alterations in coagulation and fibrinolysis (Saito et al. 2001), thrombosis being a frequent complication in DMD. In addition, impaired fibrinolysis was also shown recently to exacerbate degeneration in mdx mice (Suelves et al. 2007).
In this study, we unveil a new role for fibrin/ogen deposition in promoting the fibrotic transition of dystrophic muscle by demonstrating that blunting fibrin/ogen accumulation reduces fibrosis in mdx mice. Through binding its receptor Mac-1, fibrin/ogen can induce a proinflammatory response that subsequently leads to TGFβ production by macrophages, which in turn may potentiate their alternative activation state. Apart from reinforcing the emerging link between tissue inflammation and fibrosis, we provide evidence for a fibrin/ogen/Mac-1–IL-1β–TGFβ axis, which can drive distinct pathways leading to fibrotic transition of dystrophic muscle. Fibrin/ogen also directly stimulates the expression of collagen by mdx fibroblasts via binding its receptor αvβ3 integrin. Taken together, these findings introduce the integrative concept that fibrin/ogen promotes tissue inflammation and fibrosis in mdx muscular dystrophy (see Fig. 4).
Figure 4.
Proposed model for the role of fibrin/ogen in the muscle dystrophy-associated fibrosis. Fibrinogen promotes collagen production by three distinct but possibly overlapping mechanisms in mdx muscle. (1) TGFβ is produced in response to fibrinogen stimulation of proinflammatory, classically activated mdx macrophages (through Mac-1 binding) and via the intermediate induction of IL-1β (a direct fibrinogen-inducible gene), as an anti-inflammatory counteracting mechanism involving a macrophage activation switch. Macrophage-secreted TGFβ is free to bind and activate TGFβ receptors expressed by fibroblasts from mdx diaphragms, and directly induce collagen synthesis. (2) TGFβ can also promote the alternative activation of macrophages initiated by IL-13 Th2 cytokine. By up-regulating arginase activity in these cells, TGFβ (in conjuntion with IL-13) is known to promote fibroblast proliferation, collagen production, and ultimately, fibrosis. (3) Fibrin/ogen might also directly activate the collagen-producing machinery in fibroblasts by binding to αvβ3 receptors.
Given the importance of preserving fibrinogen-clotting function, strategies targeting only fibrinogen signaling without affecting its procoagulant properties might be suitable for human therapeutic intervention. Fibγ390-396A knock-in mice produce a modified fibrinogen protein incapable of binding Mac-1, but preserving its clotting capacity (Flick et al. 2004; Adams et al. 2007). We therefore comparatively analyzed the inflammatory response and connective tissue-remodeling in Fib−/− and Fibγ390-396A mice in experimentally injured muscle. Importantly, both Fib−/− and Fibγ390-396A mice showed reduced macrophage infiltration and less collagen deposition compared with wild-type mice (Fig. 5), corroborating the idea that blocking fibrin/ogen-Mac-1 interaction might be of therapeutic relevance in muscle dystrophy.
Figure 5.
Cardiotoxin-injured Fib−/− mice or Fibγ390–396A mice have diminished inflammation and reduced collagen deposition. Muscle injury was performed by cardiotoxin (CTX) injection in gastrocnemius muscles of Fib−/− and Fibγ390–396A, and their respective wild-type controls. (A,B) The Macrophage number per square millilmeter of damaged area at 3 and 5 d post-injury (dpi) in Fib+/+ and Fib−/− mice (left panels) or wild-type and Fibγ390–396A mice (right panels). (C) Sirius Red staining quantification in the described mice. (*) P < 0.05 versus wild-type mice. n = 4 animals per group.
