Abstract
We have recently reported that gamma tocopherol (γT) reduces allergen and zymosan-induced inflammation using rodent models. As an initial step in extending these observations to humans, we conducted an open-label, Phase I dosing study of two doses (one or two capsules/daily for one week) of a gamma tocopherol rich preparation containing 623mg of γ tocopherol, 61.1mg of d-α-tocopherol, 11.1 mg of d-β-tocopherol (11.1mg), and 231 mg of d-σ-tocopherol per capsule. Endpoints for this study include serum levels of 5-nitro-gamma tocopherol, as a marker of oxidative stress, and changes in serum gamma, alpha and delta tocopherol and γ-2′-carboxyethyl-6-hydroxychroman (CEHC) six and 24 hours after the first dose and after 1 week of treatment. To assess biological activity of this treatment, we obtained peripheral blood mononuclear cells at baseline and after 1 week of treatment with 2 capsules of a gamma tocopherol rich preparation/day, and examined the inflammatory cytokine response of these cells in culture to ex-vivo endotoxin/LPS (0.01 ng/ml) challenge. We also monitored a number of safety endpoints to examine how well this preparation is tolerated in 8 normal volunteers (4 allergic and 4 non-allergic) and 8 allergic asthmatics. We further obtained human monocytes from a subset of these volunteers and treated them ex vivo with γT, αT,γ-CEHC and α-CEHC and assessed their actions on LPS induced degradation of IkBα, and JNK signaling and ROS generation. As detailed herein, this open label study demonstrates that gamma tocopherol enriched supplementation decreased systemic oxidative stress, increased serum levels of gamma tocopherol, and inhibited monocyte responses to LPS without any adverse health effects. Further,in vitro treatment of human monocytes with γ-CEHC and α-CEHC inhibits ROS generation and LPS-induced degradation of IκB and JNK activation.
Keywords: gamma tocopherol, gamma CEHC, asthma
Introduction
Reactive oxygen and nitrogen species have been increasingly implicated in a wide variety of cellular mechanisms central to inflammation, as well as a number of specific inflammatory states, including asthma. Multiple oxidant stresses have been linked to exacerbation of airway disease, including oxidants generated by endogenous sources (activated inflammatory cells), pollutants, and cells activated by innate or acquired immune stimuli (viruses, inhaled LPS and particulate biomass, and allergens)[1]. These observations indicate that oxidant stress plays a central role in acute environmental asthma. We hypothesize that antioxidant supplementation may be a useful adjunct to asthma therapy. Vitamin E is a nutritional antioxidant which has been studied for its impact in asthma. Dietary vitamin E intake has recently been associated with decreased levels of IgE, suggesting a protective effect against development of atopy [2]. Conversely, decreased maternal intake of Vitamin E during pregnancy has been associated with increased asthma prevalence in children born from these pregnancies[3,4]. Taken together, these observations suggest that Vitamin E may be a useful adjunct for asthma therapy.
In humans, alpha tocopherol is the most highly conserved isoform of vitamin E and is the form most commonly employed for dietary supplementation. Animal studies demonstrate that supplementation of mice with α-tocopherol decreases allergen-induced airway inflammation, and airway responsiveness. Treatment studies of persons with asthma [5] and healthy volunteers [6] using α-tocopherol coupled with ascorbic acid (Vitamin C) demonstrated that antioxidant vitamins protect against changes in lung function provoked by oxidant stimuli such as inhaled ozone exposure. Romieu et al reported that anti-oxidant vitamin supplementation of asthmatic children was an effective prophylaxis intervention against the effect of ambient air ozone exposure on disease exacerbation in those children who possessed the null genotype for the antioxidant gene GSTM1 [7,8]. Moreover, asthmatics have been shown to be deficient in antioxidant levels in their airways secretions compared to non-asthmatics[9], suggesting they may be particularly responsive to antioxidant therapy. There are also case reports suggesting that vitamin E may be helpful in asthma[10]. Contrasting with the results noted above are many studies which fail to demonstrate any effect of supplemental α-tocopherol on asthma[11], causing confusion regarding the role of Vitamin E supplementation in treating allergic disease and asthma [12–14].
One potential reason for the discrepancy between the reported benefits of dietary vs. supplemental vitamin E in asthma may lie in the isoform of vitamin E present in each source. The most predominant isoform of vitamin E in dietary sources is gamma tocopherol, whereas alpha tocopherol is used for most supplements. We hypothesize that gamma tocopherol may be a better agent for treating asthma than alpha tocopherol. Alpha and gamma tocopherol differ structurally only by a single methyl group at the C-5 position. This extra methyl group confers increased antioxidant potential to αT than γT, as it promotes phenolic hydrogens (electrons) to donate to lipid radicals. However, the unmethylated C-5 position in γT makes this isoform of vitamin E a better trap for lipophilic electrophiles such as reactive nitrogen species, and thus counters the oxidative stress induced by products of the oxidative burst of eosinophils[15–17].
Gamma tocopherol also appears to be a more potent anti-inflammatory agent than αT. Investigators from our group have also shown that γT (unlike αT) specifically inhibits COX-2 in LPS-stimulated macrophages and IL-1β-stimulated epithelial cells (the cells which initially encounter ozone in asthmatics) independently of its action as an antioxidant. The inhibitory potency of γT and its metabolite γ-CEHC (2,7,8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman)competes with arachidonic acid (AA) at the active site of COX-2, resulting in a decrease in eicosanoids [18]. In carrageenan-induced inflammation in male Wistar rats, γT treatment is specifically associated with decreased prostaglandin (PGE2), leukotriene (LTB4) and TNF release , suggesting that lipoxygenase mediated production of leukotrienes (including LTC4, D4 and E4 which play a role in asthma) as well as TNF secretion is inhibited by γT [19].
Using a rodent model of ovalbumin-sensitized airway inflammation, we recently extended these studies into allergic airway disease. We observed that acute airway inflammation of sensitized animals due to either ozone alone or ozone plus ovalbumin was markedly decreased following gamma tocopherol supplementation. These observations support the rationale for use of gamma tocopherol rich preparations as adjunct treatments for inflammatory diseases such as asthma. As an initial step in exploring the utility of gamma tocopherol as an adjunctive treatment for human asthma, we conducted an open-label, Phase I dosing study of two doses (one or two capsules/daily for one week) of a gamma tocopherol rich preparation containing 623mg of γ tocopherol, 61.1mg of d-α-tocopherol, 11.1 mg of d-β-tocopherol (11.1mg), and 231 mg of d-σ-tocopherol per capsule in eight non-asthmatic and eight asthmatic volunteers.
For this study, we collected serum samples at baseline, six and 24 hours after the initial administration of the first dose of mixed tocopherols and after one week of treatment, waited during a one week washout period, repeated this sampling schedule for the higher dose of mixed tocopherols, and then recovered a sample 1 week after all dosing was complete. Serum levels of gamma, alpha and delta tocopherol and γ-CEHC were assessed for pharmacokinetic endpoints, and levels of 5-nitro-γ-tocopherol were measured as a marker of systemic oxidative stress. A number of safety endpoints were also assessed to examine how well this preparation was tolerated in the 8 non-asthmatic volunteers (4 allergic and 4 healthy non-allergic) and 8 allergic asthmatics enrolled in this study.
To assess biological activity of this treatment, we obtained peripheral blood mononuclear cells at baseline and after one week of treatment with 2 capsules of a gamma tocopherol rich preparation/day, and examined the response of these cells in culture to ex-vivo endotoxin/LPS challenge. We also conducted in vitro studies focused on the effect of γT, αT, γ-CEHC and α-CEHC on changes in NF-kB and JNK signal transduction pathways, reactive oxygen species generation, and mediator secretion on LPS-stimulated monocytes recovered from a subset of asthmatic volunteers. As detailed herein, this open label study demonstrates that gamma tocopherol enriched supplementation increases serum levels of gamma tocopherol and γ-CEHC, decreases systemic oxidative stress, and ex vivo, inhibits a number of pro-inflammatory and oxidative stress responses in peripheral blood monocytes following LPS challenge.
MATERIALS and METHODS
Subjects
Adult subjects between the ages of 18 and 50 years old were recruited through use of advertisements placed on Clintrials.gov and Center for Environmental Medicine, Asthma and Lung Biology (CEMALB) websites. Potential subjects were also identified from the CEMALB database of potential subjects that have expressed interest in participating in studies. A total of sixteen volunteers were recruited and placed into two cohorts, classified either as moderate to severe allergic asthmatic adults, or healthy adult volunteers without prior physician diagnosis of asthma. Inclusion into both cohorts required oxygen saturation of >94% at baseline, and blood pressure between 90–150mm systolic and 60–100mm diastolic. Inclusion criteria into the allergic asthmatic cohort required specific allergy as demonstrated by positive immediate skin test response to on of the following allergen mixes: two species of house dust mite (Dermatophagoides farinae and Dermatophagoides Pteryonnisuius), cockroach, tree mix, grass mix, weed mix, mold mix 1, mold mix 2, rat, mouse, guinea pig, rabbit, cat or dog.
