Abstract
TNF-induced NF-κB activity shows complex temporal regulation whose different phases lead to distinct gene expression programs. Combining experimental studies and mathematical modeling, we identify two temporal amplification steps—one determined by the obligate negative feedback regulator IκBα—that define the duration of the first phase of NF-κB activity. The second phase is defined by A20, whose inducible expression provides for a rheostat function by which other inflammatory stimuli can regulate TNF responses. Our results delineate the nonredundant functions implied by the knockout phenotypes of iκbα and a20, and identify the latter as a signaling cross-talk mediator controlling inflammatory and developmental responses.
Keywords: NF-κB signaling, negative feedback, computational modeling, temporal control, IκBα, A20
Tumor necrosis factor (TNF) is a potent cytokine and critical regulator of apoptosis, inflammation, and immunity (Wallach et al. 1999) via control of the transcription factor nuclear factor κB (NF-κB). In resting cells, NF-κB is sequestered in complexes with one of three Inhibitor of κB (IκB) isoforms (α, β, or ε). Upon cellular stimulation, the inhibitor of κB kinase (IKK) phosphorylates IκB proteins, targeting them for proteolysis, which leads to NF-κB nuclear accumulation and transcriptional activation of hundreds of genes, including inflammatory mediators such as cytokines and chemokines, and regulators of proliferation and apoptosis (Ghosh and Karin 2002; Hoffmann and Baltimore 2006).
In response to injury or infection, TNF is secreted in bursts by tissue-resident macrophages, and due to a short half-life (Beutler et al. 1985) it is sensed by responsive cells as transient, or pulse, stimulation. Termination of the resulting NF-κB activity is critical to preventing aberrant inflammatory gene expression, and its misregulation has been implicated in pathologies including cancer, heart disease, and Crohn’s disease (Kaufman and Choi 1999; Aggarwal et al. 2002; Monaco and Paleolog 2004). Several attenuators of the TNF–NF-κB axis have been described, including the rapidly and highly inducible iκbα and a20 genes (Scott et al. 1993; Song et al. 1996). IκBα attenuates NF-κB nuclear localization and DNA binding (Scott et al. 1993) and A20 inhibits signaling upstream of IKK by interfering with the K63-linked ubiquitin chains on RIP1 that are required for activation of the TNR receptor (TNFR)-associated signaling complex (Wertz et al. 2004; M.P. Boldin and D. Baltimore, unpubl.). Based on their NF-κB-dependent inducible expression, IκBα and A20 are commonly thought of as negative feedback regulators in the TNF–NF-κB signaling pathway. However, whether inducible expression is required for their function (i.e., as obligate negative feedback regulators) remains to be tested. Abolishing both constitutive and inducible expression by genetic deletion in mice results in lethality, perinatally in the case of iκbα (Beg et al. 1995; Klement et al. 1996) or within a few weeks of age in the case of a20 (Lee et al. 2000). This suggests that even though IκBα and A20 act in the same signaling axis, they apparently have nonredundant (i.e., differential or specific) functions. Indeed, mutations in the iκbα gene are linked to Hodgkins lymphoma (Cabannes et al. 1999; Krappmann et al. 1999), whereas A20 has been shown to control the severity of atherosclerosis (Wolfrum et al. 2007), the responsiveness to commensual bacteria (Boone et al. 2004; Hitotsumatsu et al. 2008; Turer et al. 2008) and of T cells (Stilo et al. 2008), as well as the development of regulatory T cells and in anti-tumor responses (Song et al. 2008).
Negative feedback regulators not only terminate cellular responses, they also modulate the dynamics of cellular signaling (Stelling et al. 2004; Kholodenko 2006). In the case of TNF signaling, the temporal profile of NF-κB activity is complex: A first phase of NF-κB activity is associated with the expression of a large number of genes that mediate nonspecific inflammatory and stress responses (such as cytokines, anti-apoptotic regulators, and heat-shock proteins), whereas longer lasting NF-κB activity elicits the expression of chemokines (e.g., MCP3, MCP5, and RANTES) and tissue proteases (e.g., MMP3, MMP9, MMP10, MMP12, MMP13) required for an effective adaptive immune response (Saccani et al. 2001; Hoffmann et al. 2002, 2003; Tian et al. 2005; Werner et al. 2005).
