Abstract
We investigated whether the MuSVts110 gag gene product (P58gag) can regulate the novel growth temperature dependence of MuSVts110 RNA splicing. MuSVts110 mutants with either frameshifts or deletions in the gag gene were tested for their ability to maintain the MuSVts110 splicing phenotype. Only small decreases in splicing efficiency and no changes in the thermosensitivity of viral RNA splicing were observed in MuSVts110 gag gene frameshift mutants. Deletions within the gag gene, however, variably decreased MuSVts110 splicing efficiency but had no effect on its thermosensitivity. Another class of MuSVts110 splicing mutants generated by treatment of MuSVts110-infected cells with NiCl2 was also examined. In these "nickel revertants," P58gag is made, but splicing of the viral transcript is nearly complete at all growth temperatures. The splicing of "tagged" viral RNA transcribed from a modified MuSVts110 DNA introduced into nickel revertant cells remained thermosensitive, arguing against trans effects of viral gene products on splicing efficiency. These experiments indicated that neither the MuSVts110 P58gag protein nor any other viral gene product acts in trans to regulate MuSVts110 splicing.
Full text
PDF
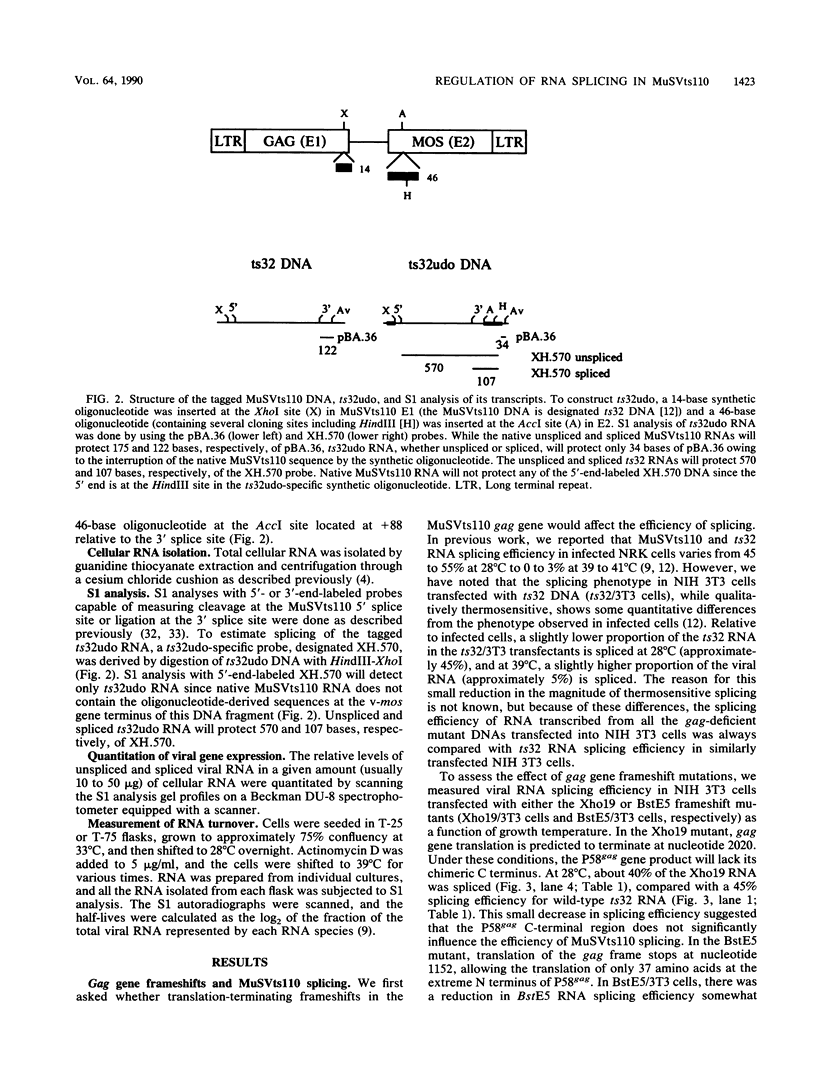





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo S., Beemon K. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988 Nov;8(11):4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham J. R., Jr, Scott M. P. Developmentally regulated alternative splicing of transcripts from the Drosophila homeotic gene Antennapedia can produce four different proteins. EMBO J. 1988 Oct;7(10):3211–3222. doi: 10.1002/j.1460-2075.1988.tb03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggart N. W., Gallick G. E., Murphy E. C., Jr Nickel-induced heritable alterations in retroviral transforming gene expression. J Virol. 1987 Aug;61(8):2378–2388. doi: 10.1128/jvi.61.8.2378-2388.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggart N. W., Murphy E. C., Jr Analysis of metal-induced mutations altering the expression or structure of a retroviral gene in a mammalian cell line. Mutat Res. 1988 Mar;198(1):115–129. doi: 10.1016/0027-5107(88)90047-4. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Chen I. S., Temin H. M. Substitution of 5' helper virus sequences into non-rel portion of reticuloendotheliosis virus strain T suppresses transformation of chicken spleen cells. Cell. 1982 Nov;31(1):111–120. doi: 10.1016/0092-8674(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Cizdziel P. E., de Mars M., Murphy E. C., Jr Exploitation of a thermosensitive splicing event to study pre-mRNA splicing in vivo. Mol Cell Biol. 1988 Apr;8(4):1558–1569. doi: 10.1128/mcb.8.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Kopchick J. J., Stacey D. W. Effect of intron size on splicing efficiency in retroviral transcripts. Nucleic Acids Res. 1982 Oct 11;10(19):6177–6190. doi: 10.1093/nar/10.19.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsert C., Morin N., Klessig D. F. cis-acting elements and a trans-acting factor affecting alternative splicing of adenovirus L1 transcripts. Mol Cell Biol. 1989 Oct;9(10):4364–4371. doi: 10.1128/mcb.9.10.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Manley J. L. Factors influencing alternative splice site utilization in vivo. Mol Cell Biol. 1987 Feb;7(2):738–748. doi: 10.1128/mcb.7.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallick G. E., Hamelin R., Maxwell S., Duyka D., Arlinghaus R. B. The gag-mos hybrid protein of ts110 Moloney murine sarcoma virus: variation of gene expression with temperature. Virology. 