Inclusion in the asthmatic cohort also required moderate to severe persistent asthma according to NHLBI definitions including history one of the following:
-
1
Episodic wheezing, chest tightness or shortness of breath consistent with asthma symptoms at least 1 time per week that affects activity
-
2
Asthma symptoms occurring at night or during sleep at least once a week
-
3
Measured FEV1 or FVC <80% of predicted
OR
-
4
Physician diagnosed moderate or severe persistent asthma that was currently controlled with maintenance medication that includes moderate or high dose inhaled corticosteroid, or any dose of inhaled corticosteroid and a longacting inhaled β2-agonist.
Exclusion criteria included pregnancy or currently nursing a baby; inability to abstain from NSAIDs, aspirin, antihistamines or anticoagulants for the length of the study; a current diagnosis of anemia or abnormal blood counts or clotting times at screening; and known vagal response to venipuncture. Volunteers were also excluded if they had any chronic medical condition considered by the PI as a contraindication to receiving the gamma tocopherol enriched preparation including significant cardiovascular disease, diabetes, chronic renal disease, chronic thyroid disease, kidney disease or coagulation defects. Volunteers were also asked to refrain from taking vitamin supplements or other dietary supplements for the length of the study.
This study was reviewed and approved by the Committee for the Protection of the Rights of Human subjects of the University of North Carolina. All study subjects signed a consent form prior to any study procedures. Participants then underwent an assessment of general health including a health history questionnaire, physical exam, measurement of baseline circulating antioxidant levels, measurement of baseline safety labs, lung function assessment, and symptom scoring prior to receiving γT.
Study Design
This was an open-label, one arm phase I study of a gamma-tocopherol enriched supplement in allergic asthmatics and healthy adults. Each capsule of the supplement contained 623mg of g tocopherol as well as d-a-tocopherol (61.1mg), d-B-tocopherol (11.1mg) and d-s-tocopherol (231mg) per capsule, with a total tocopherol content of 823 mg. The supplements were kindly provided as a gift from Yasoo Health Inc (Johnson City, TN). The dosing protocol included two escalating doses, beginning with a one capsule dose taken orally, once daily for 8 consecutive days, followed by an 8 day wash-out period and then a second 8 day dosing period with 2 capsules, with a follow-up visit 8 days after dosing was complete. Primary endpoints included blood count, liver enzyme assessments and coagulation studies, symptom questionnaire, and serum levels of γT, αT and γ CEHC a primary γT metabolite, 5-nitro-γ-tocopherol (a marker of oxidative stress), and serum levels of IL-1β, IL-6 and IL-8. Secondary endpoints included changes in peripheral blood monocyte cytokine production. The protocol scheme is depicted in Figure 1.
Figure 1.
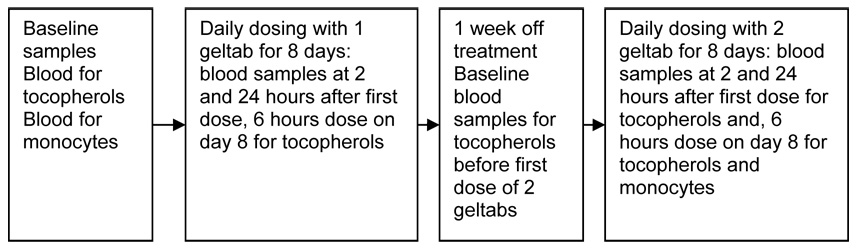
Phase I study protocol for examination of the effect of gamma tocopherol enriched capsules on blood levels of alpha, delta and gamma tocopherol, gamma CEHC, safety endpoints an effect on ex vivo response of peripheral blood monocytes to LPS in 8 allergic asthmatic and 8 non-asthmatic volunteers. There was 623mg of γ tocopherol, 61.1mg of d-α-tocopherol, 11.1 mg of d-β-tocopherol (11.1mg), and 231 mg of d-σ-tocopherol per geltab.
Blood samples were collected at baseline, 6 and 24 hours after the initial dose, after 8 consecutive days of γT treatment, and after 8 days without supplement (which served as a wash-out period) for each of the two γT doses. These blood samples were analyzed for serum levels of gamma tocopherol, alpha tocopherol, delta tocopherol, and the primary metabolite of gamma tocopherol, γCEHC. Additional blood was collected at baseline, on the 8th day of dose containing 623mg γT and the 8th day of 1246 mg γT for isolation of PBMCs for the purposes of ex-vivo LPS stimulation, supernatant cytokine analysis and mRNA extraction. Individual subject participation lasted approximately 6 weeks and required 8 visits to the study facility. Participants were compensated for their time and expenses.
Symptom Score Questionnaire
All subjects filled out a symptom questionnaire before starting each dose of γT, after taking the dose of γT for 8 days, and after washout periods. The questionnaire had the participant score any symptoms they were experiencing on a scale of 0 (none) to 3 (severe), and were given definitions of mild, moderate and severe. There were 12 symptom choices including malaise, fatigue, shortness of breath, cough, myalgia, chills, fever, headache, nausea, vomiting, indigestion/stomach ache and unusual bleeding/bruising. Minimum possible score on the questionnaire was 0, and maximum possible score was 36.
Safety Laboratory Analysis
All participants underwent serum analysis for safety endpoints before each dose, 24 hours after beginning each dose of γT, after 8 days of γT and after the washout period. The safety labs included a complete blood count with differential assessment,and blood levels of creatinine, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), alkaline phosphatase, Prothrombin Time (PT) , PT/International Normalized Ratio (INR), and Activated Partial Thromboplastin Time. All clinical labs were performed at the UNC Hospital Laboratories.
Allergy Testing
Skin allergy testing was performed before the initial dose of γT. Skin testing was performed using skin test reagents from Greer Laboratories, and included extracts for dust mites (D. pteronyssinus and D. farinae), tree mix, weed mix, grass mix, mold, cat, dog, rat, mouse, rabbit, guinea pig and cockroach. Antigen solution was placed on the anterior forearms of test subject using specialized 8-pronged applicator, and left on the on the skin for 15 minutes before the test was read. Allergic individuals developed indurations corresponding to individual allergens, and the diameter in millimeters of the resulting indurations were measured and traced. A positive allergic response is quantified as a resultant wheal diameter that is equal to or larger than the positive control wheel (histamine). A negative control is also included in the battery to aid in reading the test.
Analysis of Serum Tocopherols
Quantitation of tocopherols
α-T, γ-T , δ-T and 5-nitro-γ-T were measured by a HPLC assay with electrochemical detection [16]. Briefly, tocopherols were extracted from serum using a mixture of methanol/hexane (2:5, v/v). All extractions were carried out in the presence of 0.8 mM butylated hydroxytoluene. After centrifugation at 1000 × g for 10 min at 4°C, the top hexane layer was collected and evaporated under N2, and the dried residue was re-dissolved in ethanol. Tocopherols were separated on a 150 × 4.6 mm, 5 µm Supelcosil™ LC-18-DB column, and eluted with 95:5 (v/v) methanol/water with final 25mM of lithium acetate (pH 4.75) at a flow rate of 1.2 mL/min. Tocopherols were monitored by coulometric detection (Model Coulochem II, ESA Inc., Chelmsford, MA) at 300 (upstream) and 500 mV (downstream electrode) using a Model 5011 analytical cell.
Quantitation of γ-CEHC
γ-CEHC was analyzed using a HPLC assay with fluorescent detection [20]. Briefly, 200 µL of serum was diluted with an equal volume of PBS, acidified using 20 µL of acetic acid, and then extracted twice with 1 mL of ethyl acetate containing 40 µg/mL of ascorbic acid. The combined ethyl acetate layers were dried under nitrogen. The residue was reconstituted in 200 µL of 70% MeOH/ 30% water. Samples were injected through a Hitachi L-7200 autosampler (Hitachi, San Jose, CA) and were separated on a 5 micron Supelcosil LC-18-DB column, 4.6 × 150 mm (Supelco, Bellefonte, PA). The mobile phases included A – 35% acetonitrile, 65% 10 mM ammonium acetate at pH 4.3 and B – 96% acetonitrile, 4% 10 mM ammonium acetate at pH 4.3. Analytes were eluted at a flow rate of 1.0 mL/min with the following gradient: maintaining 100% A for 8 min, linearly increasing to 100% B from 8 to 30 min, maintaining 100% B until 55 min and then back to 100% A at 56 min. γ-CEHC was detected by a Shimadzu RF-10AXL spectrofluorometric detector (Shimadzu, Columbia, MD) with the excitation and emission wavelength at 292 nm and 327 nm, respectively. γ-CEHC was quantified using the authentic standard as the external standard.