A potential advantage of a fibrin/ogen-based therapeutic approach over current anti-inflammatory/fibrotic strategies is that targeting fibrin/ogen interactions is likely to impose a block on the activation and recruitment of leukocytes only locally; i.e., within the dystrophic muscle where fibrin/ogen is deposited. Indeed, anti-TGFβ antibody therapies in muscle dystrophy reduce fibrosis but also increase inflammation (Andreetta et al. 2006). Hence, by targeting fibrinogen, one might prevent both inflammation and fibrosis in dystrophic muscle.
Importantly, muscle fibrosis not only accelerates the clinical decline in young DMD patients, but also represents a major obstacle for successful engraftment of stem cells in ongoing experimental cell-based therapies in DMD aimed at correcting the primary defect (i.e., dystrophin replacement or rescue). Thus, reducing inflammation-mediated fibrosis by targeting fibrin/ogen might also facilitate the homing of donor stem cells to muscle, and hence, the success of these primary therapies.
Materials and methods
Mice, induction of muscle regeneration, and muscle analyses
Mouse lines; genotyping protocols; systemic defibrinogenation with ancrod; induction of muscle regeneration; morphometric, immunohistochemical, and collagen content (fibrosis) analyses of muscle sections; and functional muscle force assays are described in the Supplemental Material.
Cell culture, RNA, and protein analyses
Culture conditions for primary macrophages and fibroblasts, qRT–PCR, and antibodies for protein immunodetection are described in the Supplemental Material.
DMD patient study
Muscle biopsies were analyzed as described in Supplemental Material.
Statistical analysis
Quantitative data were analyzed by Student’s t-test, unless otherwise specified. P < 0.05 was considered statistically significant.
Acknowledgments
We are greatful to V. Lukesova, G. Cònsol, M. Humà, E. Serrano, I. Cuartas, and B. González, for excellent assistance; Drs. E. Perdiguero, R. Lopez-Alemany, J. Seoane, B. Chazaud, G. Gil-Gómez, T. Partridge, R. Bataller, P. Sancho-Bru, and C. Millán for advice or reagents; J. Martín-Caballero, S. Gómez, J. Visa, and R. Bonavia for animal care; and Drs. J.P. Barbet, J. Colomer (Hospital Sant Joan de Deu, Barcelona), I. Illa, and E. Gallardo for providing D.M.D. muscle samples. B.V., B.B.R., and V.R.B. are recipients of FPI, FIS, and FI predoctoral fellowships, respectively; A.L.S. is a Ramon y Cajal investigator; M.T. is a Leibniz Junior Group Leader, in part supported by the Belgian FWO. J.L.D. is supported by NIH grants AR049822 and HL085357. This work was supported by MDA, SAF2007-63062, BFU2007-63068, AFM, EU (7FP), Fundación MM y R Pascual, CIBERNED, and Marató-TV3.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.465908.
References
- Adams R.A., Bauer J., Flick M.J., Sikorski S.L., Nuriel T., Lassmann H., Degen J.L., Akassoglou K. The fibrin-derived γ377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 2007;204:571–582. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreetta F., Bernasconi P., Baggi F., Ferro P., Oliva L., Arnoldi E., Cornelio F., Mantegazza R., Confalonieri P. Immunomodulation of TGF-β 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: Implications for antifibrotic therapy. J. Neuroimmunol. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Bonniaud P., Margetts P.J., Ask K., Flanders K., Gauldie J., Kolb M. TGF-β and Smad3 signaling link inflammation to chronic fibrogenesis. J. Immunol. 2005;175:5390–5395. doi: 10.4049/jimmunol.175.8.5390. [DOI] [PubMed] [Google Scholar]
