Abstract
In the past few years, inflammation has emerged as a major driving force of atherosclerotic lesion development. It is now well-established that from early lesion to vulnerable plaque formation, numerous cellular and molecular inflammatory components participate in the disease process. The most prominent cells that invade in evolving lesions are monocyte-derived macrophages and T-lymphocytes. Both cell types produce a wide array of soluble inflammatory mediators (cytokines, chemokines) which are critically important in the initiation and perpetuation of the disease. This review summarizes the currently available information from mouse studies on the contribution of a specified group of cytokines expressed in atherosclerotic lesions, viz. interleukins (IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12, IL-18, IL-20) and macrophage-associated cytokines [tumour necrosis factor-α (TNF-α); macrophage migration inhibitory factor (MIF); interferon-γ (IFN-γ); colony stimulating factors G-CSF,-M-CSF,-GM-CSF) to atherogenesis. Emphasis is put on the consistency of the effects of these cytokines, i.e. inasmuch an effect depends on the experimental approach applied (overexpression/deletion, strain, gender, dietary conditions, and disease stage). An important outcome of this survey is (i) that only for a few cytokines there is sufficient consistent data allowing classifying them as typically proatherogenic (IL-1, IL-12, IL-18, MIF, IFN-γ, TNF-α, and M-CSF) or antiatherogenic (IL-10) and (ii) that some cytokines (IL-4, IL-6 and GM-CSF) can exert pro- or anti-atherogenic effects depending on the experimental conditions. This knowledge can be used for improved early detection, prevention and treatment of atherosclerosis.
KEYWORDS: Atherosclerosis, Inflammation, Cytokines, Interleukins, Macrophage
1. Introduction
It is now well-accepted that atherosclerosis is not only merely a lipid disorder, but also a chronic inflammatory disease.1 Inflammation is recognized as a major contributor to atherogenesis through adverse effects on lipoprotein metabolism and arterial wall biology, and both the innate (monocyte-derived macrophages) and the acquired immune system (T-cells) have been implicated in the atherogenic process.1,2 Monocytes and T-cells migrate from the circulation into the intima of the arterial wall where they differentiate into macrophages, which then take up modified lipoproteins thereby transforming into foam cells. Monocyte-derived macrophages are abundantly present at all stages of the disease process.1,3 Their pivotal role in atherogenesis has been demonstrated by the attenuation of lesion formation in monocyte-deficiency in both ApoE−/− mice and LDLR−/− mice.4,5
In addition to cells of the monocyte/macrophage lineage, CD4+ and CD8+ T-cells are present in atherosclerotic lesions. Macrophages and T-lymphocytes together produce a wide array of cytokines that can exert both pro- and anti-inflammatory effects (Figure 1). Pro-inflammatory cytokines of the interleukin category are considered to be key players in the chronic vascular inflammation that is typical for atherosclerosis.3 Presence of interleukins and their receptors has been demonstrated in atheromatous tissue, and the serum levels of several of these cytokines have been found to correlate positively with (coronary) arterial disease and its sequelae. Besides interleukins, macrophage-associated cytokines such as tumour necrosis factor (TNF)-α, macrophage migration inhibitory factor (MIF), interferon (IFN)-γ and colony stimulating factors (CSFs) have emerged as key factors in the pathogenesis of atherosclerosis.2,6
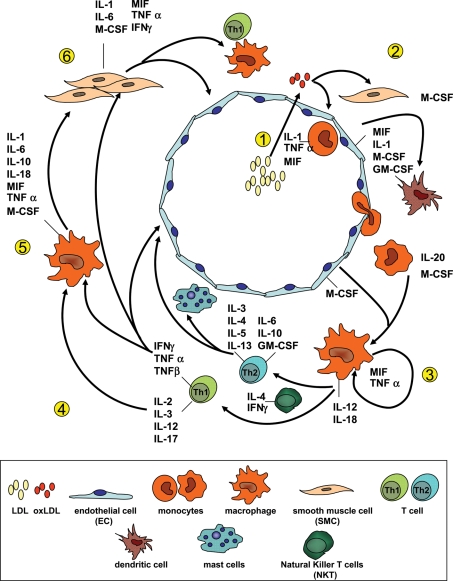
Figure 1.
Cytokines involved in atherogenesis and their cellular source and targets. (1) LDL particles penetrate the endothelial cell layer and are oxidized in the intima (oxLDL). Pro-inflammatory lipids are released from oxLDL and induce expression of cytokines in EC (e.g. IL-1, MIF, M-CSF, and GM-CSF) and SMCs (M-CSF). MIF and M-CSF can function as a chemotactic factor for monocytes and T-cells. GM-CSF is a major regulator of dendritic cell differentiation and involved in lesional dendritic cell accumulation. (3) TNF-α and MIF are involved in the autocrine activation of macrophages thereby amplifying inflammation. IL-12 and IL-18 formed by macrophages and IL-4 (e.g. from NKT) promote the differentiation of native T-cells into TH1-cells and TH2-cells, respectively. IL-12 and IL-18 are potent inducers of IFN-γ. (4) TH1-cells can further activate macrophages (inflammatory cascade) whereas TH2-cells produce anti-inflammatory mediators (e.g. IL-4, IL-10, and IL-13) with opposite effect on macrophages, T-cells and EC. IL-4 (TH2-associated) impair the development of TH1-cells from pre-TH-cells (not shown). Vice versa, IFN-γ inhibits TH2-cell development. Also, TH2-derived IL-4 and IL-13 are potent stimulators of antibody production (on B-cells, not shown), IL-5 is involved in B-cell differentiation and eosinophilic inflammation. (5) Macrophages are further stimulated by T-cell derived IFN-γ as well as IL-1 and TNF-α (also from other sources) together resulting in an amplification of the inflammatory response. (6) SMCs are targets for many of the macrophage- and T-cell-derived cytokines and participate in this self-perpetuating inflammatory cycle by producing and secreting a range of pro-inflammatory factors among which are IL-1, TNF-α, and IFN-γ.
More refined analysis of local vascular inflammation and the cytokines expressed in atherosclerotic plaques revealed that there is a balance between pro-inflammatory and anti-inflammatory cytokines and that this balance is crucial for lesion development (Figure 2A). For example, the secreted cytokines of type 1 CD4+ T-helper cells (TH1 cells) such as interleukin-2 (IL-2), IL-12, IFN-γ, TNF-α and TNF-β are pro-inflammatory and exacerbate atherosclerotic disease, whereas TH2 cytokines such as IL-4, IL-5, IL-10, and IL-13 are considered to be mainly atheroprotective and can counteract TH1 cytokine activity/production.7–9 Notably, this attractive black-and-white concept of TH1 and TH2 responses controlling the development of atherosclerosis may not be true at all stages of plaque development.10
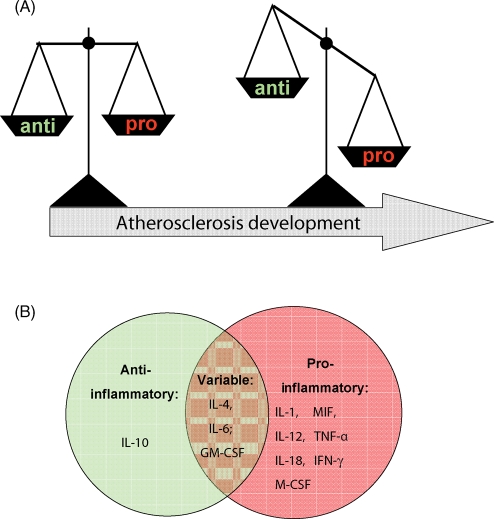
Figure 2.
Categorization of cytokines into pro-atherogenic and anti-atherogenic cytokines based on their effects in mouse atherosclerosis models. (A) The balance between pro-inflammatory and anti-inflammatory cytokines is crucial for lesion development and imbalance is colloquially referred to exacerbate atherosclerotic disease. (B) Overview of the cytokines with consistent anti-atherogenic effects (green circle), pro-atherogenic effects (red circle), or variable, dual function (intersection).
Important insight into the role of cytokines in atherogenesis has been gained from animal studies, in particular mouse studies. Mouse atherosclerosis models resemble humans in that the most prominent cells involved in lesion development are monocyte-derived macrophages and T-lymphocytes. It is important to note, however, that all standard mouse atherosclerosis models (e.g. ApoE−/− mice, LDLR−/− mice, and ApoE*3Leiden-mice)11 are on a C57/BL6 background which is prone to TH1-type immune responses and inherently underestimates the impact of TH2-type responses.
The TH1 cytokines IL-1β and TNF-α are among the most important cytokine mediators of the inflammatory response,12 and concordantly a large number of studies have attempted to delineate the role of these cytokines in atherogenesis. However, the role of other members of the interleukin family in atherogenesis is increasingly appreciated. Here, we summarize all currently available information from mouse atherosclerosis studies investigating the role of the interleukins IL-1,-2,-4,-5,-6,-10,-12,-18, and -20, the macrophage-associated cytokines TNF-α, MIF, IFN-γ, and the colony stimulating factors G-CSF, M-CSF, and GM-CSF. Particular attention is paid to the experimental conditions employed (cf, strain, gender, type of diet, disease stage, and mode of intervention; experimental details are provided in Table 1) as different or even contradictory study outcomes may be related to differences in experimental set-up.
Table 1.