1984 Dec;139(2):366–374. doi: 10.1016/0042-6822(84)90382-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Chan E. K., Tan E. M., Arlinghaus R. B. Antibodies against small nuclear ribonucleoproteins immunoprecipitate complexes containing ts110 Moloney murine sarcoma virus genomic and messenger RNAs. Virology. 1986 Jul 15;152(1):87–99. doi: 10.1016/0042-6822(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Kabat K., Blair D., Arlinghaus R. B. Temperature-sensitive splicing defect of ts110 Moloney murine sarcoma virus is virus encoded. J Virol. 1986 Jan;57(1):301–309. doi: 10.1128/jvi.57.1.301-309.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Blair D. G., Arlinghaus R. B. Partial characterization of a moloney murine sarcoma virus 85,000-dalton polypeptide whose expression correlates with the transformed phenotype in cells infected with a temperature-sensitive mutant virus. Virology. 1980 Sep;105(2):516–525. doi: 10.1016/0042-6822(80)90052-5. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Hwang L. S., Park J., Gilboa E. Role of intron-contained sequences in formation of moloney murine leukemia virus env mRNA. Mol Cell Biol. 1984 Nov;4(11):2289–2297. doi: 10.1128/mcb.4.11.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Murphy E. C., Jr, Arlinghaus R. B. Electron microscopic analysis of ts1 10 Moloney mouse sarcoma virus, a variant of wild-type virus with two RNAs containing large deletions. J Mol Biol. 1982 Oct 25;161(2):229–250. doi: 10.1016/0022-2836(82)90150-4. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Kotler M., Skalka A. M. cis-acting intron mutations that affect the efficiency of avian retroviral RNA splicing: implication for mechanisms of control. J Virol. 1988 Aug;62(8):2686–2695. doi: 10.1128/jvi.62.8.2686-2695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff S. E., Evans R. M., Rosenfeld M. G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell. 1987 Feb 13;48(3):517–524. doi: 10.1016/0092-8674(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller C. K., Embretson J. E., Temin H. M. Transforming viruses spontaneously arise from nontransforming reticuloendotheliosis virus strain T-derived viruses as a result of increased accumulation of spliced viral RNA. J Virol. 1988 Apr;62(4):1219–1226. doi: 10.1128/jvi.62.4.1219-1226.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. Insertion of several different DNAs in reticuloendotheliosis virus strain T suppresses transformation by reducing the amount of subgenomic mRNA. J Virol. 1986 Apr;58(1):75–80. doi: 10.1128/jvi.58.1.75-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M. A., Brizzard B. L., Wong J. L., Murphy E. C., Jr Murine sarcoma virus ts110 RNA transcripts: origin from a single proviral DNA and sequence of the gag-mos junctions in both the precursor and spliced viral RNAs. J Virol. 1985 Feb;53(2):624–633. doi: 10.1128/jvi.53.2.624-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M., Brown N. V., Wong J. L., Arlinghaus R. B., Murphy E. C., Jr S1 nuclease mapping of viral RNAs from a temperature-sensitive transformation mutant of murine sarcoma virus. J Virol. 1984 May;50(2):478–488. doi: 10.1128/jvi.50.2.478-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Wood T. G., Peltier-Horn J., Robey W. G., Blair D. G., Arlinghaus R. B. Characterization of virus-specified proteins present in NRK cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):747–754. doi: 10.1101/sqb.1980.044.01.080. [DOI] [PubMed] [Google Scholar]
- de Mars M., Cizdziel P. E., Murphy E. C., Jr Activation of thermosensitive RNA splicing and production of a heat-labile P85gag-mos kinase by the introduction of a specific deletion in murine sarcoma virus-124 DNA. J Virol. 1988 Jun;62(6):1907–1916. doi: 10.1128/jvi.62.6.1907-1916.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]