- Boutard V., Havouis R., Fouqueray B., Philippe C., Moulinoux J.P., Baud L. Transforming growth factor-β stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J. Immunol. 1995;155:2077–2084. [PubMed] [Google Scholar]
- Bronte V., Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Campbell K.P. Three muscular dystrophies: Loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Flick M.J., Du X., Witte D.P., Jirouskova M., Soloviev D.A., Busuttil S.J., Plow E.F., Degen J.L. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.Y., Ito K., Hayashi R., Jazrawi E.P., Barnes P.J., Adcock I.M. NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1β-induced TGF-β1 gene transcription. J. Immunol. 2006;176:603–615. doi: 10.4049/jimmunol.176.1.603. [DOI] [PubMed] [Google Scholar]
- Lluis F., Roma J., Suelves M., Parra M., Aniorte G., Gallardo E., Illa I., Rodriguez L., Hughes S.M., Carmeliet P., et al. Urokinase-dependent plasminogen activation is required for efficient skeletal muscle regeneration in vivo. Blood. 2001;97:1703–1711. doi: 10.1182/blood.v97.6.1703. [DOI] [PubMed] [Google Scholar]
- Perez R.L., Ritzenthaler J.D., Roman J. Transcriptional regulation of the interleukin-1β promoter via fibrinogen engagement of the CD18 integrin receptor. Am. J. Respir. Cell Mol. Biol. 1999;20:1059–1066. doi: 10.1165/ajrcmb.20.5.3281. [DOI] [PubMed] [Google Scholar]
- Rubel C., Gomez S., Fernandez G.C., Isturiz M.A., Caamano J., Palermo M.S. Fibrinogen-CD11b/CD18 interaction activates the NF-κB pathway and delays apoptosis in human neutrophils. Eur. J. Immunol. 2003;33:1429–1438. doi: 10.1002/eji.200323512. [DOI] [PubMed] [Google Scholar]
- Rybarczyk B.J., Lawrence S.O., Simpson-Haidaris P.J. Matrix-fibrinogen enhances wound closure by increasing both cell proliferation and migration. Blood. 2003;102:4035–4043. doi: 10.1182/blood-2003-03-0822. [DOI] [PubMed] [Google Scholar]
- Saito T., Takenaka M., Miyai I., Yamamoto Y., Matsumura T., Nozaki S., Kang J. Coagulation and fibrinolysis disorder in muscular dystrophy. Muscle Nerve. 2001;24:399–402. doi: 10.1002/1097-4598(200103)24:3<399::aid-mus1012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sitrin R.G., Pan P.M., Srikanth S., Todd R.F. Fibrinogen activates NF-κB transcription factors in mononuclear phagocytes. J. Immunol. 1998;161:1462–1470. [PubMed] [Google Scholar]
- Stedman H.H., Sweeney H.L., Shrager J.B., Maguire H.C., Panettieri R.A., Petrof B., Narusawa M., Leferovich J.M., Sladky J.T., Kelly A.M. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelves M., Lopez-Alemany R., Lluis F., Aniorte G., Serrano E., Parra M., Carmeliet P., Munoz-Canoves P. Plasmin activity is required for myogenesis in vitro and skeletal muscle regeneration in vivo. Blood. 2002;99:2835–2844. doi: 10.1182/blood.v99.8.2835. [DOI] [PubMed] [Google Scholar]
- Suelves M., Vidal B., Serrano A.L., Tjwa M., Roma J., Lopez-Alemany R., Luttun A., de Lagran M.M., Diaz-Ramos A., Jardi M., et al. uPA deficiency exacerbates muscular dystrophy in MDX mice. J. Cell Biol. 2007;178:1039–1051. doi: 10.1083/jcb.200705127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh T.T., Holmback K., Jensen N.J., Daugherty C.C., Small K., Simon D.I., Potter S., Degen J.L. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes & Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- Wehling M., Spencer M.J., Tidball J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Porter J.D., Cheng G., Gong B., Hatala D.A., Merriam A.P., Zhou X., Rafael J.A., Kaminski H.J. Temporal and spatial mRNA expression patterns of TGF-β1, 2, 3 and TβRI, II, III in skeletal muscles of mdx mice. Neuromuscul. Disord. 2006;16:32–38. doi: 10.1016/j.nmd.2005.09.009. [DOI] [PubMed] [Google Scholar]