Abstract
Aims
Renal failure is associated with aortic valve calcification (AVC). Our aim was to develop an animal model for exploring the pathophysiology and reversibility of AVC, utilizing rats with diet-induced kidney disease.
Methods and results
Sprague–Dawley rats (n = 23) were fed a phosphate-enriched, uraemia-inducing diet for 7 weeks followed by a normal diet for 2 weeks (‘diet group’). These rats were compared with normal controls (n = 10) and with uraemic controls fed with phosphate-depleted diet (‘low-phosphate group’, n = 10). Clinical investigations included serum creatinine, phosphate and parathyroid hormone (PTH) levels, echocardiography, and multislice computed tomography. Pathological examinations of the valves included histological characterization, Von Kossa staining, and antigen and gene expression analyses. Eight diet group rats were further assessed for reversibility of valve calcification following normalization of their kidney function. At 4 weeks, all diet group rats developed renal failure and hyperparathyroidism. At week 9, renal failure resolved with improvement in the hyperparathyroid state. Echocardiography demonstrated valve calcifications only in diet group rats. Tomographic calcium scores were significantly higher in the diet group compared with controls. Von Kossa stain in diet group valves revealed calcium deposits, positive staining for osteopontin, and CD68. Gene expression analyses revealed overexpression of osteoblast genes and nuclear factor κB activation. Valve calcification resolved after diet cessation in parallel with normalization of PTH levels. Resolution was associated with down-regulation of inflammation and osteoblastic features. Low-phosphate group rats developed kidney dysfunction similar to that of the diet group but with normal levels of PTH. Calcium scores and histology showed only minimal valve calcification.
Conclusion
We developed an animal model for AVC. The process is related to disturbed mineral metabolism. It is associated with inflammation and osteoblastic features. Furthermore, the process is reversible upon normalization of the mineral homeostasis. Thus, our model constitutes a convenient platform for studying AVC and potential remedies.
KEYWORDS: Aortic valve, Calcification, Renal failure, Reversibility, NFκB pathway
1. Introduction
Aortic valve disease is common in the elderly, accounting for approximately 70% of all valve diseases. The two hallmarks of aortic stenosis (AS) are aortic valve calcification (AVC) and regional valve thickening. AVC exists in 25% of the population who are 65 years old and above, and in 50% who are above the age of 85. Ultimately, about 10% of these patients will develop severe calcification and significant AS.1 In the past, AVC was considered a degenerative condition, caused by tissue necrosis and calcium precipitation. However, recent data suggest that calcification is an active process, involving osteoblast transformation in valve tissue. This results in increased formation of bone matrix.2,3 Furthermore, osteoblast differentiation is mediated by an inflammatory process that includes accumulation of activated T cells and macrophages in aortic valve lesions, together with several cytokines (transforming growth factor-β, tumour necrosis factor-α, and others).4,5
Several risk factors are currently known to be associated with AVC including age, male gender, hypertension, elevated LDL-cholesterol, and metabolic conditions such as Paget's disease and hyperparathyroidism. In addition, anatomic changes that might interfere with normal flow (e.g. bicuspid valve) are also significant risk factors for AVC.6 Once AVC has occurred, it is considered an irreversible process and efforts were made in order to slowdown its progression; recently rosuvastatin was found to be an effective treatment in slowing AS progression.7
One of the most significant risk factors for AVC is renal failure; approximately 40% of the patients who suffer from end-stage renal disease are likely to develop AVC. Renal failure induces an elevation of phosphate levels along with development of secondary hyperparathyroidism. These metabolic changes (especially hyperparathyroidism) result in diffuse calcification and formation of hydroxyapatite crystals in several tissues, including the aortic valve.6
Parathyroid hormone (PTH) is the most important regulator of calcium and phosphate metabolism. It is essential for both bone formation and osteoblast activity, and increases the conversion of vitamin D to its active metabolite.8 Vitamin D receptor genotype polymorphism is associated with increased prevalence of calcific AS, although the exact role of vitamin D in AVC is still unknown.9
As AVC is a multifactorial condition, it is essential to develop a simple animal model that may shed more light on this pathophysiological process. Initial models were based on inflicting mechanical injury to the valve during surgery. These models were complicated and had high morbidity and mortality rates. Recently, several diets were shown to cause AVC. These diets were based on high cholesterol, alone or in combination with high-dose vitamin D,10 or high fat and carbohydrate diets.11 These models were successful in causing aortic valve disease, but none of them was able to explore the reversibility of the process. As renal failure is a major risk factor of AVC, our aim was to develop a simple model based on this observation.