Analysis of serum cytokines at baseline and after supplement dosing
Serum samples were recovered on the same schedule as described above for tocopherol assessments. These were assayed for IL1α, IL1β, IL2 , IL3 , IL4, IL5 , IL6 , IL7, IL8 , IL10, IL12p40 , IL12p70 , IL13 , IL15 , GMCSF , IFNg , TNFa , Eotaxin , MCP1 , RANTES , MIP1a , and IP10 using the Luminex 100IS Multiplex flow cytometric assay (Luminex, Austin TX)
Analysis of ex vivo endotoxin-induced cytokine release from peripheral blood monocytes at baseline and after supplement dosing
70cc of blood was collected at baseline and the 8th day of 1200 mg γT. Peripheral blood monocytes were isolated from the blood through a series of gradient centrifugations, and then counted and plated in RPMI 640 + 10% FBS media , then aliquoted in cell culture tubes with 3 ×105 cells/tube. 0 ng/ml, 0.001 ng/ml or 0.01 ng/ml of lipopolysaccharaide (Sigma, Inc, St Louis, MO) was incubated with blood monocytes, respectively. Total volume of cells + media + LPS was 500ul/tube. All LPS concentrations were assayed in duplicate, which would be a total of 6 tubes per separation (3 concentrations × 2 tubes each). Cells were then incubated 24 hours, then centrifuged at 500 rcf for 10 minute. 300ul of the supernatant was collected and stored for the cytokine assay. Once samples of cellular supernatants from 14 of the 16 volunteers were thawed, levels of pro-inflammatory cytokines IL-1β IL-6, and TNFα, chemokines MCP-1 and MIP-1α and anti-inflammatory cytokines IL-1RA and IL-10 were measured using the Luminex 100IS Multiplex flow cytometric assay (Luminex, Austin TX).
In vitro analysis of γT, αT, γ-CEHC and α-CEHC effects on NFkB and JNK signaling, and intracellular reactive oxygen species (ROS) generation of PBMCs recovered from volunteers with allergic asthma
Immunoblotting for I κB proteins and JNK phosphorylation
2×106 PBMCs with or without pretreatment of tocopherols (dissolved in alcohol, 0.1%) or their metabolites were treated with LPS, washed twice with cold phosphate-buffered saline (PBS), and then lysed in RIPA buffer (1x PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitors: 20 µg/ml leupeptin, 20 µg/ml aprotinin, 0.5 mM phenylmethylsulfonyl fluoride, 200 µM sodium orthovanadate, and 20 mM sodium fluoride). Supernatants of cell lysates were subjected to SDS-PAGE. Proteins were transferred onto nitrocellulose membrane. Membrane was blocked with 5% nonfat milk, washed briefly, incubated with primary antibody (against IκBα and phosphorylated JNK) at 4°C overnight, followed by incubating with corresponding HRP-conjugated secondary antibody for 1 h at room temperature. Immunoblot images were detected using chemiluminescence reagents and the Gene Gynome Imaging System (Syngene, Frederick, MD).
Measurement of Intracellular ROS
The intracellular formation of ROS in PBMCs was detected by using the fluorescent probe 5-(and 6-)chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Molecular Probes, (Eugene, OR)). In this method, monocytic cells incorporate the CM-H2DCFDA, and the diacetate moiety is cleaved to produce the nonfluorescent compound DCFH. The ROS generated by activated PBMCs oxidize the intracellular DCFH to the fluorescent compound 2′,7′-dichlorofluorescein. The green fluorescence produced by the PBMCs is proportional to the amount of ROS produced. Briefly, 2×105 PBMCs were pre-incubated with 40 µM tocopherol for 18 h prior to loading of 10 µM CM-H2DCFDA at 37°C for 1 h. 100 ng/ml PMA was added and incubated at 37°C for 30 min. Cells were washed once with PBS and suspended in 0.5 ml PBS, and put on ice before determination of green fluorescence. Flow cytometry was performed with a FACSORT (Becton Dickinson, Miami, FL) by using an Argon-ion laser (wavelength 488nm). The FACSORT was calibrated with Calibrite beads before each use, and 6,000 events were counted for all sample runs. Relative cell size and density/granularity were quantified by analyzing light-scatter properties using Cell Quest software (Becton Dickinson), namely forward scatter for cell size and side scatter for density/granularity, and recording the mean fluorescence intensities (MFI) for each.
Statistical Analysis
Intention to treat principle was used for all analyses of in vivo studies; i.e. the data from all patients was used in the analysis regardless of whether they complied with the medication assigned. All results were expressed as mean +/− standard error of the mean. As appropriate, paired and unpaired techniques were employed to assess comparisons of outcomes between baseline and gamma tocopherol treatments. ANOVA was employed to test between multiple variables followed by focused comparisons between specific means using paired t-tests. This was used for analysis of the entire cohort. Comparisons between asthmatic and non-asthmatic groups were conducted using unpaired techniques. All assessments were conducted using Prism software (GraphPad Software Incorporated, San Diego, CA)
RESULTS
Serum Levels of α, δ and γ-tocopherol and γ-CEHC
All sixteen volunteers (eight asthmatics and eight normal volunteers) completed this open-label, Phase I dosing study in which volunteers consumed one capsules/day of a gamma tocopherol rich preparation containing 623mg of γ tocopherol, 61.1mg of d-α-tocopherol, 11.1 mg of d-β-tocopherol (11.1mg), and 231 mg of d-σ-tocopherol per capsule, followed by a one week washout period and then a second dosing period in which volunteers consumed two geltabs/day for one week. Blood samples were collected before each dosing period, 6 and 24 hours after the initial dose and 6 hours after the last dose of each dosing period. Samples were analyzed for levels of α, δ and γ– tocopherol and γ-CEHC and are described in Table 1.
Table 1.
Levels of alpha, gamma and delta tocopherol recovered from serum of 16 volunteers prior to initial dose of the tocopherol preparation, 6 and 24 hours after the initial dose of the 1 geltab preparation, 6 hours after the eighth daily dose of 1 geltab. There was a 1 week period between the 1 and 2 geltab dosing periods and then blood sampling occurred immediately prior to initial administration of the 2 geltab dose, 6 and 24 hours after that dose, 6 hours after the eighth daily dose of 2 geltabs and 1 week after dosing ended.
| 1 geltab of gamma tocopherol enriched supplement/day | 2 geltabs of gamma tocopherol enriched supplement/day | |||||||
|---|---|---|---|---|---|---|---|---|
| baseline | 6 hours | 24 hours | 8 days | baseline | 6 hours | 24 hours | 8 days | |
| α-tocopherol (µM) | 26.7±3.1 | 27.1±3.2 | 27.5±2.8 | 26.1±2.7 | 25.7±2.2 | 24.6±2.5 | 25.9±2.7 | 25.2±2.4 |
| δ-tocopherol (µM) | 0.3±0.1 | 3.5±0.9* | 1.2±0.3* | 4.6±0.8* | 0.4±0.1 | 4.8±0.9* | 1.1±0.2* | 5.1±1.1* |
| γ-tocopherol (µM) | 3.7±0.4 | 13.5±2.9* | 9.6±1.3* | 19.5±1.9* | 4.1±0.5 | 16.4±2.4* | 9.6±1.4* | 18.6±2.6* |
There was no change above baseline levels in alpha tocopherol with either dosing period. Serum levels of delta tocopherol were significantly increased 6 hours after initial dosing, ,decreased at 24 hours (though still significantly above baseline) ands elevated again six hours after the final dose with both the one and two geltab tocopherol regimens, with no difference in levels following either dose. A similar pattern of gamma tocopherol levels was observed following either dosing regimen of the gamma tocopherol enriched supplement regimen. When the cohort was segregated into groups of 8 asthmatics and 8 non-asthmatic volunteers, no differences were observed in the levels of any of the tocopehrols.
Unlike the tocopherols, levels of γ-CEHC (2,7,8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman) steadily increased across each blood sampling with each dosing regimen. Furthermore, the final levels of γ-CEHC were significantly higher after the 2 geltab regimen than with the 1 geltab regimen. As with the tocopherols, no significant differences were observed between the non-asthmatic volunteers and the asthmatic volunteers. It was also notable that levels of all measured tocopherols of γ-CEHC fell to baseline levels during the washout period of one week (Figure 2).