Summary of the reviewed atherosclerosis studies and their experimental conditions, i.e. mouse model, gender, composition of diet, animal age, and treatment period, effect on cholesterol, atherosclerotic lesion size, and lesion composition
| Cytokine | Mouse model (treatment) | Diet | Age at start of treatment + treatment period | Cholesterol | Lesion size | Composition | Reference | |
|---|---|---|---|---|---|---|---|---|
| IL-1Ra | ApoE−/− (M/F)+rec hu IL-Ra | HFD+cholate | 16% fat/1.16% chol/ 0.5% cholate | 2 m + 1 m | ↔ | 56%↓(M) | Fatty streak | 14 |
| 63%↓(F) | ||||||||
| s human IL-1Ra Tg/ApoE−/− (M) | HFD | 1.25% chol | 10 w + 10 w | ↔ | 47% ↓/53% ↓ | 15 | ||
| ic human IL-1Ra Tg/ApoE−/− (M) | HFD | 1.25% chol | 10 w + 10 w | ↔ | 40% ↓/ 67% ↓ | 15 | ||
| IL-1Ra−/−/ApoE−/− (M) | HFD | 1.25% chol | 10 w + 7 w | 42% ↓ | No atherosclerosis; massive inflammation | 15 | ||
| IL-1Ra−/−/C57Bl/6J | HFD | 7.5% fat/1.25% chol/ 0.5% cholate | 4 w + 12 w | 3 x ↓ | Tendency to increased foam cell lesion area | 16 | ||
| IL-1RaTg/LDLR−/− | HC/HF | 4 w + 10 w | 40% ↑ | ↔ | 16 | |||
| HC/HF+cholate | 4 w + 4 w | ↔ | 40% ↓ | |||||
| IL-1R−/−/ApoE+/− vs. IL-1R+/−/ApoE+/− | HFD+cholate | 15% fat/1.25% chol/ 0.5% cholate | 10 w + 14/24 w | 14 w: ↔ | Milder | 21 | ||
| 24 w:14x↓ | ||||||||
| IL-1R−/−/ApoE+/− vs. IL-1R+/−/ApoE+/− | Chow | 0.02% chol/ 4.5% fat | 10 w + 14/24 w | 14 w: ↔ | Milder | 21 | ||
| 24 w: ∼3x↓n.s. | ||||||||
| IL-1Ra+/−/ApoE−/− vs. IL-1Ra+/+/ApoE−/− (vs. IL-1R−/−/ApoE−/−) | Chow | 0.02% chol/ 4.5% fat | 16 w + 24 w | ↔ | 16 w: 30%↑ | A-SMA↓ | 17,18 | |
| 24 w: ↑n.s. | MOMA-2↑ | |||||||
| 24 w | ↑ | ↑ | ||||||
| IL-1α | IL-1α−/− C57BL/6 | HF/HC | 17% fat/1.25% chol/0.5% cholate | 6 w + 19 w | ↑ (non-HDL) | 56% ↓ | 20 | |
| C57BL/6+IL-1α−/− BMT | HF/HC | 17% fat/1.25% chol/0.5% cholate | 6 w + 16 w | ↔ | 59% ↓ | 20 | ||
| IL-1β | IL-1β−/−/ApoE−/− (M) | Chow | 4.6% fat/ <0.02% chol | 0 w + 12/24 w | ↔ | 33%↓/32%↓ | 19 | |
| IL-1β−/− C57BL/6 | HF/HC | 17% fat/1.25%chol/0.5% cholate | 6 w + 19 w | ↑ (HDL) | 50% ↓ | 20 | ||
| C57BL/6+IL-1β−/− BMT | HF/HC | 17%fat/1.25% chol/0.5% cholate | 6 w + 16 w | ↔ | 33% ↓ | 20 | ||
| IL-2 | ApoE−/− (M)+rmIL-2 | HF | 21% fat/ 0.15% chol | 6 w | ND | ↑ | More advanced | 22 |
| ApoE−/− (M)+α-mouse IL-2 | ↓ | Less advanced | ||||||
| IL-4 | IL-4−/−/ApoE−/− (M+F) | Chow | 6, 15, 30, 45 w | ↔ | 30 w:27%↓ | 23 | ||
| 45 w: ↓ | ||||||||
| IL-4T KO/C57BL/6J | Paigen | 17% fat/ 1.25 chol | 15 w | ↔ | ↔ | 24 | ||
| IL-4−/−/C57BL/6J+HSP65 (F) | HF Teklad diet (TD 90221) | 15 w | ↔ | ↓ | 26 | |||
| LDLR−/−+IL-4−/− BMT | HF | 21% fat/1.25% chol/ 0.5% cholate | 4 w | ↔ | Root: ↔ | ↔ | 25 | |
| Arch: 69%↓ | ||||||||
| LDLR−/−/IL-4−/−(F) | HF Teklad 88137 or Teklad 90221 | 21% butter fat/0.15% chol | 8–10 w + 4 w | ↔ | ↔ | 27 | ||
| 21% fat/0.15%chol/0.5% cholate | ||||||||
| ApoE−/− /IL-4−/−(M, F) | Chow or HF/HC | 21% butter fat/0.15% chol | 8–10 w + 36 w | ↔ | ↔ | 27 | ||
| ApoE−/−+i.p. IL-4 | Normal chow or HF Teklad 88137 | 21% butter fat/0.15% chol | 8 w + 30 d | ↔ | ↔ | 27 | ||
| C57BL/6J+IL-4 (M) | Teklad diet 96354 | 20% fat/ 1.5% chol/0.5% cholate | 15 w | ↔ | 90%↓ | 28 | ||
| IL-5 | LDLR−/−+IL-5−/− BMT | Teklad diet 94059 | 15.8% fat/1.25% chol | 16 w | ND | Root: ↑ | 29 | |
| Arch: ↑ | ||||||||
| IL-6 | C57Bl/6 (M)+rec mouse IL-6 | HFD+cholate | 20% fat/1.5% chol/0.5% cholate | 3 w + 21 w | ↔ | 5.1 x ↑ | 30 | |
| ApoE−/− (M)+rec mouse IL-6 | LFD (chow) | 5.67% fat/0% chol | 3 w + 21 w | ↔ | 2.4 x ↑ | 30 | ||
| ApoE−/− (M)+rec mouse IL-6 | HFD+cholate | 20% fat/1.5% chol/0.5% cholate | 3 w + 6 w | ↔ | 1.9 x ↑ | 30 | ||
| IL-6+/−/ApoE−/− (F) | LFD (chow) | 0 w + 16 w | ↔ | 28%↓(n.s.) | 32 | |||
| IL-6−/−/ApoE−/− (F) | LFD (chow) | 0 w + 16 w | 26%↑(sign.) | 29%↓(n.s.) | 32 | |||
| IL-6−/−/ApoE−/− (F) | LFD (chow) | 0 w + 1 y | ↔ | 1.9 x ↑ | Calcification area: 4.1 x ↑ | 32 | ||
| IL-6−/−/C57Bl/6 (F) | Paigen | 15% fat/1.25% chol/0.5% cholate | 8–10 w + 15 w | ND | 70%↑ | 31 | ||
| IL-6−/−/LDLR−/− (M, F) | Chow | 5 w + 16 w | ↔ | ↔ | M—45% (n.s.) | 34 | ||
| F—38% (n.s.) | ||||||||
| IL-6−/−/LDLR−/− (M, F) | Western-type diet | 20% fat/1.5% chol | 5 w + 16 w | ↔ | ↔ | M—27% (n.s.) | 34 | |
| F—19% (n.s.) | ||||||||
| IL-6−/−/LDLR−/− (M, F) | Paigen diet | 15% fat/1.25% chol/0.5% cholate | 5 w + 16 w | ↔ | ↔ | M—15% (n.s.) | 34 | |
| F—22% (n.s.) | ||||||||
| IL-6−/−/ApoE−/− (M) | Chow | 0 w + 53 ± 4 w | 60% ↑ | 1.4–1.9 x ↑ | 33 | |||
| IL-10 | mIL-10TG/C57BL/6J | HF | 15% fat/1.25% chol/0.5%cholate | 15 w | ↔ | 2.5x↓ | 40 | |
| IL-10−/−/C57BL6 | HF | 15% fat/1.25% chol/0.5%cholate | 15 w | HDL↓ | 2x↑ | Fatty streak | 40 | |
| LDLR−/−+IL-10Tg BMT | HF (TD94059) | 15.8% fat/1.25% chol | 20 w | ↔ | 2x↓ | 44 | ||
| IL-10−/− /ApoE−/− (M+F) | Chow | 16 w + 48 w | total chol ↔ | 16 w:3x↑ | SMC+inflammatory cells ↔ | 95 | ||
| VLDL↓LDL↑ | 48 w: ↔ | |||||||
| IL-10−/−/ C57BL/6J (F) | HF | 18% fat/1.25% chol/0.5% cholate | 8 w + 16 w | ↔ | SPF:3x↑ | Macrophages↔ | 41 | |
| CONV:30x↑ | T-cells 2.5x↑ | |||||||
| Collagen↓ | ||||||||
| LDLR−/−+IL-10−/− BMT | HF | 15% fat/1.25% chol | 14 w | ↔ | 35%↑ | SMC↔ | 45 | |
| Collagen 49%↓ | ||||||||
| Macrophage 62%↑ | ||||||||
| T-cells 116%↑ | ||||||||
| ApoE−/−+mIL-10-HVJ (M) | HF/HC | 7.5% fat/1.25% chol/0.5% cholate | 9 w | ↔ | 2.5x↓ | Macrophages 2x↓ | 42 | |
| T-cells 2x↑ | ||||||||
| Collagen ↔ | ||||||||
| ApoE−/−+AAV2/5-mIL-10 | HF | 8 w | chol+TG ↓ | 31%↓ | MCP-1↓ | 43 | ||
| LDLR−/−+AAV2-hIL-10 | HC | 2%chol | 18 w | ↓ | Macrophages ↓ | 39 | ||
| Nitrotyrosine ↓ | ||||||||
| IL-12 | IL-12−/−/ApoE−/− (M+F) | Chow | 6, 15, 30, 45 w | ↔ | 30 w:52%↓ | 30 w: fewer macrophages | 23 | |
| 45 w:↔ | ||||||||
| ApoE−/−+recmIL-12 (M) | Chow | 30 d | ↔ | ↑ | More advanced | 47 | ||
| CD3+ cells ↑ | ||||||||
| LDLR−/−+IL-12-PADRE (F) | Western-type diet | 15% fat/0.25% chol | 2 w diet +6 w collar | ↔ | 68.5%↓ | 4.2x↑ SM-actin 2.8x↑ collagen | 48 | |
| Macrophages↔ | ||||||||
| IL-18 | ApoE−/−+IL-18 | Chow | 30 d | ↔ | 2.2x↑ | T-cells 4.5x↑ | 49 | |
| MHCII+cells 4.4x↑ | ||||||||
| IFN-γ−/−/ApoE−/−+IL-18 | Chow | 30 d | ↔ | ↔ | ↔ | 77 | ||
| IL-18−/−/ApoE−/− | 24 w | 50%↑ | 35%↓ | I-Ab gene ↓ | 50 | |||
| CD4+ cells↓ | ||||||||
| SMactin ↑ | ||||||||
| ApoE−/−+pcDNA3-mIL-18BP (M) | 9 w | ↔ | 24%↓ | Macrophages 50%↓ | 51 | |||
| T-cells 67%↓ | ||||||||
| 2x↑SMC | ||||||||
| 85%↑ collagen | ||||||||
| TUNEL 3.5x↓ | ||||||||
| IL-20 | ApoE−/−+intramuscular electroporation of IL-20 (F) | Atherogenic diet | 0.15% chol | 10 w | ND | 1.4x↑ | Macrophages ↔ | 54 |
| Mig, I-TAC, TNF-α, IL-6 ↑ | ||||||||
| TNFR | p55−/−/ApoE−/− | Chow | 0 w + 64 w | ↔ | ↔ | ↔ | 57 | |
| p55−/− or wt grafts in ApoE−/− | Chow | 9% fat | 6 w + 8 w post operatively | not reported | Lumen 31%↑ | SMC proliferation ↓ | 58 | |
| p55−/−/ApoE−/−(F) | Chow | 9% fat | 60 w | ↔ | 12%↓ | 58 | ||
| p55−/−/C57Bl6(c.pneumoniae infected) | HF/HC | 15% fat/ 1.25% chol/ 0.5% cholate | 9 w + 12/16 w | ↔ | ↔ | 59 | ||
| p55−/−/C57Bl6 (F) | HF/HC | 15% fat/ 1.25% chol/ 0.5% cholate | 6–8 w + 14 w | ↔ | 2.3x↑ | ↔ | 55 | |
| p55−/−/ p75−/−/ p55−/− p75−/−/C57BL6 | HF/HC | 15% fat/ 1.25% chol/ 0.5% cholate | 6 w + 18 w | ↔ | 2.4x↑/↔/2.4x↑ | 56 | ||