Mechanistic mathematical modeling allows for quantitative analyses and temporal resolution of dynamic signaling events that biochemical measurements cannot achieve alone. A mathematical model of the IκB–NF-κB signaling module was first developed based upon biochemical data (Hoffmann et al. 2002) and was validated with real-time single cell fluorescence imaging (Nelson et al. 2004). The model quantitatively accounts for association and dissociation of protein complexes, synthesis of IκB proteins, IKK-dependent and -independent IκB degradation, and nuclear localization of the IκBs and NF-κB. It recapitulates homeostatic control of NF-κB in resting cells (O’Dea et al. 2007), and dynamic regulation differentially provided by the three IκB isoforms (α, β, and ε) (Kearns et al. 2006) in response to distinct inflammatory signals via differential IKK temporal profiles (Werner et al. 2005).
To examine how temporal control of NF-κB is encoded in response to TNF stimulation, we identified functional signaling modules (Hartwell et al. 1999) within the inflammatory network. First, we constructed a mathematical model for the TNFR–IKK signaling module that recapitulates the biochemical events triggered by TNFR engagement, and then integrated it with the IκB–NF-κB module to yield a mathematical description of the TNF–NF-κB signaling axis. Using the combined model in conjunction with experiments, we reveal distinct roles of IκBα and A20 in encoding NF-κB temporal control.
Results
TNF signaling to NF-κB: an integrated mathematical model of two signaling modules
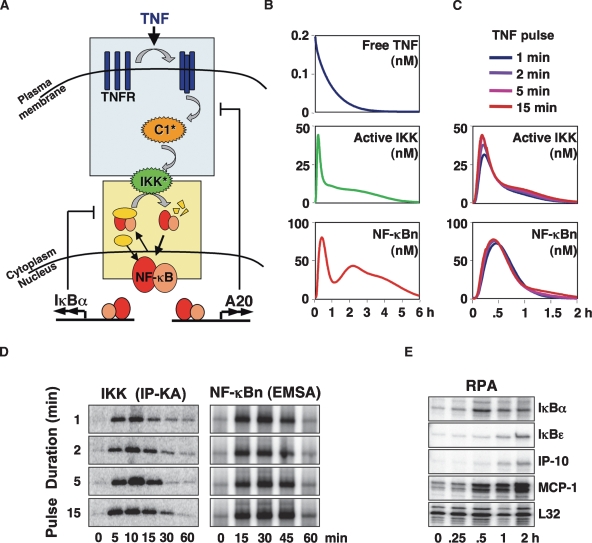
We constructed and parameterized a mathematical model that recapitulates TNF signaling to NF-κB. The model is comprised of 33 species and 98 reactions (Supplemental Tables 1, 2) and consists of a newly constructed model for the TNFR–IKK signaling module (Fig. 1A, blue box; Supplemental Material) combined with the model for the IκB–NF-κB signaling module (Fig. 1A, yellow box) that is based on previously published work (Hoffmann et al. 2002; Werner et al. 2005; Kearns et al. 2006; O’Dea et al. 2007). Our model of the TNFR–IKK signaling module includes the TNF-induced receptor trimerization step (Banner et al. 1993; Grell et al. 1998), receptor internalization (Watanabe et al. 1988), recruitment, and activation of the TRAF2, TRADD, RIP (TTR) complex to TNFR1 to form active Complex 1 (C1*) via K63 ubiquitination of RIP (Schneider-Brachert et al. 2004), and activation of the TAK1/TAB2/TAB3 kinase complex (TAK) (Wang et al. 2001). IKK is activated via phosphorylation of activation loop serines (Mercurio et al. 1997) and inactivated via an activity-dependent mechanism (Delhase et al. 1999). The combined model of the TNFR–IKK and IκB–NF-κB modules also includes the reactions that control NF-κB-dependent synthesis of A20, which directly counteracts C1 activation.
Figure 1.
A mathematical model of TNFR signaling to NF-κB. (A) Schematic depicting TNF signaling from TNFR to IKK, which functions as the input to the NF-κB signaling module. The two most rapidly NF-κB-inducible attenuators, IκBα and A20, are shown. A detailed schematic of the model is available in the Supplemental Material. (B) Computational simulations of persistent TNF stimulation depicting free TNF levels and the activities of IKK and NF-κB. (C) Computational simulations of IKK (top) and NF-κB (bottom) activities in response to 1-, 2-, 5-, or 15-min TNF pulses. (D) IKK and NF-κB activities were experimentally measured in wild-type MEFs in response to 1-, 2-, 5-, or 15-min 1 ng/mL TNF pulses via in vitro IP-kinase assay and EMSA, respectively. (E) RPA to track expression of NF-κB target genes in wild-type MEFs stimulated with a 1-min pulse of TNF (1 ng/mL).