A diet containing high levels of adenine and phosphate has been shown to cause polyuric renal failure in rats. Adenine accumulates in the renal proximal tubules which within weeks lead to elevation of serum creatinine, phosphate, and PTH.12 Phosphate is the major regulator of PTH; phosphate enrichment augments the magnitude of hyperparathyroidism while dietary phosphate restriction ameliorates it.13 We thus used high-adenine, high-phosphate diet in order to induce renal failure and AVC, and to better characterize the processes involving AVC. We also evaluated the reversibility of AVC after diet cessation and renal failure resolution. Modification of the diet regimen into high-adenine but low-phosphate was performed in order to induce renal failure accompanied by low PTH level. This modification was made in order to assess the role of PTH in AVC.
2. Methods
2.1. Animals
Forty-three male Sprague–Dawley rats, 8 weeks old, each weighing about 250 g were used for the study. The protocol was approved by the Hebrew University Ethics Committee. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
2.2. Study design
2.2.1. Diet and control groups
Thirty-three rats were divided into two groups: diet group (n = 23) and a control group (n = 10). The diet group rats were fed exclusively with high-adenine (0.75%), high-phosphate diet (1.5%) (Teklad, Madison, WI, USA) for 7 weeks, after which they were fed with normal rat chow for an additional 2 weeks. The control group daily received normal rat chow for 9 weeks. At 4 weeks, rats from the diet group were anaesthetized with ketamine/xylazine and a 1 cm3 blood sample was collected from the tail vein. After 9 weeks, all rats were anaesthetized, an echocardiogram and multislice computed tomography (MSCT) scan were performed. After the procedure, 15 rats from the diet group and all the rats from the control group were sacrificed by exsanguinations after blood sample was collected from abdominal aorta. Aortic valve tissue was excised, snap frozen in liquid nitrogen, and kept at −80°C.
Additional eight diet group rats (the reversibility subgroup) were kept alive for an additional 10 weeks, and fed with normal rat chow. MSCT scan was performed after 4 weeks and repeated after the next 6 weeks. These rats were then sacrificed, a blood sample was collected from the abdominal aorta, and aortic valve tissue was excised. Reversibility of AVC was assessed by histological examination and by comparing serial calcium scoring of each rat being its own control.
2.2.2. Low-phosphate group
In order to explore the role of renal failure and hyperparathyroidism in AVC, 10 rats were fed exclusively with high-adenine (0.75%) but low-phosphate (0.3%) diet (low-phosphate group) (Teklad) for 7 weeks, after which they were fed with normal rat chow for an additional 2 weeks. At 4 weeks, 1 cm3 blood sample was collected from the tail vein. After 9 weeks, the rats were anaesthetized and MSCT scan was performed. MSCT results and histology were compared with the diet group.
2.3. Evaluation of diet on biochemical profile
Plasma was analysed for potassium, phosphate, alkaline phosphate, creatinine, and total cholesterol using VITRO system 5.1 chemistry (Ortho-Clinical Diagnostics, Johnson & Johnson, Rochester, NY). Plasma PTH was measured by an enzyme immunoassay (Immutopics, San Clemente, CA, USA).
2.4. Echocardiography
Two-dimensional and colour Doppler echocardiography studies were performed at 9 weeks by a skilled operator blinded to the study groups using one of the following machines: a VIVID-I machine (GE Healthcare/Tirat Hacarmel, Israel) equipped with a 13 MHz linear array transducer or a Vevo 770 system (VisualSonics, Toronto, Canada) equipped with a 35 MHz linear transducer. Parasternal long-axis view and high short-axis view were used to evaluate aortic valve morphology.
2.5. Tissue analysis
Aortic valve was dissected, fixed in formalin, and embedded in paraffin. Serial cross-sections of the valve were stained using haematoxylin & eosin and with Von-Kossa stains in order to assess the structure and calcium deposits.
2.6. Immunohistochemistry studies
Formalin-fixed aortic valve tissue at 5 mm cross-sections were used for immunohistochemistry studies. The sections were incubated overnight with anti-osteopontin and anti-CD68. After phosphate buffer saline wash, the sections were incubated with goat anti-rabbit (1:200) secondary antibody conjugated with Cy5 (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) for 1 h.
2.7. Computed tomography scan
A 64-slice chest MSCT scan without contrast was performed on all rats (Brilliance, Philips Medical Systems, Groningen, The Netherlands). Study parameters were: 120 kVp, 300 mAs, slice thickness 0.67 mm, increment 0.3 mm. The scan was analysed by an operator blinded to the study groups, on an off-line CT workstation. The Agatston score was calculated by multiplying the area of a calcified lesion restricted only to aortic valve area, with a weighted CT attenuation score dependent on the maximal CT attenuation (HU) within a lesion as previously described.14
2.8. Western blot analysis
Polyclonal antibodies to osteopontin and β-actin and their secondary antibodies (Santa Cruz, CA, USA) were used according to standard procedures.15 Briefly, the tissue was hydrolyzed and homogenized under ultrasound and boiled for 5 min. After quantification of the protein concentration using the Bradford method, 12 mg of extracts were separated on 5–15% sodium dodecyl sulfate-polyacrylamide gradient gels, and transferred to nitrocellulose membranes using a Hoeffer transfer chamber. The membranes were blocked with either 0.5% donkey serum or 2% skim milk, depending on the antibody. Bands were detected after incubation with western blotting Luminol reagent (Santa Cruz Biotechnology Inc.). Osteopontin protein expression was normalized to β-actin expression. The bands on the X-ray film were quantified by scanning densitometry (ImageJ version 1.34, NIH)16 and expressed as percentage of the control.