| TNF-α | TNF−/−/E3Leiden (F) | Western-type diet | 15% fat/ 1% chol | 8 w + 20 w | ↔ | ↔ | Early↑ necrosis↓ | 64,65 |
| Necrosis ↑ | ||||||||
| Apoptosis ↑ | ||||||||
| TNFα−/−/ApoE−/− | Western-type diet | 21% fat/ 0.15% chol | 4 w + 10/40 w | ↑n.s./ ↔ | 50%↓/ 60%↓ | 60 | ||
| TNFα−/−/ApoE−/−(BMT) | Western-type diet | 21% fat/ 0.15% chol | 10 w + 25 w | ↔ | 83%↓ | 60 | ||
| TNFα−/−/ApoE−/−(murine sTNF-RI/Fc chimera) | Chow | 7 w + 25 w | ↑n.s. | 75%↓ | 60 | |||
| p55−/−/ApoE−/− | Chow | 0 w + 64 w | ↔ | ↔ | ↔ | 57 | ||
| p55−/− (M) | Atherogenic diet | 15% fat/ 1.25% chol/ 0.5%cholate | 9 w + 12/16 w | ↔ | ↔ | 59 | ||
| p55−/−/ApoE−/− (F) | Atherogenic diet | 15% fat/ 1.25% chol/ 0.5% cholate | 6–8 w + 14 w | ↔ | Lesion size 2.3x↑ | Cell number↓ | 55 | |
| Scavenger receptor expression↑ | ||||||||
| TNFα−/−/C57BL/6 (F) | HF/HC | 15% fat/ 1.25% chol/ 0.5% cholate | 6 w + 16 w | 46%↑ | ↔ | 56 | ||
| TNFα−/−/ApoE−/− (M+F) | Chow | 0 w + 12 w | ↔ | 27%↓ | 61 | |||
| TNF−/−/C57BL/6 (M) | HF/HC | 17% fat/ 1.25% chol/ 0.5% cholate | 8–10 w + 20 w | 61.5%↑HDL | 17.8x↓ | 65 | ||
| 16.9%↓IDL/LDL | ||||||||
| 21.6%↓VLDL | ||||||||
| TNF−/−/ ApoE−/− (F) | HF/HC | 17% cocoa butter, 1.25% cholesterol | 6 w + 26 w | not reported | 30%↓ | Reduced wall-thickness | 62 | |
| tmTNF/C57Bl6 | HF/HC | 17% fat/ 1.25% chol/ 0.5% cholate | 8–10 w + 20 w | ↔ | 1.8x↓n.s. | 65 | ||
| Anti-TNF | ApoE−/−+TNFbp (M+F) | HF/HC | 16% fat/1.15% chol/0.5% cholate | 8 w + 4 w | ↔ | M ↔ | 14 | |
| F: 35%↓ n.s. | ||||||||
| IFN-γ | IFNγ−/−/ApoE−/− (M+F) | Chow | 36 w | ↔ | M: 42%↓ | Neutral lipid, macrophages, T-cells, collagen ↔ | 75 | |
| F: ↔ | ||||||||
| HF (TD88137) | 0.15% chol/20% butterfat | 8 w + 12 w | ↔ | M: 74%↓ | Neutral lipid, macrophages, T-cells, collagen ↔ | |||
| F: ↔ | ||||||||
| ApoE−/− (M)+mIFNγ IP | Chow | 16 w + 4 w | 15%↓VLDL | 2x↑ | 2,7x↑ T-cells | 76 | ||
| 3.3x↑ MHCII+ cells | ||||||||
| IFN-γR−/−/ApoE−/− (F) | Western-type diet | 21% fat/0.15% chol | 5 w + 12 w | FC↑ | 59%↓ lipid content | Smaller | 53 | |
| phospholipid-rich particles↑ | Less cellular | |||||||
| apoA-IV↑ | Collagen↑ | |||||||
| IFN-γ−/−/LDLr−/− (M+F) | D12108 research diets | 40% lipid/ 1.25% chol | 5–6 w + 8 w | ↔ | 75%↓ lipid area | T-cells↔ | 74 | |
| 62%↓ lesion area | Macrophages↓ | |||||||
| En face 46%↓ | MHCII+cells ↓ | |||||||
| Collagen↓ | ||||||||
| 5–6 w + 20 w | ↔ | 43%↓ lipid area | ↔ | |||||
| Lesion area ↔ | MHCII+cells ↓ | |||||||
| en face 70%↓ | ||||||||
| Anti-IFN-γ | ApoE−/−+sIFN-γR plasmid | Western-type diet | 20% fat/ 0.15% chol | 8 w + 4 w | ↔ | en face plaque area 60%↓ | IFN-γ, Il-1β, MCP-1, VCAM-1↓ | 78 |
| Western-type diet | 20% fat/ 0.15% chol | 8 w + 8 w | ↔ | Attenuate lesion progression 50%, stabilization | Inflammatory cyto/chemokines↓ | 96 | ||
| MMP-9/13↓ | ||||||||
| Collagen I↑ | ||||||||
| 8 w + 12 w | ↔ | Attenuate lesion progression 40% | pSTAT1/STAT1↓ | |||||
| CD40/CD40L↓ | ||||||||
| MIF | ApoE−/−+α-MIF mAb | Chow | ? + 14 w | ↔ | Lesion area %↓ n.s. | 4x↓Macrophages | 71 | |
| Inflammatory mediators↓ | ||||||||
| ApoE−/−+α-MIF Ab | Atherogenic diet | ? + 16 w | Aortic root surface↓ | T-cells↓ | 2 | |||
| Macrophages↓ | ||||||||
| Mif−/−/LDLr−/− | Chow | 0 w + 30 w | Monocyte adhesion↓ | 2 | ||||
| Macrophages↓ | ||||||||
| Mif−/−/LDLr−/− (M) | Atherogenic diet | 6 w + 12/26 w | LDL↓ | 26 w: En face lipid deposition 4.5x↓ | Ki67+cells↓ | 70 | ||
| HDL↔ | Longitudinal intima 7x↓ | SMCs↓n.s. | ||||||
| TG↓ | Collagen↓n.s. | |||||||
| ApoE−/−+MIF mAb+carotid transluminal wire injury | Atherogenic diet | 21% fat | 8 w + 3 w | ↔ | Neointima/media volumes↔ | Macrophages 2x↓ | 72 | |
| SMC2x↑ | ||||||||
| LDLr−/−+α-MIF mAb+carotid injury | HF | 20.1% fat/1.25% chol | Weaning+12 w | ↔ | Neointimal thickening 54%↓ | Proliferation 56%↓ | 73 | |
| CD45+ cells 27%↓ | ||||||||
| Apoptosis↑ | ||||||||
| M-CSF | Op0/op1/op2/ ApoE−/− (M+F) | Chow | 0 w + 16 w | Op1 ↑ | Op1 ↓ | 85 | ||
| Op0 ↑↑ | Op0 ↓↓ | |||||||
| VLDL | ||||||||
| Op0/op1/op2/ ApoE−/− (M+F) | Chow | 12 w + 12 w | Op0: 2.5x↑ | Op0: M: no lesions | 86 | |||
| HDL↔ | F: 98%↓ | |||||||
| Op1:M: 60%↓ | ||||||||
| F:85%↓ | ||||||||
| Op0/op1/op2/C57Bl6 | Atherogenic diet | 15% fat/ 1.25% chol/ 0.5% cholic acid | 24 w + 15 w | Op0: 2x↑ | Op0: 84%↓ | 86 | ||
| Op1:3x↑ | Op1: 46%↓ | |||||||
| Op0/op1/op2/ ApoE−/− (M+F) | Western-type diet | 42% fat/ 0.15% chol | 3 w + 9 w | Op0: 1.5x↑ | Op0: 4x↓ | Monocytes 3x↓ | 4 | |
| Chow | 4.5% fat/0.02% chol | 4 w + 12 w | Op0:↓ | Macrophages↓ | ||||
| Op/+, op/op/ LDLr−/− (M+F) | Atherogenic diet | 15% fat/ 1.25% chol/ 0.5% cholic acid | 12 w + 16 w | Op/op 25%↑ | Op/op 99.7%↓ | 5 | ||
| Op/+16%↑ | Op/+99.4%↓ | |||||||
| ApoE−/− (F)+murine c-fms mAb | HF | 20% fat/ 0.3% chol | 6 w + 6 w diet+6 w Ab | ↔ | 70%↓ | 87 | ||
| 6 w + 12 w diet + 6 w Ab | ↔ | ↔ | Macrophages↔ | |||||
| SMC↔ | ||||||||
| GM-CSF | GM-CSF−/−, GM-CSF+/−/ LDLr−/− (M+F) | Western-type diet | TD88137, teklad | 10 w + 12/13 w | ↔ | −/−: ∼20%↓ | Macrophages↔ | 88 |
| +/−: ↓ | SMC↔ | |||||||
| Collagen↔ | ||||||||
| Elastin↓ | ||||||||
| CD11c+ cells 63%↓ | ||||||||
| CD4+ Thcells 50%↓ | ||||||||
| IL-6/MCP-1↔ | ||||||||
| ApoE−/− (M)+GM-CSF (10 µg/kg−1d−1; 5 d per w) | Paigen diet | 30 kcal % fat/ 1.25% chol/0.5% cholate | 8 w + 8 w | ↔ | 2x↑ | ↔ | 89 | |
| G-CSF | GM-CSF−/−/ApoE−/− (M) | HF | 20% fat/0.125% chol | 8 w + 12 w | ↔ | 30%↑ | Macrophages 2x↑ | 90 |
| SMC↔ | ||||||||
| Collagen 15%↓ | ||||||||
| PPARγ 50%↓ | ||||||||
| ABCA1, SR-A, CD36↓ | ||||||||
| ApoE−/− (M)+G-CSF (10 µg/kg−1d−1; 5 d per w) | Paigen diet | 30 kcal % fat/ 1.25% chol/0.5% cholate | 8 w + 8 w | ↔ | 44%↑ | ↔ | 89 | |
2. Interleukin-1
IL-1 is a prototypic pro-inflammatory cytokine that exists in two forms, IL-1α and IL-1β, both of which exert similar but not completely overlapping biological functions mediated through the IL-1 receptor (IL-1R). After binding to IL-1R, IL-1 induces the production of a broad spectrum of cytokines and chemokines as well as the expression of adhesion molecules on endothelial cells (EC), thus leading to the recruitment of inflammatory cells. In addition, IL-1 contributes to the development of vascular damage by stimulating cell proliferation and differentiation and the release of matrix-degrading enzymes. The IL-1R antagonist (IL-1Ra) is a structural homologue of IL-1 that binds to IL-1R but does not induce any cellular responses and is therefore a natural inhibitor of IL-1-activity.13
The role of IL-1 in atherogenesis has been investigated through influencing its level or activity (Table 1). Elhage et al.14 showed that short-term perfusion with a recombinant IL-1Ra preparation reduces fatty-streak formation in male and female ApoE−/−-mice fed a cholesterol/cholate-rich diet, without evident interference with lipid metabolism. This study lacked, however, proper controls (e.g. no sham minipump). Merhi-Soussi et al.15 demonstrated that atherosclerosis development is inhibited in ApoE−/−-mice overexpressing either secreted IL-1Ra or intracellular IL-1Ra and fed a cholesterol-rich diet, thus providing more solid proof to the observations by Elhage.