Rate constants for the TNFR–IKK module were derived either from the literature or our own experimental data (Supplemental Tables 1, 2). For example, parameter values for A20 mRNA/protein synthesis and half-life were derived from experimentally determined mRNA (Supplemental Fig. 3A) and protein expression profiles (Supplemental Fig. 1C). Other rate constants were derived via parameter-fitting techniques and ascribed values within ranges that satisfied a set of experimentally determined constraints (Supplemental Material).
Simulation of chronic TNF stimulation results in curves for the concentrations over time of “free” TNF protein, IKK, and NF-κB activities (Fig. 1B). In vitro kinase activity measurements of immunoprecipitated IKK complexes (Supplemental Fig. 1A) confirmed that peak activation occurs at 10 min followed by a rapid attenuation to a plateau that is just a few fold above baseline. Similarly, TNF-induced IκBα protein degradation and rapid resynthesis is accurately reproduced in the model simulation, as is the induction of A20 protein synthesis (Supplemental Fig. 1B). The model correctly describes the experimentally measured NF-κB activity profile, which peaks at 30 min, followed by post-induction repression at 90 min, and a subsequent plateau of late activity (Fig. 1B; Supplemental Fig. 1A).
Given that TNF secretion by macrophages occurs in transient bursts, we first examined signaling to NF-κB by very short pulses of TNF. In simulations, we found that both IKK and NF-κB activity profiles are strikingly similar no matter how long the TNF pulses are: A 1-min TNF pulse was predicted to provide the same degree of activation as a 15-min pulse (Fig. 1C). We addressed this prediction experimentally. Treating cells with TNF pulses of 1, 2, 5, and 15 min, we found that the temporal profile of IKK and nuclear NF-κB activities did not change with the duration of the TNF pulse (Fig. 1D). The results suggest that the TNF-NF-κB signaling axis ensures that NF-κB activity lasts at least 45 min to provide for robust stress response gene expression program. Indeed, we found that even a 1-min pulse of TNF stimulation was able to induce inflammatory gene expression (Fig. 1E).
This first invariant phase of NF-κB activity may be described as “hardwired,” as it is not only independent of the TNF pulse duration, but also the TNF concentration (Werner et al. 2005; Cheong et al. 2006). The hardwired sensitivity of the pathway to transient TNF bursts is due to two temporal amplification steps (Supplemental Fig. 1D): The first, within the TNFR-IKK signaling module, is determined by the autoinhibitory mechanism of the IKK complex that was proposed to involve C-terminal phosphorylation (Delhase et al. 1999) and ensures that IKK activity lasts at least 15 min no matter how short the TNF pulse duration; the second, within the IκB–NF-κB signaling module, is a function of the IκBα feedback. This explains why the first mathematical model of NF-κB activation (Hoffmann et al. 2002), which did not include the TNF-IKK module, correctly recapitulated NF-κB responses to transient TNF stimuli of >15 min, but erroneously predicted reduced NF-κB activation with shorter TNF stimulation durations.
Distinguishing between obligate and nonobligate feedback regulation
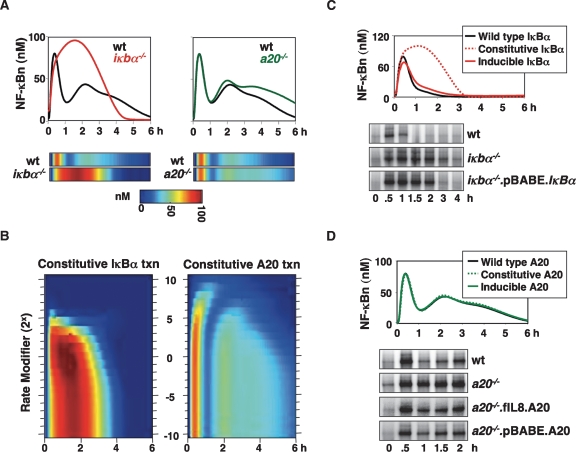
Both IκBα and A20 protein expression are rapidly induced in response to TNF, and have been reported to play important roles in regulating NF-κB responses (Beg et al. 1995; Klement et al. 1996; Lee et al. 2000). In our computational simulations (Fig. 2A), post-induction repression of NF-κB activity occurs at 1 h in wild-type cells, but is delayed to 3 h in iκbα−/− cells. In a20−/− cells, NF-κB activity is sustained and elevated between 3 and 6 h. These simulations conform to experimental results (Hoffmann et al. 2002; Werner et al. 2005), where only a20−/− cells exhibited elevated IKK and NF-κB activity at late (>3-h) time points (Supplemental Fig. 2A,B). One consequence of a greatly enhanced first phase of NF-κB activity in iκbα−/− cells is elevated A20 induction, which results in complete attenuation of late IKK (Supplemental Fig. 2C). This, together with elevated IκBε induction (Kearns et al. 2006), diminishes the second phase of NF-κB activity.