2.9. Reverse transcriptase-polymerase chain reaction
Transcription of osteopontin, osteocalcin, receptor activator of nuclear factor κB (NFκB), ligand (RANKL), Runx-2, and actin genes were analysed by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) using rat aortic valve tissue.
2.10. Statistical analysis
Data are presented as mean ± SEM. Statistical differences between the diet group and control group rats, and between the diet group and the low-phosphate group rats were calculated using the analysis of variance, followed by the Student Neumann-Keuls test. Serial calcium scores calculated for reversibility assessment were compared using dependent t-test for matched variables. All P-values were two-tailed. P-value < 0.05 was considered significant.
3. Results
3.1. Electrolytes, kidney function, and parathyroid hormone levels
At 4 weeks, all rats in the diet group demonstrated a significant elevation in creatinine and phosphate levels, reflecting renal failure. In addition, severe hyperparathyroidism developed in the diet group. There were no significant differences in potassium, alkaline phosphate, or total cholesterol levels. At 9 weeks (2 weeks after cessation of the adenine diet), both renal failure and hyperphosphataemia had completely resolved, although a moderate increase in the PTH level remained in the diet group.
In the reversibility subgroup, creatinine and PTH levels returned to normal after 19 weeks.
The low-phosphate group developed significant elevation in creatinine reflecting the same degree of renal failure as the diet group. PTH and phosphate levels were similar to the controls and significantly lower than the diet group (Table 1).
Table 1.
Biochemical profiles of the study groups
| Diet (high phosphate) |
Control | Low phosphate | |||
|---|---|---|---|---|---|
| Week 4 (n = 15) | Week 9 (n = 15) | Week 19 (n = 8)‡ | Week 9 (n = 10) | Week 4 (n = 10) | |
| Creatinine (mmol/L) | 213 ± 3.78* | 90 ± 1.47 | 80 ± 2.7 | 70 ± 3.6 | 207 ± 2.58 |
| Phosphate (mmol/L) | 4.20 ± 0.06* | 2.62 ± 0.03 | 1.6 ± 0.02 | 2.5 ± 0.03 | 2.2 ± 0.06** |
| PTH (pg/mL) | 2800 ± 40† | 750 ± 123† | 414 ± 37 | 281 ± 53 | 210 ± 40** |
| Total cholesterol (mmol/L) | 3.14 ± 0.01 | 2.83 ± 0.03 | — | 2.1 ± 0.04 | — |
| Alkaline phosphatase (U/L) | 189 ± 6.33 | 173 ± 2.33 | — | 189 ± 1.2 | — |
| Potassium (mmol/L) | 5.92 ± 0.02 | 4.93 ± 0.07 | — | 5.8 ± 0.09 | — |
Biochemical profile obtained from three groups. In the diet group: at 4 weeks (during the adenine diet), at 9 weeks (2 weeks after cessation of diet), and at 19 weeks (the reversibility subgroup). In the low-phosphate group at 4 weeks and the control group at 9 weeks. At 4 weeks there was significant increase in creatinine, phosphate, and parathyroid hormone (PTH) levels in the diet group blood compared with the control; creatinine was similar to the low-phosphate group, while the phosphate and PTH were significantly higher. After 9 weeks, creatinine and phosphate level in the diet group decreased, while the PTH levels were still significantly higher than the controls. After 19 weeks, creatinine, phosphate, and PTH levels were normalized.
*P < 0.05 for the comparison of diet group with the control group.
†P < 0.01 for the comparison of diet group with the control group.
**P < 0.05 for the comparison of diet group with the low-phosphate group.
‡The reversibility subgroup.
3.2. Echocardiography
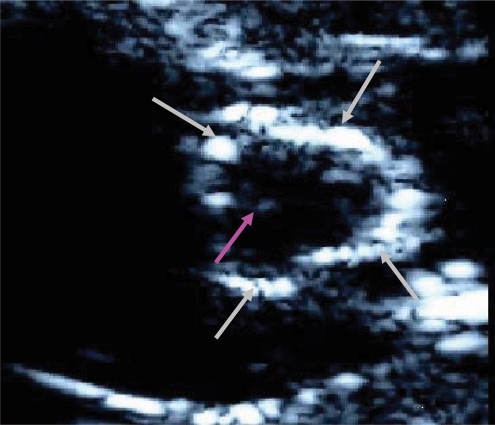
Echocardiography showed that rats in the diet group (n = 10) developed thickening and calcification of the aortic valve (Figure 1). In nine animals of the diet group, the calcification was diffuse and involved all parts of the valve, including the annulus and the leaflets. Calcification was not found in any of the control and reversibility group's rats.