Devlin et al.16 used two strains of mice, either with deficient or excess IL-1Ra. IL-1Ra−/−/C57/BL6/J-mice fed a cholesterol/cholate-diet had a 3-fold decrease in non-HDL-cholesterol and a trend toward increased foam-cell lesion area compared to wild-type littermate controls. LDLR−/−-mice expressing high levels of murine IL-1Ra and fed a cholesterol-saturated fat diet showed a 40% increase in non-HDL-cholesterol, consistent with the IL-1Ra−/− data, but there was no significant change in lesion size. However, when IL-1Ra-overexpressing LDLR−/−-mice were fed a high-cholesterol(HC)/high-fat (HF) diet containing cholate, lesion area was 40% lower than in non-transgenic controls; no significant differences in plasma cholesterol or lipoproteins between the experimental groups were observed under these conditions.
Studies by Isoda et al.17,18 further clarified the role of IL-1Ra in atherosclerotic lesion development. The studies focused on the comparison of atherosclerotic lesion between IL-1Ra+/+/ApoE−/− and IL-1Ra+/−/ApoE−/−-mice, because IL-1Ra−/−/ApoE−/−-mice were significantly leaner, had significantly elevated total plasma cholesterol, decreased HDL-cholesterol levels, and severe fatty livers when compared with the other mice. The lowering of serum levels of IL-1Ra in IL-Ra+/−/ApoE−/−-mice to approximately half of those in IL-1Ra+/+/ApoE−/− was associated with a significant increase in lesion development during early atherogenesis. At later stages, the differences of lesion size between these mice disappeared, but, most importantly, (lack of) IL-1Ra appeared to have modulated plaque composition during lesion progression, with a significant increase in macrophages and depletion of smooth muscle cells (SMC) in IL-1Ra+/−/ApoE−/−, suggesting reduced plaque stability.
Merhi-Soussi et al.15 also noticed massive vascular inflammation in IL-1Ra−/−/ApoE−/− mice with marked macrophage infiltration in the adventitia and severe destruction of elastic lamina, paralleled by severe illness and high death rate.
Taken together, the reported effects of IL-1Ra on early lesion development and plaque composition point to an important role of IL-1 signalling in atherogenesis. In line, IL-1β−/−/ApoE−/− showed a significant 30% decrease in atherosclerosis at 12 and 24 weeks of age compared with that in IL-1β-expressing ApoE−/− mice.19 There was no significant difference in plasma lipid levels between the two genotypes. Kamari et al.20 confirmed these observations using IL-1β−/−/C57/BL6-mice and additionally demonstrated that genetic deletion of IL-1α reduced lesion area by 56%. Of note, IL-1α−/− mice displayed high levels of non-HDL-cholesterol whereas IL-1β−/−-mice had higher levels of HDL-cholesterol. Transplantation of IL-1α−/− or IL-1β−/− bone marrow (BM) in irradiated C57/BL6-mice reduced early lesion formation by 59 and 33%, respectively, when compared with wild-type BM transplanted mice, without changing plasma lipids.20
The evidence for an important role of IL-1 signalling in atherosclerosis is further strengthened by a study by Chi et al.21 who showed that the absence of IL-1R markedly reduces the progression of atherosclerosis in ApoE+/−-mice under experimental conditions known to favour the aggravation of vascular lesions, i.e. 24 weeks of exposure to HF-diet and P. gingivalis. The effect of IL-1R absence persisted whether the inciting factors were genetic, dietary, or infectious, alone or in combination, thus highlighting the prominent role of IL-1 signalling in the atherosclerotic process and emphasizing its attractiveness as a therapeutic target.
3. Interleukin-2
IL-2 is a pro-inflammatory cytokine produced by TH1 lymphocytes. Intraperitoneal injection of IL-2 into ApoE−/−-mice fed with HF-diet enhances atherosclerosis, while anti-IL-2 antibody treatment has a protective effect, indicating that IL-2 is atherogenic.22
4. Interleukin-3
See below under CSFs.
5. Interleukin-4
IL-4, a TH2 cytokine with a key role in TH2-cell differentiation, is produced by activated T-lymphocytes and mast cells, and can exert both pro- and anti-inflammatory effects.9,23,24
The role of IL-4 in atherosclerosis has been investigated in several mouse models (Table 1).23,25–27
Davenport and Tipping23 found that IL-4−/−/ApoE−/−-mice had a significant reduction in plaque area in the aortic root compared to ApoE−/−-mice at 30 weeks of age. At 45 weeks, there were no significant differences in lesion sizes in the aortic root between the strains; however, IL-4−/−/ApoE−/−mice showed a marked decrease in atherosclerosis in their aortic arch compared to ApoE−/− mice.
King25 demonstrated that immune cell-specific deficiency of IL-4 in hypercholesterolemic, cholate-fed female LDLR−/− mice, produced by repopulation with BM stem cells from IL-4−/− mice, led to a reduction in atherosclerosis in the arch/thoracic regions of the aorta, but not in the aortic root. The same group reported no effect of genetic IL-4-depletion in models of cholesterol-induced atherosclerosis (female LDLR−/− mice; after 4 weeks), angiotensin-II-induced atherosclerosis (male ApoE−/− mice; after 4 weeks), and spontaneous atherogenesis (male and female ApoE−/− mice; up to 36 weeks).27 I.p. administration of mouse IL-4 protein during 30 days had also no effect on atherogenesis in ApoE−/− fed a normal chow or a HF-diet.27 In none of these experiments modulation of IL-4 altered plasma cholesterol.
Using a model of accelerated fatty streak formation induced by heat-shock-protein-65 or Mycobacterium tuberculosis, George et al.26 found that fatty streak formation in IL-4−/− mice was significantly reduced compared with lesions in wild-type C57/BL6 mice, lending support to a pro-atherogenic character of IL-4.
However, in another study by the same group, fatty streak formation in the aortic root of C57/BL6 fed a HC-diet was not influenced by IL-4 deficiency.24 Notably, the lesions in this study were not as advanced as in the above studies using ApoE−/−.
At odd with these findings, IL-4 injection decreased aortic lesion size in mice under conditions of mild hypercholesterolemia, suggesting that exogenous and endogenous IL-4 may have opposite effects.28
Overall, the data show that IL-4 can play a role in the development of atherosclerosis at various vascular sites. The net effect of IL-4 may depend on the disease stage, reflecting its dual, i.e. pro- and anti-inflammatory, character.
6. Interleukin-5
IL-5 is mainly produced by TH2 cells and mast cells. In LDLR−/− mice which were reconstituted with wild-type or IL-5−/− BM cells, Binder et al.29 established that IL-5 links adaptive and natural immunity specific for epitopes of oxidized low-density lipoprotein (oxLDL) and had an overall atheroprotective role.
7. Interleukin-6
IL-6 is a multifunctional cytokine that regulates various aspects of the immune response, acute-phase reaction, and haematopoiesis. IL-6 is inducible by IL-1, and consequently concentrations in serum are often a reflection of IL-1 activity in vivo. Nonetheless, IL-6 has been identified as an independent risk factor for coronary artery disease. Direct involvement of IL-6 in atherosclerotic lesion development was suggested by experiments in which mice had been treated for 6–21 weeks with recombinant IL-6 (rIL-6).30 rIL-6 treatment increased lesion size in HF/cholate-fed C57/BL6 and ApoE−/− mice 5.1- and 1.9-fold, respectively when compared to lesions in saline-treated animals (Table 1). Total cholesterol levels were unchanged between rIL-6-treated and non-treated groups. Seemingly contrasting these observations with supra-physiological injections of rIL-6 is a study reporting that IL-6−/− mice developed 1.7 times larger fatty streak lesions than C57/BL6 after 15 weeks on atherogenic Paigen diet.31
In another study, using chow-fed ApoE−/− mice that were IL-6 mono- or double-deficient, there was a tendency for decreased fatty streak formation when compared with ApoE−/− mice, despite a significant increase in plasma non-HDL cholesterol in IL-6−/−/ApoE−/−.32 However, when female IL-6−/−/ApoE−/− mice were maintained for 1 year on a normal mouse-chow, the animals showed similar hypercholesterolemia to IL-6+/+/ApoE−/− but presented significantly larger and more calcified lesions.32
In a very similar study, Schieffer et al.33 used male IL-6−/−/ApoE−/− double knockout mice on a normal chow diet for 1 year. A lifetime deficiency of IL-6 in the male ApoE−/− model resulted in enhanced atherosclerotic lesion formation, similarly as reported by Elhage et al.,32 but significantly higher levels of total cholesterol, LDL, and VLDL, which is deviant from the findings with female IL-6−/−/ApoE−/−. Schieffer et al. also reported reduced collagen metabolism and decreased recruitment of inflammatory cells to the atherosclerotic plaque in male IL-6−/−/ApoE−/− mice.