Figure 2.
Inducible expression is critical for the function of IκBα but not A20. (A) Simulations of nuclear NF-κB activity in wild-type, iκbα−/−, and a20−/− MEFs in response to TNF. The data are presented as graphs (top) and as heat maps (below) in which the level of NF-κB is color-coded from 0 nM (blue) to 100 nM (red). (B) Simulations of nuclear NF-κB activity in models with altered constitutive transcription rates of IκBα (left) or A20 (right) in the absence of inducible transcription. In each simulation the constitutive transcription rate was multiplied by one of 21 constitutive transcriptional modifiers, ranging from 2−10, 2−9, 2−8. . .1. . .28, 29, 210. The results were graphed over time (X-axis), with the value of the rate modifier on the Y-axis. NF-κB activity is color-coded as in Figure 2A. (C) Simulations of nuclear NF-κB activity in models possessing both constitutive and inducible expression of IκBα (wild type), or each individually. Nuclear NF-κB activity was then measured via EMSA in wild-type cells, or in iκbα−/− cells reconstituted with a constitutively expressing iκbα transgene (pBABE.IκBα.puro) or an empty vector control (pBABE.EV.puro, labeled iκbα−/−) in response to a 15-min pulse of TNF (1 ng/mL). (D) Simulations predict NF-κB activity in TNF-treated cells that have either constitutive or inducible A20 expression, or both (wild type). Nuclear NF-κB activity was measured via EMSA in wild-type cells, or in a20−/− cells reconstituted with a constitutively expressing a20 transgene (pBABE.A20.puro), an NF-κB-inducible transgene (fIL8.A20.puro), or an empty vector control (pBABE.EV.puro, labeled a20−/−) in response to persistent TNF stimulation (1 ng/mL).
We asked whether feedback control by these regulators via inducible synthesis is important, or whether constitutive transcription alone could provide proper control of NF-κB activity. Using a parameter sensitivity analysis, we set the inducible IκBα or A20 transcription rates to zero and repeatedly ran the model with constitutive transcription rates modified by factors of 2x (where –10 ≤ × ≤ 10). We then plotted the simulation outputs (nuclear NF-κB activity) over time (Fig. 2B, X-axis) using a color heat map, ranging from blue (0 nM) to red (100 nM) (Fig. 2B), with the constitutive transcription rate modifier on the Y-axis.
For IκBα, removing inducible transcription while maintaining the original constitutive transcription parameter value results in NF-κB activity similar to that seen in iκbα−/− cells, where peak activation occurs between 1 and 2 h, and is attenuated by 3 h (Fig. 2B, left panel). Although turning down the parameter had little effect, increasing it impacts NF-κB activation severely. At a 16-fold (24) increase, NF-κB activation is limited to 1 h as in wild-type cells; however, the amplitude of this first phase peak is significantly reduced and no late activity is seen. Interestingly, increasing the constitutive IκBα transcription rate by ≥25 (a level that is similar to the maximal inducible rate) completely abrogates NF-κB activation. We conclude that inducible IκBα synthesis is critical for stimulus-responsive activation and timely post-induction repression of NF-κB.
In the case of A20, model simulations also predict that removing A20 inducible transcription results in IKK and NF-κB activity resembling that in a20−/− cells (Fig. 2B, right panel; Supplemental Fig. 2C). Although the temporal profiles of IKK and NF-κB activity are largely invariant to turning down the constitutive A20 transcription rate, increasing it has two effects (Fig. 2B, right panel). First, late NF-κB activity (3–6 h) is dampened as in wild-type cells. Second, further increases of A20 expression results in a delay and reduction of the peak of NF-κB activity. In other words, our computational simulations predict that, unlike the case of IκBα, a specific range of the constitutive transcription rate of A20 (fourfold to 16-fold higher than that estimated for murine embryonic fibroblasts [MEFs]) allows for a TNF-induced NF-κB activity profile that is similar to that observed in wild-type cells.