Figure 1.
Echocardiogram of a diet group rat. Echocardiogram of one of the diet group rats in high short-axis view. Aortic valve calcification involves the annulus (grey arrows) and the commisures (purple arrow).
3.3. Multislice computed tomography scan
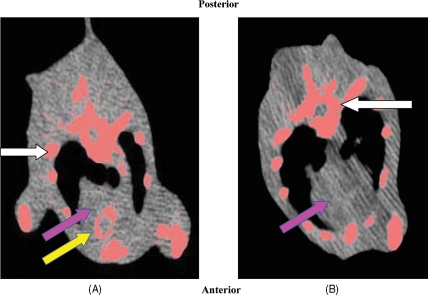
AVC was found in all diet group rats (Figure 2A). In contrast, none of the animals in the control group had calcium deposits (Figure 2B). Calcium scores were calculated using the Agatston score; all diet group animals developed calcification with a mean Agatston score significantly higher when compared with control group (145 ± 118 vs. 0, P < 0.01). The mean Agatston score of the low-phosphate group valves was an order of magnitude lower when compared with the diet group (14 ± 2 vs. 145 ± 118, P < 0.01).
Figure 2.
Chest multislice computed tomography (MSCT) of the diet and control group rats. Chest MSCT a diet group rat (A) and a control group rat (B) showing the heart (purple arrow) and bony structures (white arrows). Calcified tissue: ribs and vertebrae are stained in pink. Calcium aggregates are demonstrated in aortic valve annulus (yellow arrow). No calcification is observed in the control.
3.4. Aortic valve calcification reversibility using multislice computed tomography scan
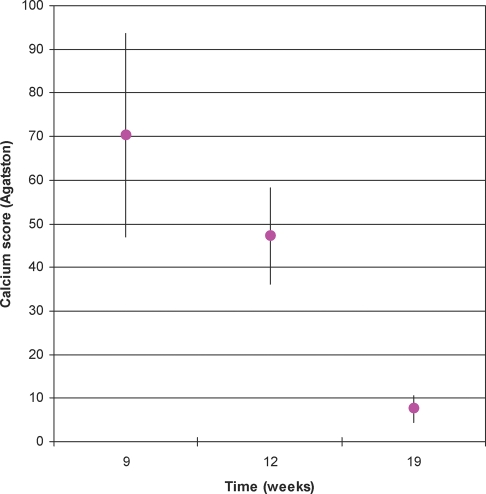
To assess the reversibility of AVC, eight diet group rats were followed using serial MSCT scans. Although three of the rats showed an increase in calcium score at 13 weeks, the overall valve calcification was reduced at 13 weeks when compared with 9 weeks (42 ± 11 vs. 70 ± 23; P = 0.19). At 19 weeks, all the rats showed a significant and impressive decrease in calcium score when compared with the scoring of the ninth week (7 ± 2.8 vs. 70 ± 23; P < 0.05) (Figure 3).
Figure 3.
Average calcium score at 9, 13, and 19 weeks. Serial average calcium scores (mean ± SEM) for reversibility evaluation after 9, 13, and 19 weeks. All rats' calcium score were significantly lowered at the end of the period. After 13 weeks, calcium score was reduced when compared with 9 weeks. A significant impressive reduction in calcium score occurred at 19 weeks.