Figure 2.
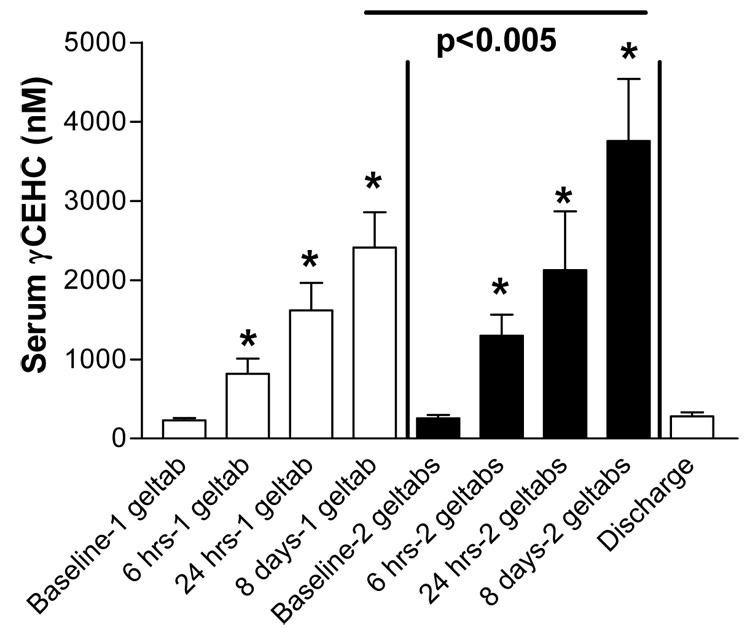
Levels of gamma CEHC recovered from serum of 16 volunteers prior to initial dose of the tocopherol preparation, 6 and 24 hours after the initial dose of the 1 geltab preparation, 6 hours after the eighth daily dose of 1 geltab. There was a 1 week period between the 1 and 2 geltab dosing periods and then blood sampling occurred immediately prior to initial administration of the 2 geltab dose, 6 and 24 hours after that dose, 6 hours after the eighth daily dose of 2 geltabs and 1 week after dosing ended.
Serum Levels of 5-NITRO-γ-tocopherol
Serum levels of 5-NITRO-γ-tocopherol were present in all recovered samples and significantly decreased after 1 week of intervention with the one geltab and two geltab dose of the gamma tocopherol rich preparation employed in this study (Figure 3). As this molecule is generated via nitration of γ-tocopherol by peroxynitrite and other reactive nitrogen species,[17] we interpret this as a sign of decreased systemic oxidative/nirtrosative stress after supplementation.
Figure 3.
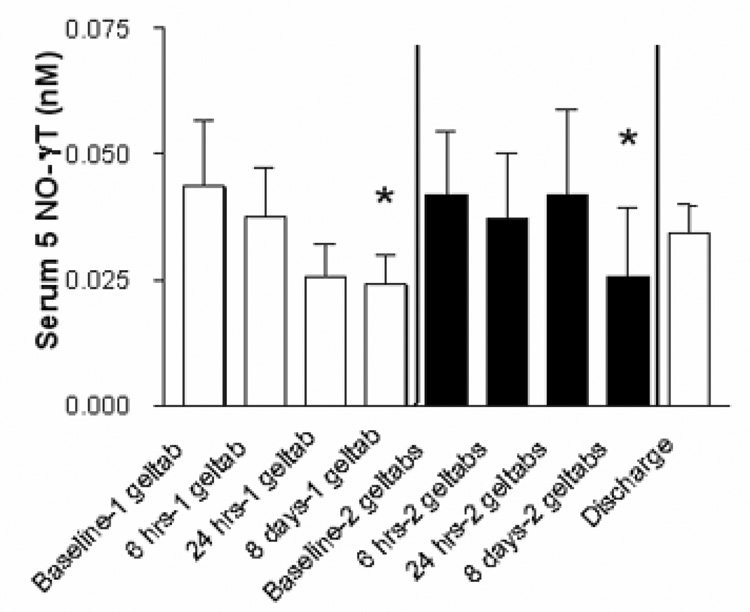
Levels of 5-nitro-gamma tocopherol recovered from serum of 16 volunteers prior to initial dose of the tocopherol preparation, 6 and 24 hours after the initial dose of the 1 geltab preparation, 6 hours after the eighth daily dose of 1 geltab. There was a 1 week period between the 1 and 2 geltab dosing periods and then blood sampling occurred immediately prior to initial administration of the 2 geltab dose, 6 and 24 hours after that dose, 6 hours after the eighth daily dose of 2 geltabs and 1 week after dosing ended.
Serum Levels of cytokines
Serum levels of IL-1α, Il-1β, IL-12p40, GM-CSF, TNF, Eotaxin, Rantes, MIP1α and IP-10 were all measurable, but did not change as a result of supplementation. All other cytokines were not detected in serum (data not shown).
Effect of tocopherol supplementation on cytokine and chemokine release from peripheral blood monocytes exposed to lipopolysaccharide ex vivo
We recovered PBMCs from 14 of 16 volunteers at baseline and 6 hours after the final dose of the 2 geltab regimen. PBMCs were then challenged with 0, and 0.01 ng/ml of lipopolysacharride and supernatants were collected for measurement of the pro-inflammatory cytokines IL-1β , IL-6 and TNFα the chemokines MCP1 and MIP1β, and the anti-inflammatory cytokines IL-1RA and IL-10. All assessed mediators were significantly increased following challenge with 0.01 ng/ml of LPS with cells collected after baseline and after the 2 geltab dose. However, comparing the cytokine levels in the supernatants of PBMCs treated with 0.01 ng/ml LPS revealed that the LPS-induced secretion of the proinflammatory cytokines IL-1β, IL-6 and TNFα (Figure 4) and chemokines MCP-1 and MIP1α (Figure 5) response was significantly decreased after treatment with gamma tocopherol enriched supplementation, with no significant effect being observed on secretion of the anti-inflammatory cytokines IL-1RA and IL-10. (Figure 6). There was no difference between cytokine responses seen between the 8 non-asthmatic volunteers and 6 asthmatic volunteers.
Figure 4.
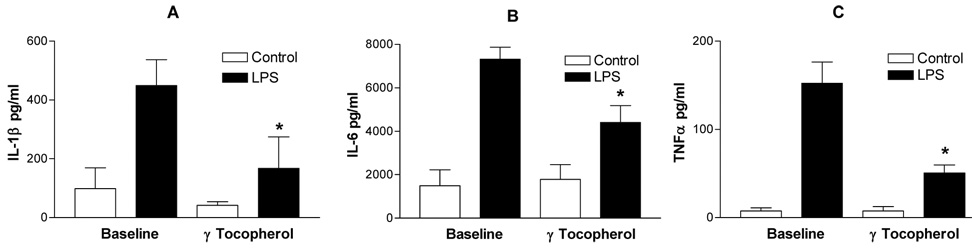
IL-1β (Panel A), IL-6 (Panel B) and TNFα (Panel C) secretion of PBMCs recovered at baseline and after gamma-tocpherol enriched supplementation following control (open bars) and LPS (closed bars) treatment of PBMCs. *=p<0.05 comparing LPS response at baseline vs, after supplementation.
Figure 5.
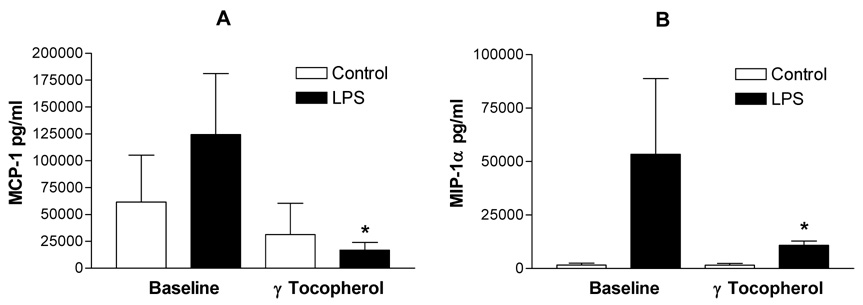
MCP-1 (Panel A) and MIP-a (Panel B) secretion of PBMCs recovered at baseline and after gamma-tocpherol enriched supplementation following control (open bars) and LPS (closed bars) treatment of PBMCs. *=p<0.05 comparing LPS response at baseline vs, after supplementation.
Figure 6.