To re-examine the hypothesis that IL-6 and the acute- phase-response play an important role in atherogenesis, Song and Schindler34 crossbred IL-6−/− mice with LDLR−/− mice because loss of ApoE protein in ApoE−/− mice has been associated with decreased immune and inflammatory responses.11,35 Although there was a trend for modestly smaller lesions in IL-6−/−/LDLR−/− throughout the course of the study of 16 weeks on chow, Western-type, and Paigen diets, these differences never achieved statistical significance. This despite a significant defect in the expression of acute-phase-response genes in IL-6−/−/LDLR−/− Paigen diet fed mice. Similar to the development and structure of atheromata, no significant differences in lipid/lipoprotein were noted, indicating that IL-6 does not contribute significantly either to the regulation of lipid metabolism on chow, Western-type, or Paigen diet fed LDLR−/− mice.
In all, reported studies support both a positive and negative role for IL-6 in the development of atherosclerosis, while some groups failed to identify a substantial role for IL-6 at all in either the development or structure of atheromata. Part of the explanation for the different findings may lie in the experimental set-up (mouse model, male vs. female, diet, age of the animals, duration of the experiment; Table 1). Also, IL-6 may have a biphasic effect on the atherosclerotic process as a reflection of its pro-inflammatory or anti-inflammatory nature activities.36–38 Furthermore, redundancy may play an important role. IL-6 belongs to a family of cytokines, which include IL-11, leukaemia inhibitory factor, oncostatin M, and ciliary-neurotropic factor, which are expressed in the arterial wall. Since these IL-6-type cytokines all use the same receptor gp130 subunit for signal transduction, they may induce similar (patho)physiological responses as IL-6 and overcome IL-6 deficiency (‘redundancy’).
8. Interleukin-10
IL-10 is a pleiotropic cytokine produced by a variety of immune cells (e.g. TH2 cells, macrophages and CD8+ cells) that can inhibit a broad array of immune and inflammatory responses.39 Because of its anti-inflammatory properties, IL-10 is thought to be atheroprotective and have anti-atherogenic potential.
Indeed, atherosclerosis is reduced in IL-10-transgenic C57/BL6-mice fed a cholate-containing HF-diet,40 whereas their IL-10-deficient counterparts exhibited increased early atherosclerotic lesion formation (Table 1).40,41 Caligiuri et al.95 also showed a protective effect of IL-10 by using ApoE−/−/IL-10−/− mice: IL-10-deficiency increased atherosclerosis, LDL, thrombosis, and plaque vulnerability. In line with this, intramuscular gene transfer of IL-10 cDNA reduced atherosclerosis in ApoE−/− mice.42 Inhibition of atherogenesis was also found by delivery of adeno-associated virus vector-mediated IL-10 (AAV/IL-10) gene transfer into the tibial muscle of ApoE−/− mice43 or by AAV/IL-10 tail vein injection in LDLR−/− mice.39 Because IL-10 is produced by BM cells, two groups44,45 examined the direct role of leukocyte-derived IL-10 on the inflammatory process and development of advanced atherosclerotic lesions in irradiated LDLR−/− mice transplanted with BM cells from IL-10 transgenic,44 wild-type45 or IL-10−/− mice. After feeding a HF/cholate-free diet, IL-10 deficiency significantly enhanced development of advanced atherosclerotic lesions, reiterating that leukocytes are a major source of IL-10, active in protection against excessive growth of advanced lesions.
9. Interleukin-12
IL-12 is a heterodimeric (p70) cytokine consisting of a 35 kDa light chain (p35) and a 40 kDa heavy-chain (p40). IL-12 is produced by various inflammatory cell types present in the atherosclerotic vasculature, among which macrophages. Because of its early production in response to stimuli and its ability to enhance TH1-cell differentiation, IL-12 forms a bridge between innate and adaptive immunity.46
IL-12 p40−/− /ApoE−/− mice show markedly less atherosclerosis in the aortic root at 30 weeks of age than ApoE−/− controls (Table 1).23 In addition, daily administration of IL-12 protein promotes atherosclerosis in young ApoE−/− mice compared with control-treated mice.47 Hauer et al.48 reported that functional blockade of endogenous IL-12 by vaccination resulted in a significant 69% reduction of atherogenesis induced in carotid arteries by bilateral perivascular collar placement using LDLr−/− mice; vaccination against IL-12 also improved parameters for plaque stability.
These studies, in which the pro-atherosclerotic role of IL-12 is described, indicate that IL-12 may be a suitable target in the treatment of atherosclerosis.
10. Interleukin-18
IL-18 and IL-1β are members of the same structural family (IL-1 family). Animal experiments support the concept that IL-18 participates in the pathogenesis of atherosclerosis (Table 1). When injected for 30 days with IL-18, ApoE−/− mice exhibit a doubling of the lesion size through release of the pro-inflammatory cytokine IFN-γ, and without a change in serum cholesterol.49
Elhage et al.50 found reduced atherosclerosis in IL-18-deficient ApoE−/− mice, despite an increase in serum cholesterol. Overexpression of the IL-18-binding protein, a naturally occurring, specific inhibitor of IL-18, prevents the spontaneous development of atherosclerosis in ApoE−/− mice,51 thus confirming the pro-atherogenic character of IL-18. Part of the pro-atherogenic effect of IL-18 may be indirect since IL-18, in particular in combination with IL-12, is a potent inducer of the production of IFN-γ, which has aggravating effects on atherosclerosis (see below).52,53
11. Interleukin-20
IL-20 belongs to the IL-10 family and is a proinflammatory cytokine, preferentially expressed in monocytes. Systemic delivery of IL-20 by intramuscular electroporation of IL-20 expression vector resulted in increased atherosclerotic lesion areas in ApoE−/− mice, suggesting that IL-20 acts as a pro-atherogenic cytokine.54
12. Tumour necrosis factor-α
TNF-α is a pleiotropic cytokine that exerts potent pro-inflammatory effects in atherosclerosis and other metabolic and inflammatory disorders such as obesity and insulin resistance which are also risk factors for cardiovascular diseases. TNF-α is primarily produced by monocytes and macrophages. The involvement of TNF-α in the pathogenesis of atherosclerosis is supported by its presence in human atherosclerotic plaques. Furthermore, circulating TNF-α levels are associated with increased risk of recurrent myocardial infarction, atherosclerotic thickening of carotid intima-media, disturbances in triglyceride and glucose homeostasis, and with age-related atherosclerosis.
Lymphotoxin-α (LTα) is another member of the TNF ligand family and is synthesized predominantly by activated T- and B-lymphocytes. Like TNF-α, LTα is expressed in atherosclerotic plaques. TNF-α and LTα elicit responses through two receptors termed p55 (TNFR1) and p75 (TNFR2), of which p55 activates the majority of biological responses. The role of TNF-α (and LTα) in atherosclerosis is not fully clear at present because results in animal models are controversial (Table 1).
In female C57/BL6 wild type mice lacking p55 (p55−/− mice), Schreyer et al. found an unexpected 2.3-fold increase in atherosclerosis when feeding the mice an atherogenic diet.55 In a subsequent study, the same group found that loss of p75 did not alter lesion development, and loss of both p55 and p75 resulted in lesion areas comparable with those for p55−/− mice.56 No significant changes in plasma lipid levels were observed between genotypes. By contrast, in older p55−/− mice on an ApoE−/− background no effect on lesion progression or composition of very advanced plaques was observed.57 Using an arterial grafting model (i.e. transplantation of lesion-free wild-type- or p55−/−-donor carotid grafts in ApoE−/− recipient mice) Zhang et al. demonstrated that expression of p55 in just the arterial wall (SMC and EC) contributed substantially to both early-stage and late-stage atherosclerosis by enhancing adhesion molecule and chemokine expression as well as SMC proliferation.58 The authors also analyzed atherosclerosis in 60-week-old p55−/−/ApoE−/− and ApoE−/− mice and showed that p55-deficiency results in 12% less thoracic atherosclerosis at comparable cholesterol levels.
Campbell et al. also reported diminished atherosclerotic lesion development in male p55−/− mice fed a HF/HC-diet.59 In addition, these investigators showed that signalling through the p55 receptor may also play a role in the atherogenic effects of C. pneumoniae in hyperlipidaemic mice. Whereas C. pneumoniae-infected male C57/BL6J−mice on HF/HC-diet showed a 2.5- to 3.3-fold enlarged lesion size in the aortic sinus in comparison to uninfected mice, no acceleration of atherosclerotic lesion development was observed in infected p55−/− mice compared to uninfected controls. No differences in total plasma cholesterol values were observed between infected and sham-inoculated animals.
Elhage et al.14 used repeated subcutaneous injections of a specific TNF/LT inhibitor (TNF-binding-protein; TNFbp) consisting of two molecules of the extracellular domain of p55 linked to a polyethylene glycol molecule, which binds with equal affinity to both TNF-α and LTα. This treatment of ApoE−/− mice with TNFbp did not affect total plasma cholesterol, but there was a tendency to a decrease in fatty streak area in females, not in males. In this study, however, the duration of TNFbp treatment was relatively short (1 month) and the cytokine antagonist was delivered subcutaneously.
Involvement of TNF-α and/or LTα in the progression of atherosclerosis was further substantiated by Branen et al.60 Mice deficient in both ApoE and TNF-α exhibited a 50% reduction in lesion size after 10 weeks of Western-style diet feeding. In ApoE−/− mice treated with recombinant soluble p55-receptor-releasing pellets, the lesion size was 75% reduced (after 25 weeks).
Similarly, Ohta et al.61 reported that disruption of the TNF-α gene diminishes atherosclerosis development in ApoE−/− mice without affecting serum cholesterol. Kober et al.62 confirmed this observation and further demonstrated by MRI that lesion progression and atherosclerotic wall-thickening is reduced in TNF−/−/ApoE−/−mice.
Chew et al.63 administered thalidomide, an inhibitor of TNF-α production, to female ApoE−/− mice fed a HF Western-type diet without cholate and reported a significantly smaller lesion size by thalidomide as compared to placebo-treatment.
Boesten et al.,64 using TNF-α-deficient ApoE*3Leiden mice fed a cholesterol-rich diet, found that the absence of TNF-α did not affect atherosclerotic lesion area, but inhibited lesion progression towards an advanced phenotype.