3.5. Histopathology, Von Kossa, osteopontin, and CD68 staining
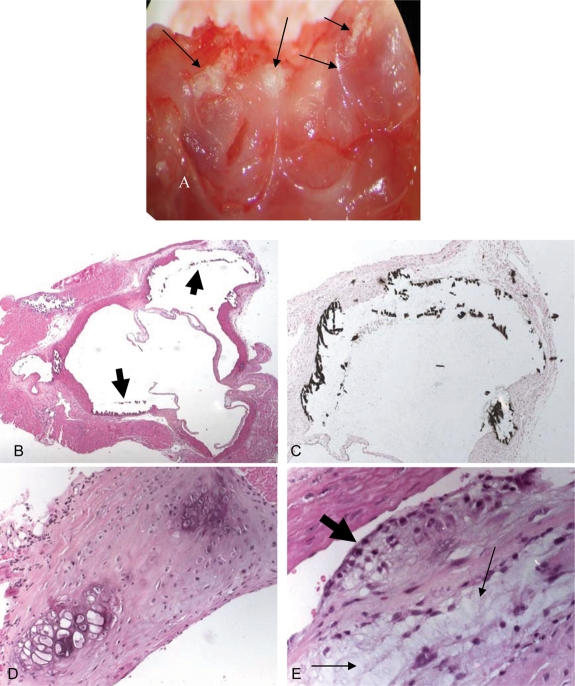
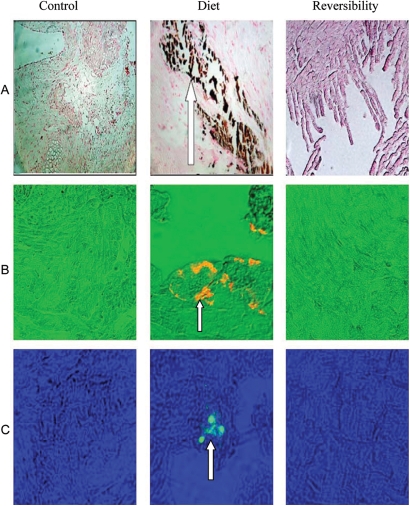
Tissues obtained from the aortic valves of the diet group rats revealed cartilaginous metaplasia of aortic valve annulus and focal subendothelial macrophage aggregates accompanied by myxoid degeneration of leaflets. Von Kossa stain demonstrated calcium precipitate involving the valve annulus (Figure 4). Calcium precipitation reflecting AVC was diffuse, and resolved after diet cessation (Figure 5A). Osteopontin and CD68 stains were positive only in valves obtained from the diet group, reflecting reversible osteoblast features and macrophages accumulation in aortic valve tissue (Figure 5B and C, respectively).
Figure 4.
Aortic valve calcification in diet group rat. (A) En-face view of open aortic valve showing diffuse calcification of valve's annulus and commisures (black arrows). (B) Cross-section through the aortic sinus showing coarse calcification of aortic valve annulus (arrows) with normal appearing leaflets (haematoxylin & eosin, original magnification ×25). (C) Higher magnification of calcified annulus (Von Kossa, original magnification ×50). (D) Focal cartilaginous metaplasia of aortic valve annulus (haematoxylin & eosin, original magnification ×200). (E) Focal subendothelial macrophage aggregate (large arrow) and myxoid degeneration of leaflet (small arrow) (haematoxylin & eosin, original magnification ×400).
Figure 5.
Von Kossa stain, immunohistochemistry staining for osteopontin and anti-CD68 stains in diet, control, and reversibility group valves. Von Kossa stain (A) for calcium aggregates (brown) demonstrates calcification only in diet group (white arrow). Staining for osteopontin (B) in orange (white arrow) is expressed only in diet group. Staining using anti-CD68 antibody (C) for macrophages (in green) and marked with white arrow is demonstrated only in diet group.
Tissues obtained from low-phosphate group's valves showed no significant calcification and both osteopontin and CD68 stains were negative (data not shown).
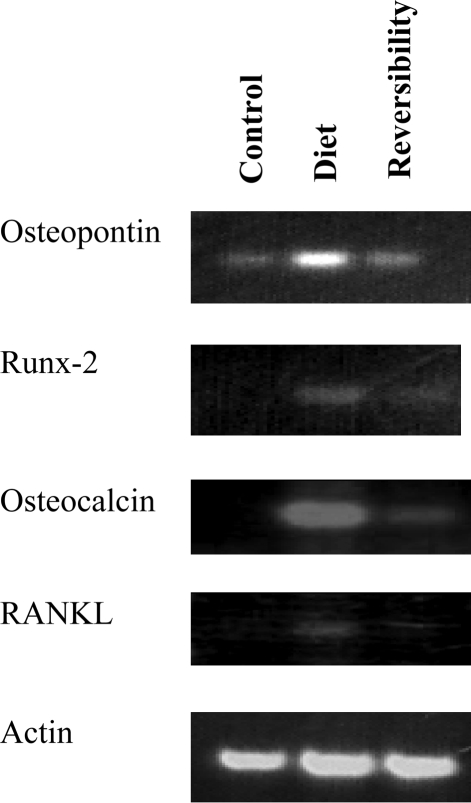
3.6. Reverse transcriptase-polymerase chain reaction for osteoblast markers and for RANKL
There was a significant increase of osteopontin, osteocalcin, the transcription factor Runx-2, and RANKL RNA levels in the diet group. These changes reflect expression of osteoblast markers and activation of NFκB family members in the valve tissue. Valves obtained from the reversibility subgroup showed downregulation of osteoblast markers expression and NFκB pathway (Figure 6).
Figure 6.
Reverse transcriptase-polymerase chain reaction (RT-PCR) for bone markers in diet, control, and reversibility group valves. RT-PCR for osteopontin, Runx-2, osteocalcin, and RANKL obtained from valves in all groups, demonstrating a significant up-regulation in expression of all four mRNA in the diet group valve tissue compared with control. There is downregulation of all four mRNA at the valves obtained from the reversibility subgroup.