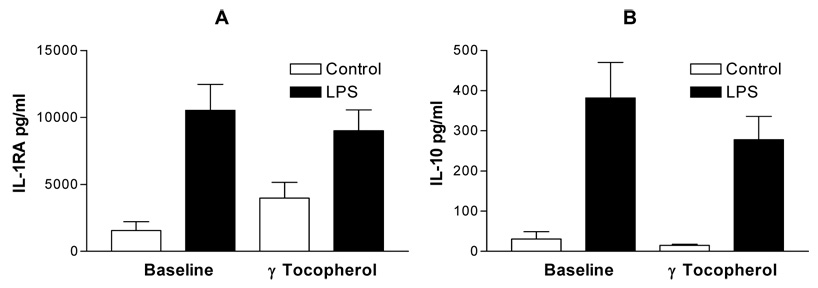
IL-1RA (Panel A) and IL-10 (Panel B) secretion of PBMCs recovered at baseline and after gamma-tocpherol enriched supplementation following control (open bars) and LPS (closed bars) treatment of PBMCs. There was no significant change in LPS response at baseline vs, after supplementation.
Effects of γT, αT, γ-CEHC and α-CEHC on intracellular reactive oxygen species (ROS) generation of PBMCs recovered from volunteers with allergic asthma and stimulated in vitro with PMA
We report that 40 µM concentrations of γT, αT, (Figure 7A) γ-CEHC and α-CEHC (Figure 7B) inhibit reactive oxygen species generation by PMA-stimulated PBMCs
Figure 7.
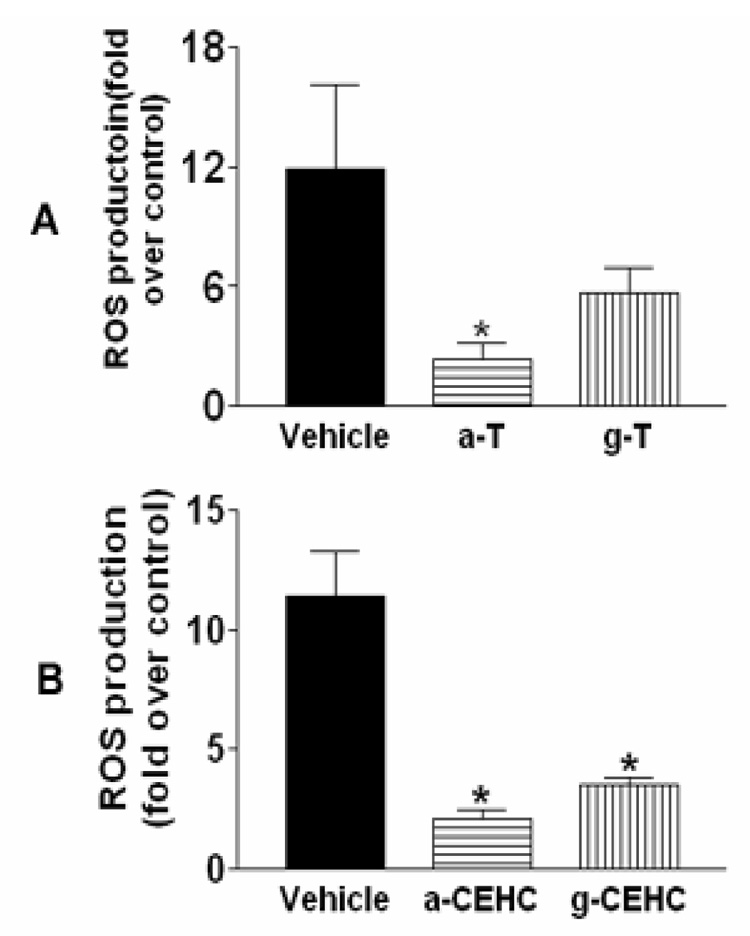
Effect of tocopherol species on PMA-Induced ROS generation, A. Effect of α and γ tocopherol (α-T and γ-T) on PMA-induced ROS production in PBMCs. α-T could significantly inhibit PMA-induced ROS production. B, Effect of α and γ CEHC (α-CEHC and γ-CEHC) on PMA-induced ROS production in PBMCs. α-CEHC and γ-CEHC significantly inhibited PMA-induced ROS production.
In vitro analysis of γT, αT, γ-CEHC and α-CEHC effects on NFkB and JNK signaling of PBMCs recovered from volunteers with allergic asthma and stimulated in vitro with LPS
As shown in Figure 8A, γ-tocopherol, but not α-tocopherol, moderately inhibited LPS-induced IkBα degradation in recovered PBMCs after LPS stimulation. Both α-CEHC and γ-CEHC differentially inhibited LPS-induced IkBα degradation (Figure 8B). As shown in Figure 9A, γ-tocopherol but not α-tocopherol moderately inhibited LPS-induced JNK phosphorylation. As shown in Figure 9B, α-CEHC and γ-CEHC differentially inhibited LPS-induced JNK phosphorylation.
Figure 8.
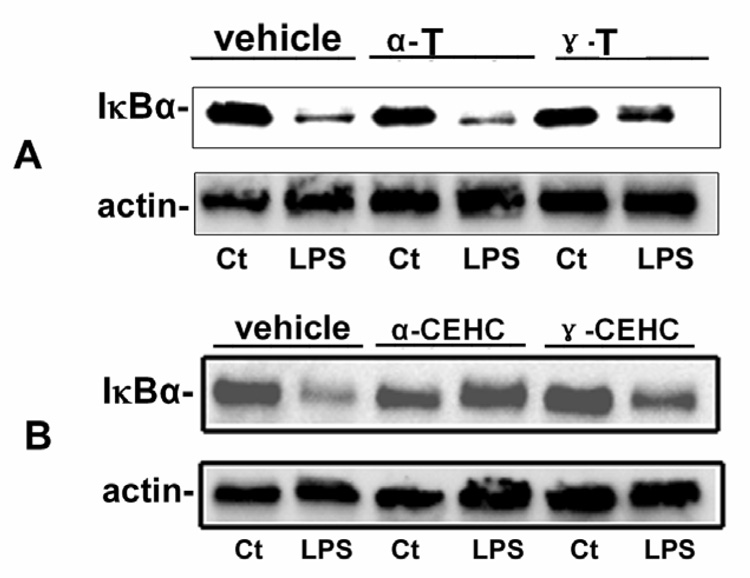
Effect of tocopherols on IκBα degradation. A, Effect of α and γ tocopherol (α-T and γ-T) on LPS-induced IkBα degradation (NFkB activation) in PBMCs. γ-T could moderately inhibit LPS-induced IkBα degradation. B, Effect of α and γ CEHC (α-CEHC and γ-CEHC) on LPS-induced IkBα degradation in PBMCs. α-CEHC and γ-CEHC could differentially inhibit LPS-induced NFkB activation.
Figure 9.
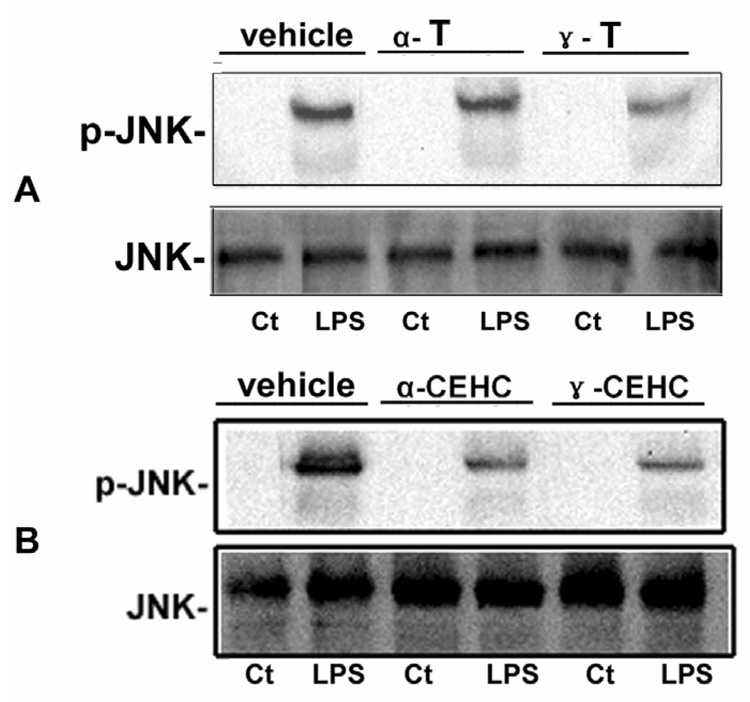
Effect of tocopherols on JNK phosphorylation. A, Effect of α and γ tocopherol (α-T and γ-T) on LPS-induced JNK phosphorylation in PBMCs. γ-T could moderately inhibit LPS-induced JNK phosphorylation. B, Effect of α and γ CEHC (α-CEHC and γ-CEHC) on LPS-induced JNK phosphorylation in PBMCs. α-CEHC and γ-CEHC could differentially inhibit LPS-induced JNK phosphorylation.