Canault et al.65 investigated the effect of a transmembrane form of TNF-α on atherosclerosis in male mice. They compared the development of early atherosclerotic lesions in (i) TNF−/− mice that express only a non-cleavable transmembrane form of TNF (tmTNF mice), (ii) wild-type C57/BL6 (WT) mice, and (iii) TNF−/− mice, all fed an atherogenic diet for 20 weeks. Lipid deposition was most prominent in WT, tended to be lower in tmTNF mice, and rare in TNF−/− mice. Macrophage accumulation was five-fold lower in tmTNF than in WT mice.
In WT mice, the plasma lipid profile was more atherogenic than that of TNF−/− mice, but not significantly different from that of tmTNF mice. These results indicate that in contrast to TNF−/− mice, mice expressing exclusively tmTNF are not completely protected from early atherosclerotic lesion formation, although their lesions have a less inflammatory state than those of WT mice, which underlines the stronger pro-inflammatory potential of soluble TNF.
In contrast to Canault et al., and seemingly at variance with their findings with p55−/− mice,55 loss of TNF-α did not alter lesion development in female C57/BL6 mice in experiments described by Schreyer et al., while LTα deficiency resulted in a marked 62% reduction in lesion size.56 The disparity in results obtained by Schreyer et al. between ligand- and receptor-deficient mice suggests there are undefined members of the TNF ligand and receptor signalling pathway involved with regulating atherogenesis.
13. Macrophage migration inhibitory factor
MIF is a pleiotropic cytokine with hormone-like and enzymatic properties.66 Unique among cytokines is MIF’s uptake into target cells where it controls cell cycle and inflammation.67,68 MIF is predominantly released from macrophages, T-lymphocytes, and SMC, and was shown to be increasingly expressed during atherogenesis,69 with oxLDL being a major inducer.
Genetic MIF-deletion in LDLR−/− mice retarded the atherosclerotic process and resulted in reduced intimal thickening, SMC proliferation, lipid deposition, and protease expression (both cathepsins and matrix metalloproteinases)70 (Table 1). After 26 weeks on an atherogenic diet, 20% of the MIF−/−/LDLR−/− mice had developed only early, fatty-streak-like lesions, whereas >80% of the LDLR−/− mice displayed advanced lesions containing calcification and lipid cores. Antibody blockage of MIF significantly reduced (by 75%) intimal macrophage infiltration in ApoE−/− mice undergoing spontaneous atherosclerosis and diminished the aortic plaque area.71 Schober et al.72 showed that MIF antibody treatment also diminishes neointimal macrophage/foam cell content after endothelial denudation. In the same study, SMC and collagen content were increased, reflecting a shift in plaque composition towards a stable phenotype. Decreased neointimal thickening and cellular proliferation was also shown in LDLR−/− mice on a C57/BL6J background undergoing carotid injury.73 Seven days after surgery, anti-MIF treatment reduced the number of inflammatory CD45-positive cells invading the intima by 27%. In accordance with this anti-inflammatory effect achieved with MIF antibody treatment, Burger-Kentischer et al.71 reported that immunoneutralization of MIF reduces parameters of aortic (CD40L, ICAM, and MMP-2) and systemic inflammation (SAA, fibrinogen, and IL-6).
A recent study demonstrates that blockage of MIF in ApoE−/− mice with established advanced atherosclerotic lesions leads to plaque regression and reduces monocyte and T-cell content in atherosclerotic lesions.2 Using ApoE−/− mice, MIF−/−/LDLR−/− mice, and anti-MIF antibodies, the authors showed that MIF functions as a non-cognate CXCR ligand and plays a prominent role in the recruitment of macrophages, T-cells, and neutrophils. The proatherogenic effects of MIF may thus mainly be explained by its cell arrest and chemoattractant functions.
14. Interferon-γ
The pleiotropic cytokine IFN-γ is a pro-inflammatory mediator that is expressed at high levels in atherosclerotic lesions by various cells, including monocytes/macrophages, TH1 cells, and natural killer T-cells (NKT).6 Activated NKT can produce significant amounts of IFN-γ and are pro-atherogenic in mice (Hansson and Libby1 and references cited therein). IFN-γ has been implicated in the atherosclerotic process via direct effects53,74–76 and indirectly via IL-12 and IL-1823,50,77 (Table 1).
Daily injection of recombinant IFN-γ in ApoE−/− mice significantly increased lesion size 2-fold, despite a 15% decrease in serum cholesterol levels.76 IFN-γ administration also significantly increased the number of T-cells within lesions. The pro-inflammatory role of IFN-γ was further underscored in studies using IFN-γ−/−/ApoE−/− mice.75 Deficiency of endogenous IFN-γ decreased atherosclerosis development in male ApoE−/− mice fed a normal or HF-diet. This decrease was associated with a decreased abundance and activation of T-cells, without changes in serum cholesterol. Deficiency of IFN-γ has also been shown to substantially decrease extent and influence phenotype of atherosclerosis in male and female LDLR−/− mice, without concomitant changes in lipoprotein profiles.74
In line with this, female IFN-γR−/−/ApoE−/− mice fed a Western-type diet showed a substantial reduction in lesion size, a 60% reduction in lesion lipid accumulation, a decrease in lesion cellularity, and a marked increase in lesion collagen content. Notably, the IFNγR−/−/ApoE−/− mice exhibited a significant increase in potentially atheroprotective phospholipid/apoA-IV rich particles.53 Koga et al.78 investigated blocking of IFN-γ function by overexpressing a soluble mutant of IFN-γ receptor (sIFNγR) in ApoE−/− mice and found a reduced luminal plaque area.
There is also indirect evidence for a role of IFN-γ in atherogenesis. One of the primary factors regulating IFN-γ secretion is IL-18. Whitman et al.77 demonstrated that the absence of IFN-γ in IFN-γ−/−/ApoE−/− mice ablated the effects of recombinant IL-18 administration on atherosclerosis. Elhage et al.50 reported that IL-18−/−/ApoE−/− mice exhibited a substantial, 35% reduction in lesion size when compared with IL-18-competent littermates, while the compound knockout mice displayed reduced I-Ab gene expression, implying reduced local IFN-γ stimulation.
IL-18 functions synergistically with other co-stimulators, particularly IL-12, to induce pronounced secretion of IFN-γ by macrophages and T-cells. Compared to ApoE−/− mice, IL-12−/−/ApoE−/− mice displayed a 52% reduction in plaque area in the aortic root at 30 weeks of age,23 similar to that reported in IFN-γR−/−/ApoE−/− mice (which also have impaired TH1 responses).53
15. Colony stimulating factors
CSFs are key cytokines involved in the survival, proliferation, and differentiation of myeloid progenitor cells as well as the functional activation of mature myeloid cells. Four CSFs have been cloned and characterized: multi-CSF/IL-3 (produced by TH1 and TH2-cells), granulocyte macrophage-CSF (GM-CSF), macrophage-CSF (M-CSF=CSF-1), and granulocyte-CSF (G-CSF).79 The CSFs have a significant overlap in their biological actions. G-CSF and M-CSF are essentially lineage-restricted in their actions on neutrophil and macrophage precursors while GM-CSF and IL-3 also can stimulate other cell types. A comprehensive body of evidence now exists that CSFs have actions in cardiovascular diseases, among which atherosclerosis80,81 (Table 1).
M-CSF has been implicated in a variety of inflammatory disorders, including atherosclerosis (see references5,80 and references therein). Atherogenic oxidized LDLs induce aortic EC and SMC expression of M-CSF, partly through activation of NF-κB.5,82–84 The localized expression of M-CSF in the vessel wall may be critical in promoting the survival of lipid-laden foam cells observed in early and advanced stages of atherosclerosis. M-CSF selectively regulates the growth and survival of mononuclear phagocytes by binding to a specific cell surface receptor, c-fms. In addition, M-CSF functions as a chemotactic factor for monocytes and regulates the effector functions of mature monocytes and macrophages, i.e. it modulates inflammatory responses by stimulating the production of other cytokines (e.g. IL-6, IL-8, IL-12, IL-18, and TNF-α) and growth factors.
Osteopetrotic (op/op) mice that lack M-CSF due to a point mutation in the M-CSF gene have proven to be useful in examining the role of M-CSF in atherogenesis. Smith et al.85 found that op/ApoE−/− mice fed a low-fat diet had significantly smaller proximal aortic lesions than their ApoE−/− littermates, despite an almost 3-fold increase in plasma cholesterol. Qiao et al.86 reported experiments in which atherogenesis was induced either by feeding op/op mice a HF/HC-diet or by crossbreeding these mice with ApoE−/− mice to generate M-CSF−/−/ApoE−/− mice. In both cases, M-CSF deficiency significantly reduced atherogenesis. The effect of the M-CSF deficiency appears not to be mediated by changes in lipoproteins, because deficient mice exhibited higher levels of atherogenic lipoprotein particles. De Villiers et al.4 also reported that mice deficient in M-CSF (op) and ApoE display elevated cholesterol levels but are protected from atherosclerosis, thereby illustrating the importance of both M-CSF and M-CSF-dependent monocytes/macrophages in maintaining cholesterol homeostasis and in atherogenesis.
Using LDLR−/− mice, Rajavashisth et al.5 showed that atheroma development depends on M-CSF concentration, as not only did homozygous op/op mice have dramatically reduced lesions (∼0.3% of control lesion size) but heterozygous (op/+) mice had lesions <1% of controls. Plasma M-CSF levels in heterozygous (op/+) mice were about half of those in controls (+/+). The finding that a ∼2-fold reduction in M-CSF expression reduced lesion size ∼100-fold suggests the requirement for a threshold level of M-CSF. The effects of M-CSF on atherosclerosis in LDLR−/− mice did not appear to be mediated by changes in plasma lipoproteins or alterations in the number of circulating monocytes, since both op/op and op/+ mice exhibited higher levels of atherogenic lipoprotein particles,86 and (op/+) mice showed a near normal number of circulating monocytes. Taken together, these results suggest that the effects of M-CSF on atherogenesis are not mediated by systemic M-CSF or by changes in monocyte numbers, but that local M-CSF plays an essential role in the arterial wall itself. Importantly, M-CSF is critical for promoting the early stages of atherosclerosis since administration of a monoclonal antibody against c-fms, the receptor of M-CSF, protects from early atherogenesis but does not attenuate progression of already developed lesions in ApoE−/− mice.87 SMC can also express c-fms and it has been suggested that activation of these cells by M-CSF may promote development of smooth-muscle-derived foam cells.