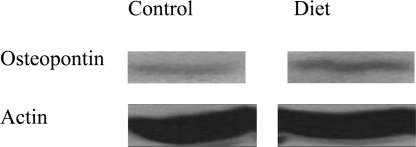
3.7. Western blot for osteopontin
There was a significant increase in osteopontin protein level in the diet group valves compared with control valves (Figure 7). Image software used to quantify the difference revealed a 50% increase of osteopontin expression in diet group valves.
Figure 7.
Western blot of osteopontin in diet and control group valves. Western blot of osteopontin obtained from both diet and control group valve tissues demonstrate a significant increase in osteopontin protein level in the diet group valves.
4. Discussion
AVC is a central component in the pathology of AS. However, the pathogenesis of the process is not clearly understood. Recent data suggest that it is mediated by cellular changes coupled with active inflammatory processes. As understanding the pathogenesis of AVC is crucial for finding efficient treatment for AS, many efforts have been directed to develop animal models for this disease.
We developed a unique diet-induced animal model for AVC, based on renal failure and secondary hyperparathyroidism. The diet contains high level of adenine that precipitates in the renal tubules, and forms 2,8-dihydroxyadenine aggregates. The tubular insult results in polyuric renal failure and secondary hyperparathyroidism that develops within weeks. Two weeks after diet cessation, the rats had normal kidney function, but elevated PTH and significant AVC. Both echocardiography and histological evaluation demonstrated calcium aggregates in all parts of the valve. In addition, we were able to quantify the calcium content using MSCT, which is an emerging tool for assessing valve calcification.17 We were able to show that AVC is an active inflammatory process, which involves the activation of the NFκB system, macrophages accumulation, and osteoblast phenotype in valve tissue. In addition, the process is reversible after PTH levels return to normal range. The reversibility was demonstrated using imaging modalities and confirmed by histology. Inflammation and osteoblast's features were resolved along with the decalcification process. We showed that calcification was minimal in the low-phosphate group; therefore the process is related to elevated PTH rather than uraemia.
Previous diet-induced AVC animal models were all based on various components of the metabolic syndrome: hyperlipidaemia, hypertension, and hypercholesterolaemia, thus emphasizing the role of atherogenesis in AVC.10,11,18 Using one of those models, Rajamannan et al. were the first to demonstrate upregulation of osteoblast markers and bone matrix production.2 Furthermore, they showed that aortic valve osteoblast-like phenotype is inhibited by statins.19 Several animal models based on renal failure have previously been reported, and some of them were used for the study of vascular calcification.20 Initial models consisted of surgical nephrectomy, and others were based on renal ischaemia, toxins, and sepsis induction. Using those models, recent studies showed that vascular calcification is a regulated process, and that several metabolic changes like serum phosphate induce osteoblast differentiation of vascular smooth muscle cells.21 Nevertheless, in none of these models was renal failure reversible, and none was used to specifically study aortic valve disease.
Primary and secondary hyperparathyroidism (the latter prevalent in kidney diseases) have been shown to induce both AVC and AS.22,23 In patients undergoing dialysis, AVC and AS are frequently found; furthermore, those conditions progress more rapidly in these patients.24 The exact mechanism of this phenomenon is not clearly understood, but may be related to the metabolic changes induced by hyperparathyroidism. In addition, lack of calcification inhibitors may also be important, as dialysis patients who develop vascular calcification were found to have low levels of serum fetuin A. It was suggested that fetuin A is a circulating inhibitor of mineralization, which was able to inhibit mineralization of vascular smooth muscle cells in vitro.25
Our results suggest that when PTH levels drop after diet cessation and the resolution of renal failure, the calcification process is reversed. We evaluated the effect of renal failure of AVC itself by inducing renal failure with low PTH level using high-adenine, low-phosphate diet. Interestingly, we showed that although severe renal failure was achieved, AVC was minimal. We suggest that the metabolic and electrolytes changes induced by PTH rather than renal failure itself are the major mediators of AVC pathophysiology. These findings, together with the fact that patients who suffer from primary hyperparathyroidism have increased risk for AVC and AS, suggest that PTH plays a major role in AVC.26
Activation of PTH receptor induces several osteoblast transcription factors (e.g. Runx-2) and proteins (e.g. osteopontin) that stimulate osteoblast maturation and calcification.27 Runx-2 is crucial in the differentiation of mesenchymal cells to an osteoblastic phenotype,28 a process that may contribute to AVC. Osteopontin and osteocalcin are the most abundant glycoproteins produced by osteoblasts, which compose the organic part of the bone and are essential for calcification.29 In our model, we demonstrated elevated osteopontin, osteocalcin, and Runx-2 levels in the diet group, reflecting the aortic valve's osteoblast features. The lack of osteoblast features in the reversibility subgroup's valves suggests that the process is not permanent and requires continuous activation by PTH.