Safety and Dose Tolerance Endpoints
There were no changes in CBC with differential, platelet counts, PT, PT/INR, and APTT, liver enzymes across the entire study, nor did any volunteer sustain an abnormal value for any of these endpoints. There were no adverse or serious adverse events observed in any volunteer during the study. Symptom scores were assessed at baseline, after the first dose of the 1 geltab dosing regimen, at the follow-up visit for the 1 geltab regimen, the first dose of the 2 geltab regimen, the follow-up period for the 2 geltab regimen and the discharge visit one week after the 2 geltab regimen was complete. Scores were obtained for Malaise, Fatigue, SOB, Cough, Myalgia, Chills, Fever, Headache, Nausea, Vomiting, Stomach ache and, bruising or bleeding events with a score for each domain ranging from 0–3 for a total possible score of 36. The single highest mean total score being 1.6 ± 1.7 (SEM) upon study discharge.
DISCUSSION
The overarching hypothesis our group is pursuing is that gamma tocopherol is an important anti-inflammatory isoform of the antioxidant Vitamin E. This hypothesis is supported by preclinical studies by our group and others which show that gamma tocopherol has notable anti-inflammatory actions in animal models of airway allergy and innate immune response[18,19,21,22]. The primary goals of the current study were to determine if one and two geltab doses of a gamma tocopherol-rich preparation were associated with changes in serum levels of α, δ and γ tocopherols as well as γ-CEHC, a primary metabolite of gamma tocopherol with anti-inflammatory action. We also assessed serum for levels of 5-nitro-γ-tocopherol, which is generated through the action of reactive nitrogen oxide species (RNOS) on γ-tocopherol and is thought to be a marker of RNOS stress in a number of diseases.
To assess the anti-inflammatory effect of γ-tocopherol, we examined serum cytokine levels, responsiveness of PBMCs recovered from volunteers before using supplements and after using 2 geltabs of gamma tocopherol enriched supplements for 1 week, and the effect of vitamin E isoforms on JNK and NFκB signaling in vitro using recovered PBMCs from allergic asthmatic volunteers. We also examined safety data from our volunteers with both doses of gamma tocopherol. There was no difference in symptom scores observed at baseline and after each dose of gamma tocopherol. There was no change in lung function or any of the systemic parameters we assessed during this study. These observations are an initial step in designing Phase I Proof of Concept studies to determine if gamma tocopherol has utility as an intervention for asthma and other respiratory tract and inflammatory diseases.
We were somewhat surprised that we did not observe some increase in circulating α-tocopherol levels following either dose of gamma tocopherol-rich supplementation. While our preparation had a relatively small amount of α-tocopherol present in our supplement preparation compared to other tocopherols, our daily doses of α-tocopherol were 61 and 122 mg/day (for the 1 and 2 geltab doses) and we would have expected to see an increase in these levels at 6 hours. This is especially true given the role that α-tocopherol transfer protein (αTTP) has in facilitating secretion of α-tocopherol from the liver into plasma [23–25].
In contrast, we observed a notable increase in delta and gamma tocopehrol, though this appeared to drop significantly 24 hours after the first dose. With regard to γ tocopherol, these observations are not inconsistent with what has been previously reported, in which there is a relatively rapid increase in γ tocopherol levels at 12 hours with a gradual decline over 72 hours following consumption of a single dose of gamma tocopherol[26]. We did find that the level of serum delta and gamma tocopherol tended to be higher six hours after the last dose was consumed for the initial dose in each eight day dosing schedule. While our phlebotomy schedule did not allow us to determine daily pharmacokinetics of these tocopherols, it seems likely that repeated dosing would gradually result in an increased level of gamma tocopherol following repeated dosing.
We also observed that there was a steady increase in γ-CEHC, a primary metabolite of γ-tocopherol, across the weekly dosing schedule for both the 1 geltab and 2 geltab test periods. The increases in γ-CEHC were gradual and consistent, and did not completely mimic the levels of γ-tocopherol. However, once consumption of gamma tocopherol stopped, there was a rapid decrease in circulating levels of gamma tocopherol, such that within one week after stopping supplementation (with either 1 or 2 geltabs), serum levels of gamma tocopherol returned to baseline levels. We found in both dosing regimens that there was a steady rise in gamma tocopherol which was maximal following the last dose of each dosing period. The higher (2 geltab) dose yielded the highest level of γ-CEHC. Of practical importance for our studies was the observation that circulating levels of δ tocopherol, γ tocopherol and γ CEHC all returned to baseline levels after stoping supplementation for one week, suggesting that it would be feasible to conduct relatively short phase I/II proof of concept studies examining the effect of gamma tocopherol supplementation on experimental exacerbation of lung disease in humans in a randomized,placebo controlled fashion.
Levels of γ-CEHC resulting from γ-tocopherol consumption may be very important as we have observed that γ-CEHC has been found to have substantial anti-inflammatory activity in animal studies and in vitro, and likely accounts for a significant portion of the anti-inflammatory action of gamma tocopherol [17,27–29]. γ-CEHC has anti-inflammatory activity as demonstrated using in vivo rodent models of neutrophilic inflammation, as well as challenge of RAW cells (a macrophage cell line) with endotoxin[27]. We did not find any notable change in any serum level of pro-inflammatory cytokines and chemokines. However, we found notably decreased IL-1β, IL-6, TNFα , MCP and MIP1α secretion from peripheral blood mononuclear cells following ex vivo LPS challenge after volunteers had completed high dose supplementation when compared to PBMC responses at baseline.
To better appreciate mechanisms by which tocopherol species may exert anti-inflammatory responses, we performed in vitro studies with PBMCs recovered from a sub-set of allergic asthmatic volunteers. Here we found that γ-tocopherol, α-CEHC and γ-CEHC but not α-tocopherol, moderately inhibited LPS-induced IκBα degradation in recovered PBMCs after LPS stimulation. We also found that γ-tocopherol but not α-tocopherol moderately inhibited LPS-induced JNK phosphorylation, and α-CEHC and γ-CEHC differentially inhibited LPS-induced JNK phosphorylation. Taken together, these observations suggest that gamma (and alpha) tocopherol species inhibit NFκB activation by interfering with IκBα degradation. This would certainly account for decreased cytokine generation after tocopherol supplementation. We also found an inhibition of JNK activation, which would also result in decreased cytokine production.
We chose this approach for assessing anti-inflammatory actions of γT in the current study for several reasons. First, LPS is a standard innate immune stimulus which initiates a well characterized response in monocytic cells, with Il-1β, IL-6, and TNFα being important pro-inflammatory cytokines secreted in these responses[30]. Second, environmentally encountered LPS (or endotoxin) is an important cause of exacerbation of asthma in occupational and domestic settings [31]. Third, monocytic cells of the airway are crucial elements of the initial response to airborne irritants and exacerbants of asthma[31]. Finally, we have developed an LPS inhalation challenge protocol which can be employed in normal volunteers, asthmatics, smokers and mild COPD patients to determine if gamma tocopherol inhibits endotoxin-induced responses in the airway [32–35].
There are a number of reasons to hypothesize that gamma tocopherol would be a useful adjunct for treatment of airway diseases. First, as noted in the introduction, consumption of dietary vitamin E (which is predominantly gamma tocopherol) is associated with decreased occurrence of allergy and asthma [2,4,36]. Secondly, exacerbation of asthma and other lung diseases is associated with activation of innate immune processes and exposure to oxidative stress, resulting from either innate inflammatory processes or exposure to environmental stressors [31,37–39]. Amongst the most common environmental causes for exacerbation of lung disease is exposure to tobacco smoke hence studies examining the effect of smoking on tocopherol metabolism is relevant to the evaluation of gamma tocpoherol as an adjunct approach to decreasing asthma exacerbations [40–42].