Similar to M-CSF, GM-CSF is induced in EC by oxidized lipids84 and abundantly present in atherosclerotic lesions.88 Its function overlaps with that of M-CSF. GM-CSF appears a major regulator specifically of dendritic cell differentiation and aortic lesional dendritic cell accumulation. So far, reported findings on the effect of GM-CSF on atherosclerosis are inconsistent. Hyperlipidaemic LDLR−/− mice with a GM-CSF deficiency exhibited ∼20–50% decrease in aortic lesion size, depending on the location of the lesions and the sex of the animals.88 Similarly, treatment of male ApoE−/− mice (fed a cholate-containing diet) with murine GM-CSF (10 µg kg−1 day−1 subcutaneously) increased the atherosclerotic lesion area by 2-fold.89 In contrast to both studies, genetic deletion of GM-CSF in ApoE−/− mice increased atherosclerotic lesion size, without affecting plasma cholesterol.90 In the latter study, animals were not fed cholate. Furthermore, the collagen content in the lesions was reduced, suggesting the formation of unstable plaques.
While both M-CSF and, to some lesser extent, GM-CSF have been extensively studied in atherosclerosis and other inflammatory disorders, G-CSF and multi-CSF/IL-3 have received hardly any attention hitherto. Haghighat et al.89 showed that subcutaneous administration of G-CSF (10 µg kg−1 day−1) in ApoE−/− mice over an 8-week period resulted in a 44% increase in atherosclerotic lesion extent, without alterations in plasma lipids or vascular and systemic inflammation markers. No studies on the role of IL-3 in atherosclerosis have been described yet.
In summary, of the four CSFs characterized, only endogenous M-CSF has been consistently found proatherogenic, most probably by influencing the lesional macrophage phenotype. GM-CSF has been attributed both pro-atherogenic and anti-atherogenic properties, while the role of G-CSF and multi-CSF/IL-3 in atherosclerosis needs further research.
16. Summary and conclusions
Inflammatory processes are involved at all stages of the atherosclerotic process, from lesion initiation to plaque rupture. Potentially, cytokines play an important role in the pathogenesis of atherosclerosis and thus could act as important targets for anti-atherosclerotic strategies, on top of those directed at cholesterol-lowering.
Here, we have summarized all currently available information from mice studies on the contribution of interleukins, macrophage-related cytokines, and CSFs to lesion evolution. Table 2 provides a global overview on the overall effects of these cytokines on plasma cholesterol and atherosclerosis in C57/BL6, ApoE−/−, and LDLr−/− mice. An important outcome of this survey is that several cytokines show explicit and consistent pro-atherogenic effects, generally independent of the experimental approach and conditions chosen: IL-1, IL-12, IL-18, TNF-α, MIF, IFN-γ, and M-CSF all display pro-atherogenic characteristics while IL-10 clearly has anti-atherogenic properties (Figure 2B). Some cytokines have a dual character and show variable effects: IL-4, IL-6, and GM-CSF can have pro- or anti-atherogenic properties, depending on the mouse model and gender used, disease stage analysed, and dietary conditions applied. Only a few studies have tested IL-2, IL-20, G-CSF (all showing pro-atherogenic characteristics so far), or IL-5 (anti-atherogenic to this point), and additional experimental evidence is required before definitive conclusions can be drawn. Another key result of this review is that several of the pro-atherogenic cytokines (IL-1, IL-18, TNF-α, IFN-γ, MIF, and M-CSF) affect plasma cholesterol levels, underscoring the notion that inflammation and lipid metabolism are interlinked processes.2,91–93
Table 2.
Comparison of the overall effects of the cytokines reviewed on plasma cholesterol levels and atherosclerosis in C57BL6 mice, ApoE−/− mice, and LDLr−/− mice
| Mouse model | Approach | C57BL6 |
ApoE−/− |
LDLr−/− |
|||
|---|---|---|---|---|---|---|---|
| Cytokine | Chol | Athero | Chol | Athero | Chol | Athero | |
| IL-1 | IL-1R antagonist (IL1Ra) exogenous | x | x | ↔ | ↓ | x | x |
| tg IL-1Ra overexpression | x | x | ↔ | ↓ | ↑ (↔ with cholate) | ↔ (↓ with cholate) | |
| IL-1Ra−/− | ↓ | ↑ | ↑ | ↑ | x | x | |
| IL-1α−/− | ↑ (↔ BM) | ↓ | x | x | x | x | |
| IL-1β−/− | ↔ | ↓ | ↔ | ↓ | x | x | |
| IL-1R | x | x | x | ↓ | x | x | |
| IL-2 | Exogenous | x | x | x | ↑ | x | x |
| Anti-IL-2-AB | x | x | x | ↓ | x | x | |
| IL-4 | Exogenous | ↔ | ↓ | x | x | x | x |
| −/− | ↔ | va | ↔ | ↓ | ↔ | ↔ | |
| IL-5 | −/− | x | x | x | x | x | ↑ |
| IL-6 | Recombinant | ↔ | ↑ | ↔ | ↑ | x | x |
| −/− | x | ↑ | va (↔↑) | va (↔↑) | ↔ | ↔ | |
| IL-10 | Transgenic | ↔ | ↓ | x | x | ↔ | ↓ |
| −/− | ↔ | ↑ | ↔ | ↑ | ↔ | ↑ | |
| Gene transfer | x | x | va | ↓ | x | ↓ | |
| IL-12 | Exogenous | x | x | ↔ | ↑ | ↔ | ↓ |
| p40−/− | x | x | ↔ | ↓ | x | x | |
| Anti-IL-12-AB | x | x | x | x | x | ↓ | |
| IL-18 | Exogenous | x | x | ↔ | ↑ | x | x |
| −/− | x | x | ↑ | ↓ | x | x | |
| IL-18 bpm | x | x | x | ↓ | x | x | |
| IL-20 | Exogenous | x | x | x | ↑ | x | x |
| TNF | TNFR−/− | ↔ | ↑p55−/−↔p75−/− | ↔ | ↔↓ (stage-dependent) | x | x |
| TNFα−/− | ↑ | ↔ ↓ | ↔ | ↓ | x | x | |
| tmTNF | ↔ | ↓ | x | x | x | x | |
| Anti-TNF | x | x | ↔ | ↓ | x | x | |
| TNFbp | x | x | ↔ | ↓ (f)↔(m) | x | x | |
| IFN-γ | IFNγ−/− | x | x | ↔ | ↓ | ↔ | ↓ |
| IFNγR−/− | x | x | ↑ | ↓ | x | x | |
| Exogenous | x | x | ↓ | ↑ | x | x | |
| Soluble IFNγR | x | x | ↔ | ↓ | x | x | |
| MIF | −/− | x | x | x | x | ↓ | ↓ |
| Anti-MIF-AB | x | x | ↔ | ↓ | ↔ | ↓ | |
| M-CSF | −/− | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ |
| Anti-M-CSF receptor | x | x | ↔ | ↓ | x | x | |
| GM-CSF | −/− | x | x | ↔ | ↑ | ↔ | ↓ |
| Exogenous | x | x | ↔ | ↑ | x | x | |
| G-CSF | Exogenous | x | x | ↔ | ↑ | x | x |
x, no data available; ↑, increase; ↓, decrease; ↔, no effect; va, variable despite comparable experimental conditions; AB, antibody; BM, bone marrow transplantation; tm, transmembrane; f/m, female/male.
Overall, our survey provides support for the notion that modulating the activity of selected cytokines (either systemically or locally) can block or retard the development of atherosclerotic lesions and thus positively influence disease outcome. The significant impact of selected cytokines on atherogenesis could be of significance for early detection and prevention of the disease, and also provides new opportunities for anti-atherosclerotic treatment regimens, alone or in combination with established hypolipidaemic or hypotensive strategies. Importantly, in the studies described in this review, the activity of the cytokine of interest was modulated by techniques such as genetic deletion, overexpression, immunoneutralization, or in vivo administration of the cytokine or its receptor or inhibitor, techniques not easily applicable to humans. The chronic nature of the atherosclerotic disease process and the generally pleiotropic effects of cytokines will demand high specificity of action and/or effective targeting to prevent the emergence of adverse side effects with clinical applications.94
Conflict of interest: none declared.
Funding
The Dutch Organization for Scientific Research (NWO; grant VENI 016. 036.061 to R.K.); the European Nutrigenomics Organisation (NuGO, CT-2004-505944 to S.Z.); and the TNO research program Personalized Health VP9 (to R.K. and T.K.).