We tested these predictions experimentally by reconstituting individual knockout cells with IκBα or A20 expressing transgenes containing heterologous promoters. In a cell line that constitutively expresses IκBα (iκbα−/−.pBABE.IκBα) (Supplemental Fig. 2D), we did indeed find, as computationally predicted (Fig. 2C, top panel), that NF-κB activity induced by a 15-min TNF pulse was equivalent to that seen in iκbα−/− (Fig. 2C, bottom panel). In contrast, either constitutive or inducible transcription of A20 was able to restore late NF-κB attenuation in silico and in vivo (Fig. 2D; Supplemental Fig. 2E). Constitutive A20 transcription was modeled fourfold higher than that of wild-type cells, consistent with our experimental work where resting a20−/−.pBABE.A20 cells contained 3.6-fold more A20 mRNA than wild-type cells (data not shown). Overall, our analysis makes a strong case for the necessity for feedback control for IκBα, but not for A20. We surmised that there might be additional physiological functions for inducible A20 expression.
Signaling cross-talk mediated by A20
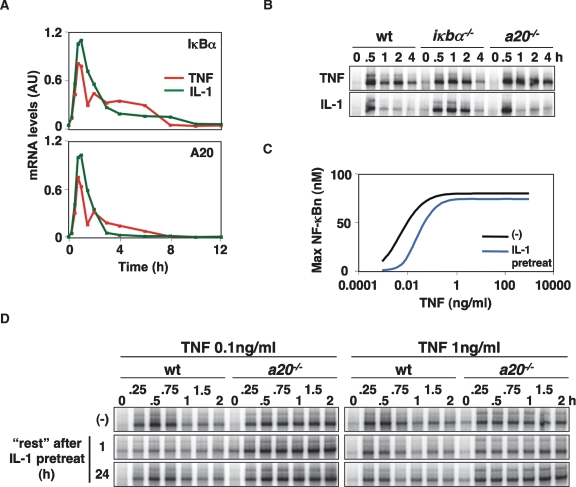
Both iκbα and a20 are highly inducible genes whose expression is activated in response to several NF-κB-inducing inflammatory stimuli (Dixit et al. 1990). Interestingly, IL-1 induces A20 expression (Fig. 3A; Supplemental Fig. 3A), but A20, unlike IκBα, does not affect IL-1 signaling (Fig. 3B; Lee et al. 2000). What may be the functional role of IL-1-induced A20? In vivo, cells are continuously exposed to a variety of stimuli, and we speculated that cells that are “primed” by IL-1 to increase cellular A20 expression might be less responsive to subsequent TNF stimulations. We employed the computational model to examine potential A20-dependent cross-talk between IL-1 and TNF for a range of TNF concentrations. Graphing the TNF dose response curve (ranging from 10−3 to 103 ng/mL) for naïve and IL-1-pretreated cells indicated that NF-κB activity induced by low TNF stimulations (0.1 ng/mL or less) is more affected than higher TNF doses (1 ng/mL or more) (Fig. 3C; Supplemental Fig. 3D,E).
Figure 3.
A20 can mediate signaling cross-talk between inflammatory stimuli. (A) Quantitated A20 and IκBα mRNA expression in MEFs stimulated with 1 ng/mL TNF or IL-1, as measured by RPA. (B) Nuclear NF-κB activity was measured via EMSA in wild-type, a20−/−, and iκbα−/− cells in response to 1 ng/mL TNF or IL-1 stimulation. (C) Simulation of TNF–NF-κB dose response in naïve (black) and IL-1 pretreated (blue) cells. Computational simulations calculated the maximal nuclear NF-κB activity for TNF doses ranging from 10−3 to 103 ng/mL. IL-1 pretreatment was simulated as a 1-h stimulation followed by 1 h of “rest” prior to TNF stimulation. (D) Nuclear NF-κB activity in response to persistent TNF stimulation (0.1 or 1 ng/mL) was measured by EMSA in wild-type and a20−/− cells that were naïve (−) or pretreated with IL-1 for 1 h, followed by 1 h or 24 h of “rest.”
We tested these predictions experimentally by measuring TNF-induced NF-κB activity in cells exposed to the IL-1 pretreatment regime (Fig. 3D; Supplemental Fig. 3C). For a 0.1 ng/mL TNF treatment, NF-κB activation was severely diminished in wild-type cells, but not in a20−/− cells (Fig. 3D; Supplemental Fig. 3B). At 1 ng/mL of TNF, IL-1 pretreatment also had a small effect, but this was A20-independent and possibly due to TNFRI ectodomain shedding (Islam et al. 2006). We confirmed that the cellular memory to inflammatory signaling provided by A20 is transient in silico and in vivo; allowing cells to rest for 24 h after IL-1 pretreatment resulted in normal TNF responsiveness (Fig. 3D; Supplemental Fig. 3F).