In addition to osteoblast and osteoclast activation, PTH has immune modulatory and pro-inflammatory roles that were demonstrated in various tissues. These effects include induction of cytokine secretion and inflammatory cell recruitment.30,31 Studies in dialysis patients showed significant correlation between PTH level, valve calcification, and inflammatory markers.32 Our results suggest that PTH has two major effects. The first is a direct effect on osteoblast and osteoclast, leading to osteoblast maturation and bone formation. The second is activation of the inflammatory process that plays an important role in AVC pathogenesis.
Another role of PTH is the activation of vitamin D (1,25-OH2D3), which plays an important role in calcium and phosphate metabolism, and also has important immune-regulatory effects. It was suggested that vitamin D may suppress several autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel diseases.33 The precise effect of vitamin D on AVC is still unknown, although it has been shown that polymorphism of vitamin D receptor is associated with higher prevalence of AVC.9
The molecular basis of PTH-induced calcification was studied in various tissues, and several cellular pathways, especially the tumour necrosis factor family pathway.34 PTH is a major activator of the RANK/RANK–ligand complex that is part of the NFκB pathway;35 the exact role of the complex in the calcification process is not clear. It was shown to trigger osteoblast and osteoclast differentiation, and a recent study showed high expression of RANKL in human calcified aortic valves.36 In our model, the calcification is involved in increased levels of RANKL, which may express the activation of the NFκB pathway.
Thus, PTH induces calcification through several mechanisms, including direct osteoblast activation, induction of vitamin D metabolism, and indirectly through its pro-inflammatory activity. Nevertheless, the exact mechanism of PTH effect requires further studies.
Interestingly, adenine derivate [adenine triphosphate (ATP)] and other nucleotides have the ability to regulate in vitro the osteoblast and osteoclast functions. ATP effect is mediated through purinergic receptors (P2 receptor) that activate intracellular pathways of osteoclast and osteoblast cells.37 A recent study conducted in aortic valve myofibroblasts showed that ATP (through P2 receptors) activates the NFκB system, induces the formation of the RANK/RANK–ligand complex and consequently enhancing AVC.38 Accordingly, it was suggested that nucleotides, released at sites of inflammation or in response to mechanical injury may regulate osteoclast formation and activity and induce calcification. This may explain why both the inflammatory process and turbulent flow occurring in deformed valves may accelerate AVC. Thus, we suggest that in addition to the role of adenine in causing renal failure, it may also have a direct effect of enhancing osteoclast and osteoblast activity and inducing calcification. The direct effect of adenine may explain the mild AVC observed in the low-phosphate group.
In conclusion, we demonstrated that adenine- and phosphate-enriched diet serves as a unique model for AVC as it simulates typical pathological changes: osteoblast transformation, expression of bone-specific proteins, and accumulation of macrophages as part of an inflammatory process. The model is simple: it does not require genetically modified animals, and AVC is achieved in short duration, facilitating long-term follow-up. We are the first to show that AVC is a dynamic and a reversible process, our model enables continuous assessment of the molecular and inflammatory processes involving AVC. Furthermore, better understanding of the molecular cascade of AVC and its reversibility might enable the development of new treatment options, by modulating key points along the calcification cascade. This model may allow precise evaluation of these future treatment modalities using calcification quantification by imaging.
Funding
The Chief Scientist Office of the Ministry of Health, Israel, the Berman Foundation for Cardiovascular Research of Hadassah Medical Center, the Joint Research Fund of the Hebrew University and Hadassah Medical Center.
Acknowledgements
The authors are grateful to Prof. Zvi Bar-Shavit for his assistance.
Conflict of interest: none declared.