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 3.Mangge H, Hubmann H, Pilz S, Schauenstein K, Renner W, Marz W. Beyond cholesterol–inflammatory cytokines, the key mediators in atherosclerosis. Clin Chem Lab Med. 2004;42:467–474. doi: 10.1515/CCLM.2004.081. [DOI] [PubMed] [Google Scholar]
- 4.de Villiers WJ, Smith JD, Miyata M, Dansky HM, Darley E, Gordon S. Macrophage phenotype in mice deficient in both macrophage-colony-stimulating factor (op) and apolipoprotein E. Arterioscler Thromb Vasc Biol. 1998;18:631–640. doi: 10.1161/01.atv.18.4.631. [DOI] [PubMed] [Google Scholar]
- 5.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, et al. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey EJ, Ramji DP. Interferon-gamma and atherosclerosis: pro- or anti-atherogenic? Cardiovasc Res. 2005;67:11–20. doi: 10.1016/j.cardiores.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421–2432. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- 8.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 9.Walch L, Massade L, Dufilho M, Brunet A, Rendu F. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis. 2006;187:285–291. doi: 10.1016/j.atherosclerosis.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Mallat Z, it-Oufella H, Tedgui A. Regulatory T cell responses: potential role in the control of atherosclerosis. Curr Opin Lipidol. 2005;16:518–524. doi: 10.1097/01.mol.0000182532.11512.90. [DOI] [PubMed] [Google Scholar]
- 11.Zadelaar S, Kleemann R, Verschuren L, de Vries van der Weij, van der Hoorn J, Princen HM, et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706–1721. doi: 10.1161/ATVBAHA.107.142570. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- 13.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 14.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 15.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, et al. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Devlin CM, Kuriakose G, Hirsch E, Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc Natl Acad Sci USA. 2002;99:6280–6285. doi: 10.1073/pnas.092324399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, et al. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 18.Isoda K, Ohsuzu F. The effect of interleukin-1 receptor antagonist on arteries and cholesterol metabolism. J Atheroscler Thromb. 2006;13:21–30. doi: 10.5551/jat.13.21. [DOI] [PubMed] [Google Scholar]
- 19.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 20.Kamari Y, Werman-Venkert R, Shaish A, Werman A, Harari A, Gonen A, et al. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195:31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 22.Upadhya S, Mooteri S, Peckham N, Pai RG. Atherogenic effect of interleukin-2 and antiatherogenic effect of interleukin-2 antibody in apo-E-deficient mice. Angiology. 2004;55:289–294. doi: 10.1177/000331970405500308. [DOI] [PubMed] [Google Scholar]
- 23.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George J, Mulkins M, Shaish A, Casey S, Schatzman R, Sigal E, et al. Interleukin (IL)-4 deficiency does not influence fatty streak formation in C57BL/6 mice. Atherosclerosis. 2000;153:403–411. doi: 10.1016/s0021-9150(00)00418-4. [DOI] [PubMed] [Google Scholar]
- 25.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 26.George J, Shoenfeld Y, Gilburd B, Afek A, Shaish A, Harats D. Requisite role for interleukin-4 in the acceleration of fatty streaks induced by heat shock protein 65 or Mycobacterium tuberculosis. Circ Res. 2000;86:1203–1210. doi: 10.1161/01.res.86.12.1203. [DOI] [PubMed] [Google Scholar]
- 27.King VL, Cassis LA, Daugherty A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am J Pathol. 2007;171:2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 29.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 31.Van Lenten BJ, Wagner AC, Navab M, Fogelman AM. Oxidized phospholipids induce changes in hepatic paraoxonase and ApoJ but not monocyte chemoattractant protein-1 via interleukin-6. J Biol Chem. 2001;276:1923–1929. doi: 10.1074/jbc.M004074200. [DOI] [PubMed] [Google Scholar]
- 32.Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, et al. Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis. 2001;156:315–320. doi: 10.1016/s0021-9150(00)00682-1. [DOI] [PubMed] [Google Scholar]
- 33.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 34.Song L, Schindler C. IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis. 2004;177:43–51. doi: 10.1016/j.atherosclerosis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39:1740–1743. [PubMed] [Google Scholar]
- 36.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 37.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–432. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 38.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Li D, Chen J, Xie J, Bandyopadhyay S, Zhang D, et al. Inhibition of atherogenesis in LDLR knockout mice by systemic delivery of adeno-associated virus type 2-hIL-10. Atherosclerosis. 2006;188:19–27. doi: 10.1016/j.atherosclerosis.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 41.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 42.Namiki M, Kawashima S, Yamashita T, Ozaki M, Sakoda T, Inoue N, et al. Intramuscular gene transfer of interleukin-10 cDNA reduces atherosclerosis in apolipoprotein E-knockout mice. Atherosclerosis. 2004;172:21–29. doi: 10.1016/j.atherosclerosis.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Yoshioka T, Okada T, Maeda Y, Ikeda U, Shimpo M, Nomoto T, et al. Adeno-associated virus vector-mediated interleukin-10 gene transfer inhibits atherosclerosis in apolipoprotein E-deficient mice. Gene Ther. 2004;11:1772–1779. doi: 10.1038/sj.gt.3302348. [DOI] [PubMed] [Google Scholar]
- 44.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 45.Potteaux S, Esposito B, van OO, Brun V, Ardouin P, Groux H, et al. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 46.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 47.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 48.Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, et al. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112:1054–1062. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- 49.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 50.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 51.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, et al. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–E45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 52.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen WY, Cheng BC, Jiang MJ, Hsieh MY, Chang MS. IL-20 is expressed in atherosclerosis plaques and promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2090–2095. doi: 10.1161/01.ATV.0000232502.88144.6f. [DOI] [PubMed] [Google Scholar]
- 55.Schreyer SA, Peschon JJ, Leboeuf RC. Accelerated atherosclerosis in mice lacking tumor necrosis factor receptor p55. J Biol Chem. 1996;271:26174–26178. doi: 10.1074/jbc.271.42.26174. [DOI] [PubMed] [Google Scholar]
- 56.Schreyer SA, Vick CM, Leboeuf RC. Loss of lymphotoxin-alpha but not tumor necrosis factor-alpha reduces atherosclerosis in mice. J Biol Chem. 2002;277:12364–12368. doi: 10.1074/jbc.M111727200. [DOI] [PubMed] [Google Scholar]
- 57.Blessing E, Bea F, Kuo CC, Campbell LA, Chesebro B, Rosenfeld ME. Lesion progression and plaque composition are not altered in older apoE−/− mice lacking tumor necrosis factor-alpha receptor p55. Atherosclerosis. 2004;176:227–232. doi: 10.1016/j.atherosclerosis.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, et al. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1087–1094. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell LA, Nosaka T, Rosenfeld ME, Yaraei K, Kuo CC. Tumor necrosis factor alpha plays a role in the acceleration of atherosclerosis by Chlamydia pneumoniae in mice. Infect Immun. 2005;73:3164–3165. doi: 10.1128/IAI.73.5.3164-3165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 61.Ohta H, Wada H, Niwa T, Kirii H, Iwamoto N, Fujii H, et al. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Kober F, Canault M, Peiretti F, Mueller C, Kopp F, Alessi MC, et al. MRI follow-up of TNF-dependent differential progression of atherosclerotic wall-thickening in mouse aortic arch from early to advanced stages. Atherosclerosis. 2007;195:e93–e99. doi: 10.1016/j.atherosclerosis.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Chew M, Zhou J, Daugherty A, Eriksson T, Ellermann-Eriksen S, Hansen PR, et al. Thalidomide inhibits early atherogenesis in apoE-deficient mice. APMIS Suppl. 2003;109:113–116. [PubMed] [Google Scholar]
- 64.Boesten LS, Zadelaar AS, van Nieuwkoop A, Gijbels MJ, de Winther MP, Havekes LM, et al. Tumor necrosis factor-alpha promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc Res. 2005;66:179–185. doi: 10.1016/j.cardiores.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Canault M, Peiretti F, Mueller C, Kopp F, Morange P, Rihs S, et al. Exclusive expression of transmembrane TNF-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis. 2004;172:211–218. doi: 10.1016/j.atherosclerosis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 67.Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408:211–216. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- 68.Kleemann R, Grell M, Mischke R, Zimmermann G, Bernhagen J. Receptor binding and cellular uptake studies of macrophage migration inhibitory factor (MIF): use of biologically active labeled MIF derivatives. J Interferon Cytokine Res. 2002;22:351–363. doi: 10.1089/107999002753675785. [DOI] [PubMed] [Google Scholar]
- 69.Burger-Kentischer A, Goebel H, Seiler R, Fraedrich G, Schaefer HE, Dimmeler S, et al. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation. 2002;105:1561–1566. doi: 10.1161/01.cir.0000012942.49244.82. [DOI] [PubMed] [Google Scholar]
- 70.Pan JH, Sukhova GK, Yang JT, Wang B, Xie T, et al. Macrophage migration inhibitory factor deficiency impairs atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:3149–3153. doi: 10.1161/01.CIR.0000134704.84454.D2. [DOI] [PubMed] [Google Scholar]
- 71.Burger-Kentischer A, Gobel H, Kleemann R, Zernecke A, Bucala R, Leng L, et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF) Atherosclerosis. 2006;184:28–38. doi: 10.1016/j.atherosclerosis.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 72.Schober A, Bernhagen J, Thiele M, Zeiffer U, Knarren S, Roller M, et al. Stabilization of atherosclerotic plaques by blockade of macrophage migration inhibitory factor after vascular injury in apolipoprotein E-deficient mice. Circulation. 2004;109:380–385. doi: 10.1161/01.CIR.0000109201.72441.09. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z, Sakuma M, Zago AC, Zhang X, Shi C, Leng L, et al. Evidence for a role of macrophage migration inhibitory factor in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:709–714. doi: 10.1161/01.ATV.0000119356.35748.9e. [DOI] [PubMed] [Google Scholar]
- 74.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 75.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 76.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 78.Koga M, Kai H, Yasukawa H, Kato S, Yamamoto T, Kawai Y, et al. Postnatal blocking of interferon-gamma function prevented atherosclerotic plaque formation in apolipoprotein E-knockout mice. Hypertens Res. 2007;30:259–267. doi: 10.1291/hypres.30.259. [DOI] [PubMed] [Google Scholar]
- 79.Nicola NA. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- 80.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Kovacic JC, Muller DW, Graham RM. Actions and therapeutic potential of G-CSF and GM-CSF in cardiovascular disease. J Mol Cell Cardiol. 2007;42:19–33. doi: 10.1016/j.yjmcc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Rajavashisth TB, Yamada H, Mishra NK. Transcriptional activation of the macrophage-colony stimulating factor gene by minimally modified LDL. Involvement of nuclear factor-kappa B. Arterioscler Thromb Vasc Biol. 1995;15:1591–1598. doi: 10.1161/01.atv.15.10.1591. [DOI] [PubMed] [Google Scholar]
- 83.Liao F, Andalibi A, Qiao JH, Allayee H, Fogelman AM, Lusis AJ. Genetic evidence for a common pathway mediating oxidative stress, inflammatory gene induction, and aortic fatty streak formation in mice. J Clin Invest. 1994;94:877–884. doi: 10.1172/JCI117409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajavashisth TB, Andalibi A, Territo MC, Berliner JA, Navab M, Fogelman AM, et al. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990;344:254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- 85.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, et al. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 87.Murayama T, Yokode M, Kataoka H, Imabayashi T, Yoshida H, Sano H, et al. Intraperitoneal administration of anti-c-fms monoclonal antibody prevents initial events of atherogenesis but does not reduce the size of advanced lesions in apolipoprotein E-deficient mice. Circulation. 1999;99:1740–1746. doi: 10.1161/01.cir.99.13.1740. [DOI] [PubMed] [Google Scholar]
- 88.Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2007;115:2049–2054. doi: 10.1161/CIRCULATIONAHA.106.665570. [DOI] [PubMed] [Google Scholar]
- 90.Ditiatkovski M, Toh BH, Bobik A. GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2337–2344. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- 91.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 92.Kleemann R, Verschuren L, van Erk MJ, Nikolsky Y, Cnubben NH, Verheij ER, et al. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 2007;8:R200. doi: 10.1186/gb-2007-8-9-r200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steinberg D. Hypercholesterolemia and inflammation in atherogenesis: two sides of the same coin. Mol Nutr Food Res. 2005;49:995–998. doi: 10.1002/mnfr.200500081. [DOI] [PubMed] [Google Scholar]
- 94.von der Thüsen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 95.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 96.Koga M, Kai H, Yasukawa H, Yamamoto T, Kawai Y, Kato S, et al. Inhibition of progression and stabilization of plaques by postnatal interferon-gamma function blocking in ApoE-knockout mice. Circ Res. 2007;101:348–356. doi: 10.1161/CIRCRESAHA.106.147256. [DOI] [PubMed] [Google Scholar]