Cigarette smokers and nonsmokers exposed to cigarette smoke have significantly increased levels of gamma tocopherol despite having lower levels of other plasma antioxidants such as ascorbate and beta carotene[43,43]. However, investigators using labeled alpha and gamma tocopherol to follow the pharmacokinetics of these agents found that that smokers have increased depletion of these isoforms of vitamin E compared to non-smokers [44–46]. This may be due to increased metabolism of the tocopherols to their respective CEHC products. It is also possible that they are being directly modified by smoke-related oxidants. Exposure of γ tocopherol to tobacco smoke in vitro results in production of 5 nitro γ-tocopherol, and smokers have double the level of 5 nitro γ-tocopherol than non-smokers [47]. Production of 5 nitro γ-tocopherol likely protects cell membranes from reactive nitrogen stress[46]. Increased levels of γ-tocopherol in smokers may allow for more production of γ-CEHC and serve as an antioxidant defense mechanism, both of which should reduce inflammation associated with smoke exposure.
Nitrosative stress is emerging and an important process in the pathophysiology of asthma[48–51]. Exhaled nitric oxide (eNO), which derives primarily from inducible nitric oxide synthase (iNOS), has emerged as a marker of asthma severity, and is associated with increased airway eosinophilia. eNO is likely able to participate in nitrosative modification of airway and cell surface proteins. Acute endotoxin challenge has been shown enhance eNO in asthmatic, but not normal volunteers[52], and children with asthma also have increased levels of 3-nitrotyrosine, a marker of nitrosative stress[48]. It seems likely that increased consumption of γ tocopherol, which is a much more effective trap for reactive nitrogen species at its unmethylated C-5 position, may have specific advantages as an anti-asthma agent than other tocopherol isoforms, including the much more extensively studied α-tocopherol[15]. Consistent with this observation is a report which demonstrates that γ tocopherol prevents protein nitration and ascorbate oxidation in rodents with zymosan-induced inflammation[28].
We determined whether dosing with one and two geltab doses of a gamma tocopherol-rich preparation were associated with changes in serum 5-nitro-γ-tocopherol, and if in vitro treatment with αT, γT, αCEHC or γ CEHC modified radical generation by PMA activated PBMCs. We observed that both supplement doses were associated with decreased serum 5-nitro-γ-tocopherol levels. We interpret these findings as a general decreased in RNOS generation, with decreased nitrosative stress. We also found that all tested tocopherol species inhibited ROS generation by PMA stimulated PBMCs. Whether this is due to direct scavenging of radicals by tocopherol species, γT's anti-inflammatory activity via iNOS down-regulation, or inhibition of cell signaling, which would also likely decrease NADPH oxidase and other oxidant generation systems present in PBMCs, these findings indicate that γ-tocopherol has potent antioxidant and anti-nitrosative activity. It is important to note that other oxidation products of tocopherols also elicit biological effects, and these include tocopheryl quinones and nitrite esters. Key oxidation products of αT include α-tocopherylquinone (αTQ), 5, 6-epoxy-α-tocopherylquinone (αTQE1) and 2,3-epoxy-α-tocopherylquinone (αTQE2). [56] αTQH2 has been demonstrated to have strong anti-oxidant properties and may protect against lipid oxidation [57]. γ-tocopherylquinone (γ TQ), the quinone metabolite for γT, is associated with producing cytotoxic effects [58] that are most likely due to its ability to give rise to Michael adducts which result in endoplasmic reticulum stress [59]. In contrast to the negative cytotoxic effects, γTQ, particularly partially substituted ones, are also capable of functioning as beneficial biological antioxidants that can destroy multi-drug resistant cancer cells by inducing apoptosis [60]. Nitrite esters are nitrosating agents and can potentially induce certain types of cancers such as gastrointestinal cancer [61].
Given the effect of gamma tocopherol in inhibiting endotoxin induced inflammation in vitro and in vivo, as well as its effect on endotoxin induced responses of peripheral blood monocytes recovered from human volunteers after consumption of gamma tocopherol rich supplements, we propose to pursue studies examining the effect of gamma tocopherol on the response to inhaled endotoxin in normal and asthmatic volunteers. Endotoxin inhalation at doses that exceed 10,000 EU causes a neutrophilic inflammation in these volunteers[32], and low level endotoxin which does not induce changes directly in the lung, have been shown to enhance response to allergen in allergic asthmatics after nasal[32,53] and inhalational challenge [54]. We have recently examined the effect of low doses of inhaled endotoxin on resident airway inflammatory cells of normal volunteers and asthmatics and found increased expression of mCD14, CD80 and CD86 on airway monocytes and macrophages, suggesting that innate immune stimulation enhances antigen presenting cell function and ability to response to airway irritants, events which likely play a role in asthma exacerbation [33,55].
The results of the current study, coupled with our recent examination of the ability of gamma tocopherol to inhibit allergen and ozone induced eosinophilic airway inflammation in a rodent model of asthma, suggest that gamma tocopherol enriched preparations are an excellent candidate as a supplemental therapy to decreased asthma exacerbations and severity. Gamma tocopherol is a potent trap for reactive nitrogen species which is an important element of eosinophilic airway inflammation and is metabolized to the anti-inflammatory molecule γ-CEHC. To further determine the feasibility of using gamma tocopherol as an adjunct treatment for asthma, we are currently examining the effect of gamma tocopherol supplementation on allergen-induced inflammation following nasal allergen challenge in allergic volunteers. We have also initiated a double blinded placebo control crossover study of the effect of gamma tocopherol in decreasing inflammatory responses to inhaled endotoxin in both normal volunteers and asthmatics.
Acknowledgements
This publication was made possible by grant number P01AT002620 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM. Ms. Wiser was supported by the Doris Duke Foundation for a 1 year translational medicine research fellowship for medical students at the UNC Center for Environmental Medicine, Asthma and Lung Biology. The mixed tocopherol preparation used in this study was supplied as a gift by Andreas Papas, PhD of Yasoo Health, Inc. We also thank Fernando Dimeo, Jessica Spencer, Xinmin Yin and Helene Freiser for their technical assistance in this project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cross CE, Valacchi G, Schock B, Wilson M, Weber S, Eiserich J, van der Vliet A. Environmental Oxidant Pollutant Effects on Biologic Systems: A Focus on Micronutrient Antioxidant-Oxidant Interactions. Am. J. Respir. Crit. Care Med. 2002;166:44S–450. doi: 10.1164/rccm.2206015. [DOI] [PubMed] [Google Scholar]
- 2.Fogarty A, Lewis S, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 2000;356:1573–1574. doi: 10.1016/S0140-6736(00)03132-9. [DOI] [PubMed] [Google Scholar]
- 3.Devereux G, Turner SW, Craig LCA, McNeill G, Martindale S, Harbour PJ, Helms PJ, Seaton A. Low Maternal Vitamin E Intake during Pregnancy Is Associated with Asthma in 5-Year-Old Children. Am. J. Respir. Crit. Care Med. 2006;174:499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 4.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr, Weiss ST, Gillman MW, Gold DR. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–911. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch. Environ. Health. 2001;56:242–249. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- 6.Samet JM, Hatch GE, Horstman D, Steck-Scott S, Arab L, Bromberg PA, Levine M, McDonnell WF, Devlin RB. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am. J. Respir. Crit Care Med. 2001;164:819–825. doi: 10.1164/ajrccm.164.5.2008003. [DOI] [PubMed] [Google Scholar]
- 7.David GL, Romieu I, Sienra-Monge JJ, Collins WJ, Ramirez-Aguilar M, Rio-Navarro BE, Reyes-Ruiz NI, Morris RW, Marzec JM, London SJ. NAD(P)H: Quinone Oxidoreductase and Glutathione S-Transferase M1 Polymorphisms and Childhood Asthma. Am. J. Respir. Crit Care Med. 2003 doi: 10.1164/rccm.200305-684OC. [DOI] [PubMed] [Google Scholar]
- 8.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, Hernandez-Avila M, London SJ. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- 9.Kongerud J, Crissman K, Hatch G, Alexis N. Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhal. Toxicol. 2003;15:101–109. doi: 10.1080/08958370304477. [DOI] [PubMed] [Google Scholar]