References
- 1.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 2.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 4.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kiliç R, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Ryden M, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 6.Faggiano P, Antonini-Canterin F, Baldessin F, Lorusso R, D'Aloia A, Cas LD. Epidemiology and cardiovascular risk factors of aortic stenosis. Cardiovasc Ultrasound. 2006;4:27. doi: 10.1186/1476-7120-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge NC, Li X, Qin L. Understanding parathyroid hormone action. Ann NY Acad Sci. 2006;1068:187–193. doi: 10.1196/annals.1346.024. [DOI] [PubMed] [Google Scholar]
- 9.Ortlepp JR, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P. The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. Heart. 2001;85:635–638. doi: 10.1136/heart.85.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. J Am Coll Cardiol. 2003;41:1211–1217. doi: 10.1016/s0735-1097(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 11.Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J Am Coll Cardiol. 2006;47:850–855. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Levi R, Ben-Dov IZ, Lavi-Moshayoff V, Dinur M, Martin D, Naveh-Many T, et al. Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: correlation with posttranslational modification of the transacting factor AUF1. J Am Soc Nephrol. 2006;17:107–112. doi: 10.1681/ASN.2005070679. [DOI] [PubMed] [Google Scholar]
- 13.Slatopolsky R, Finch J, Denga M, Ritter C, Zhong M, Dusso A, et al. Phosphate restriction prevents parathyroid cell growth in uremic rats. High phosphate directly stimulates PTH secretion in vitro. J Clin invest. 1995;96:327–333. doi: 10.1172/JCI118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Catty D. Antibodies: a practical approach. In: Rickwood HB, editor. Practical Approach Series. Vol. 1. Oxford, MA, USA: IRL Press; 1988. [Google Scholar]
- 16.ImageJ: Image processing and analysis in Java. Provider: NIH Edition: 1.29. [Google Scholar]
- 17.Feuchtner GM, Muller S, Grander W, Alber HF, Bartel T, Friedrich GJ, et al. Aortic valve calcification as quantified with multislice computed tomography predicts short-term clinical outcome in patients with asymptomatic aortic stenosis. J Heart Valve Dis. 2006;15:494–498. [PubMed] [Google Scholar]
- 18.Cuniberti LA, Stutzbach PG, Guevara E, Yannarelli GG, Laguens RP, Favaloro RR. Development of mild aortic valve stenosis in a rabbit model of hypertension. J Am Coll Cardiol. 2006;47:2303–2309. doi: 10.1016/j.jacc.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 19.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, et al. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persy V, Postnov A, Neven E, Dams G, De Broe M, D'Haese P, et al. High-resolution X-ray microtomography is a sensitive method to detect vascular calcification in living rats with chronic renal failure. Arterioscler Thromb Vasc Biol. 2006;26:2110–2116. doi: 10.1161/01.ATV.0000236200.02726.f7. [DOI] [PubMed] [Google Scholar]
- 21.Ketteler M, Giachelli C. Novel insights into vascular calcification. Kidney Int Suppl. 2006;105:S5–S9. doi: 10.1038/sj.ki.5001996. [DOI] [PubMed] [Google Scholar]
- 22.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the ADQI Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malergue MC, Urena P, Prieur P, Guedon-Rapoud C, Petrover M. Incidence and development of aortic stenosis in chronic hemodialysis. An ultrasonographic and biological study of 112 patients. Arch Mal Coeur Vaiss. 1997;90:1595–1601. [PubMed] [Google Scholar]
- 24.Kume T, Kawamoto T, Akasaka T, Watanabe N, Toyota E, Neishi Y, et al. Rate of progression of valvular aortic stenosis in patients undergoing dialysis. J Am Soc Echocardiogr. 2006;19:914–918. doi: 10.1016/j.echo.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Chen NX, Moe SM. Uremic vascular calcification. J Invest Med. 2006;54:380–384. doi: 10.2310/6650.2006.06017. [DOI] [PubMed] [Google Scholar]
- 26.Niederle B, Stefenelli T, Glogar D, Woloszczuk W, Roka R, Mayr H. Cardiac calcific deposits in patients with primary hyperparathyroidism: preliminary results of a prospective echocardiographic study. Surgery. 1990;108:1052–1057. [PubMed] [Google Scholar]
- 27.Malluche HH, Koszewski N, Monier-Faugere MC, Williams JP, Mawad H. Influence of the parathyroid glands on bone metabolism. Eur J Clin Invest. 2006;36:23–33. doi: 10.1111/j.1365-2362.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- 28.Lian JB, Stein GS. Runx2/Cbfa1: a multifunctional regulator of bone formation. Curr Pharm Des. 2003;9:2677–2685. doi: 10.2174/1381612033453659. [DOI] [PubMed] [Google Scholar]
- 29.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 30.Griveas I, Visvardis G, Papadopoulou D, Mitsopoulos E, Kyriklidou P, Manou E, et al. Cellular immunity and levels of parathyroid hormone in uremic patients receiving hemodialysis. Renal Fail. 2005;27:275–278. [PubMed] [Google Scholar]
- 31.Angelini D, Carlini A, Giusti R, Grassi R, Mei E, Fiorini I, et al. Parathyroid hormone and T-cellular immunity in uremic patients in replacement dialytic therapy. Artif Organs. 1993;17:73–75. doi: 10.1111/j.1525-1594.1993.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang A, Woo J, Wang M, Sea MM, Ip R, Li PK, et al. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol. 2001;12:1927–1936. doi: 10.1681/ASN.V1291927. [DOI] [PubMed] [Google Scholar]
- 33.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2985. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 34.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–253. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 35.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, et al. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 36.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoç A, Kiliç R, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Buckley KA, Hipskind RA, Gartland A, Bowler WB, Gallagher JA. Adenosine triphosphate stimulates human osteoclast activity via up-regulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone. 2002;31:582–590. doi: 10.1016/s8756-3282(02)00877-3. [DOI] [PubMed] [Google Scholar]
- 38.Osman L, Chester AH, Amrani M, Yacoub MH, Smolenski RT. A novel role of extracellular nucleotides in valve calcification: a potential target for atorvastatin. Circulation. 2006;114:I566–I572. doi: 10.1161/CIRCULATIONAHA.105.001214. [DOI] [PubMed] [Google Scholar]