- 10.Ahlrot-Westerlund B, Norrby A. Remarkable success of antioxidant treatment (selenomethionine and vitamin E) to a 34-year old patient with posterior subcapsular cataract, keratoconus, severe atopic eczema and asthma. Acta Ophthalmol. (Copenh) 1988;66:237–238. doi: 10.1111/j.1755-3768.1988.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 11.Pearson PJK, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur B, Rowe BH, Ram FS. Vitamin C supplementation for asthma. Cochrane. Database. Syst. Rev. 2001:CD000993. doi: 10.1002/14651858.CD000993. [DOI] [PubMed] [Google Scholar]
- 13.McDermott JH. Antioxidant nutrients: current dietary recommendations and research update. J. Am. Pharm. Assoc. (Wash. ) 2000;40:785–799. doi: 10.1016/s1086-5802(16)31126-3. [DOI] [PubMed] [Google Scholar]
- 14.Ovesen L. Vitamin therapy in the absence of obvious deficiency. What is the evidence? Drugs. 1984;27:148–170. doi: 10.2165/00003495-198427020-00003. [DOI] [PubMed] [Google Scholar]
- 15.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christen S, Jiang Q, Shigenaga MK, Ames BN. Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection. J. Lipid Res. 2002;43:1978–1985. doi: 10.1194/jlr.d200023-jlr200. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J. Lipid Res. 2007;48:1221–1230. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic. Biol. Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free Radic. Biol. Med. 2007;43:1176–1188. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaraj S, Traber MG. Gamma-tocopherol, the new vitamin E? Am. J. Clin. Nutr. 2003;77:530–531. doi: 10.1093/ajcn/77.3.530. [DOI] [PubMed] [Google Scholar]
- 24.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 25.Traber MG. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 26.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic. Biol. Med. 2005;38:857–866. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic. Biol. Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 30.Myrianthefs P, Karatzas S, Venetsanou K, Grouzi E, Evagelopoulou P, Boutzouka E, Fildissis G, Spiliotopoulou I, Baltopoulos G. Seasonal variation in whole blood cytokine production after LPS stimulation in normal individuals. Cytokine. 2003;24:286–292. doi: 10.1016/j.cyto.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Peden DB. Air pollution in asthma: effect of pollutants on airway inflammation. Ann. Allergy Asthma Immunol. 2001;87:12–17. doi: 10.1016/s1081-1206(10)62334-4. [DOI] [PubMed] [Google Scholar]
- 32.Alexis N, Eldridge M, Reed W, Bromberg P, Peden DB. CD14-dependent airway neutrophil response to inhaled LPS: role of atopy. J. Allergy Clin. Immunol. 2001;107:31–35. doi: 10.1067/mai.2001.111594. [DOI] [PubMed] [Google Scholar]
- 33.Alexis NE, Lay JC, Almond M, Bromberg PA, Patel DD, Peden DB. Acute LPS inhalation in healthy volunteers induces dendritic cell maturation in vivo. J Allergy Clin Immunol. 2005;115:345–350. doi: 10.1016/j.jaci.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 34.Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2003;112:353–361. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- 35.Alexis NE, Lay JC, Almond M, Peden DB. Inhalation of low-dose endotoxin favors local T(H)2 response and primes airway phagocytes in vivo. J Allergy Clin Immunol. 2004;114:1325–1331. doi: 10.1016/j.jaci.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Devereux G, Turner SW, Craig LCA, McNeill G, Martindale S, Harbour PJ, Helms PJ, Seaton A. Low Maternal Vitamin E Intake during Pregnancy Is Associated with Asthma in 5-Year-Old Children. Am. J. Respir. Crit. Care Med. 2006;174:499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. Health effects of air pollution. J Allergy Clin Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Kleeberger SR, Peden D. GENE-ENVIRONMENT INTERACTIONS IN ASTHMA AND OTHER RESPIRATORY DISEASES *. Annu. Rev. Med. 2005;56:383–400. doi: 10.1146/annurev.med.56.062904.144908. [DOI] [PubMed] [Google Scholar]
- 39.Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol. 2005;115:213–219. doi: 10.1016/j.jaci.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Bjorksten B. The environmental influence on childhood asthma. Allergy. 1999;54 Suppl 49:17–23. doi: 10.1111/j.1398-9995.1999.tb04383.x. [DOI] [PubMed] [Google Scholar]
- 41.Eisner MD. Environmental tobacco smoke and adult asthma. Clin. Chest Med. 2002;23:749–761. doi: 10.1016/s0272-5231(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 42.Eisner MD, Blanc PD. Environmental tobacco smoke exposure during travel among adults with asthma. Chest. 2002;122:826–828. doi: 10.1378/chest.122.3.826. [DOI] [PubMed] [Google Scholar]
- 43.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, Packer L. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am. J. Clin. Nutr. 2003;77:160–166. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 44.Bruno RS, Traber MG. Cigarette smoke alters human vitamin E requirements. J. Nutr. 2005;135:671–674. doi: 10.1093/jn/135.4.671. [DOI] [PubMed] [Google Scholar]
- 45.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 2006;40:689–697. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 46.Bruno RS, Traber MG. Vitamin E biokinetics, oxidative stress and cigarette smoking. Pathophysiology. 2006;13:143–149. doi: 10.1016/j.pathophys.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Leonard SW, Bruno RS, Paterson E, Schock BC, Atkinson J, Bray TM, Cross CE, Traber MG. 5-nitro-gamma-tocopherol increases in human plasma exposed to cigarette smoke in vitro and in vivo. Free Radic. Biol. Med. 2003;35:1560–1567. doi: 10.1016/j.freeradbiomed.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Baraldi E, Giordano G, Pasquale MF, Carraro S, Mardegan A, Bonetto G, Bastardo C, Zacchello F, Zanconato S. 3-Nitrotyrosine, a marker of nitrosative stress, is increased in breath condensate of allergic asthmatic children. Allergy. 2006;61:90–96. doi: 10.1111/j.1398-9995.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 49.Corradi M, Montuschi P, Donnelly LE, Pesci A, Kharitonov SA, Barnes PJ. Increased nitrosothiols in exhaled breath condensate in inflammatory airway diseases. Am J Respir Crit Care Med. 2001;163:854–858. doi: 10.1164/ajrccm.163.4.2001108. [DOI] [PubMed] [Google Scholar]
- 50.Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur. J. Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- 51.Kharitonov SA, Barnes PJ. Nitric oxide, nitrotyrosine, and nitric oxide modulators in asthma and chronic obstructive pulmonary disease. Curr. Allergy Asthma Rep. 2003;3:121–129. doi: 10.1007/s11882-003-0024-7. [DOI] [PubMed] [Google Scholar]
- 52.Kitz R, Rose MA, Borgmann A, Schubert R, Zielen S. Systemic and bronchial inflammation following LPS inhalation in asthmatic and healthy subjects. J. Endotoxin. Res. 2006;12:367–374. doi: 10.1179/096805106X153934. [DOI] [PubMed] [Google Scholar]
- 53.Eldridge MW, Peden DB. Allergen provocation augments endotoxin-induced nasal inflammation in subjects with atopic asthma. J. Allergy Clin. Immunol. 2000;105:475–481. doi: 10.1067/mai.2000.104552. [DOI] [PubMed] [Google Scholar]
- 54.Boehlecke B, Hazucha M, Alexis NE, Jacobs R, Reist P, Bromberg PA, Peden DB. Low-dose airborne endotoxin exposure enhances bronchial responsiveness to inhaled allergen in atopic asthmatics. J Allergy Clin Immunol. 2003;112:1241–1243. doi: 10.1016/j.jaci.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 55.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J. Allergy Clin. Immunol. 2006;117:1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 56.Liebler DC, Burr JA, Philips L, Ham AJ. Gas chromatography–mass spectrometry analysis of vitamin E and its oxidation products. Anal. Biochem. 1996;236:27–34. doi: 10.1006/abio.1996.0127. [DOI] [PubMed] [Google Scholar]
- 57.Neuzil J, Witting PK, Stocker R. Alpha-tocopheryl hydroquinone is an efficient multifunctional inhibitor of radical-initiated oxidation of low density lipoprotein lipids. Proc. Natl. Acad. Sci. USA. 1997;94:7885–7890. doi: 10.1073/pnas.94.15.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornwell DG, Williams MV, Wani AA, Wani G, Shen E, Jones KH. Mutagenicity of tocopheryl quinones: evolutionary advantage of selective accumulation of dietary alpha-tocopherol. Nutr. Cancer. 2002;43:111–118. doi: 10.1207/S15327914NC431_13. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Thomas B, Sachdeva R, Arterburn L, Frye L, Hatcher PG, Cornwell DG, Ma J. Mechanism of arylating quinone toxicity involving Michael adduct formation and induction of endoplasmic reticulum stress. Proc. Natl. Acad. Sci. USA. 2006;103:3604–3609. doi: 10.1073/pnas.0510962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thornton DE, Jones KH, Jiang Z, Zhang H, Liu G, Cornwell DG. Antioxidant and cytotoxic tocopheryl quinones in normal and cancer cells. Free Radic. Biol. Med. 1995;18:963–976. doi: 10.1016/0891-5849(94)00210-b. [DOI] [PubMed] [Google Scholar]
- 61.Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995 Jun 29;93(1):17–48. doi: 10.1016/0304-3835(95)03786-V. Review. Erratum in: Cancer Lett Nov 6;97(2):271; 1995. [DOI] [PubMed] [Google Scholar]