A temporal dose response analysis reveals distinct roles of IκBα and A20
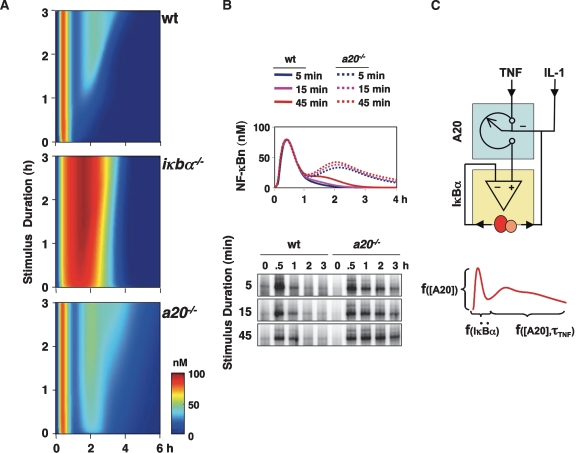
As a paracrine mediator with a short half-life, cellular exposure to TNF is usually transient but of variable duration. Using the model, we investigated the roles of IκBα and A20 in mediating NF-κB signal processing in response to TNF stimulations of varying durations. Using a three-dimensional plot, we graphed IKK and NF-κB activity (Fig. 4A, color heat map) over time (Fig. 4A, X-axis) for TNF pulses of various durations (Fig. 4A, Y-axis; Supplemental Fig. 4A). In wild-type cells, even very short TNF pulses provide for almost 1 h of NF-κB activity (Figs. 1C,D, 4A, top panel). Longer lasting TNF stimulations do not change the duration of the first phase of NF-κB activity, but allow for a second phase of NF-κB activity of proportionally increasing duration. However, in the absence of IκBα, the first invariant phase of NF-κB activity balloons to 3 h (Fig. 4A, middle panel). In A20-deficient cells, our model simulations indicate a largely intact first phase of NF-κB but a second phase that is enhanced (Fig. 4A, bottom panel). Even very short stimuli elicit a second phase that is predicted to last at least 3 h.
Figure 4.
Temporal dose response analysis of TNF-induced NF-κB activity. (A) Simulation of NF-κB activity in wild-type, iκbα−/−, and a20−/− cells in response to TNF pulses ranging from 1 min to 180 min in 1-min increments. The results were graphed over time (hours), with the pulse duration (hours) on the Y-axis. NF-κB activity (nanomolar) was color-coded as in Figure 2. (B) NF-κB activity profiles were simulated in response to 5-, 15-, or 45-min TNF pulses in wild-type and a20−/− cells (top), and were then measured experimentally via EMSA (bottom). (C) Schematic summary of how negative feedback regulators IκBα and A20 encode NF-κB activity dynamics in the TNF signaling pathway. Whereas dynamic feedback (yellow box) is critical to IκBα’s function, inducible expression of A20 confers a tunable rheostat (blue box) function. Via this rheostat function, A20 mediates signaling cross-talk, for example, from prior cellular exposure to IL-1. (Below) TNF produces a typically biphasic NF-κB activity that is encoded by the differential functions of A20 and IκBα. The duration of the first phase is a function of the inducibility (change in synthesis rate, or second-order derivative, denoted by “..”) of IκBα, but is not a function of the TNF stimulus duration or concentration. The duration of the second phase is a function of the concentration of the A20 protein at that time. High concentrations of A20 protein during the early phase (as a result of prior NF-κB activity) may also affect the amplitude of the first phase, but not its duration.
Given that A20 protein expression is markedly up-regulated only after 1 h following stimulation onset (Supplemental Fig. 1B), the prediction that A20 plays a role in regulating responses to much shorter pulses of TNF seemed surprising. Single simulations of 5-, 15-, and 45-min TNF pulses reiterated that A20-deficient cells were predicted to have elevated NF-κB activity at 2 h and longer (Fig. 4B, top panel). Experimentally, we found this to indeed be the case: An electrophoretic mobility shift assay (EMSA) analysis revealed that NF-κB activity is elevated in a20−/− cells during the later phase in response to short TNF pulse stimulations (Fig. 4B, bottom panel). This effect is correlated with incomplete attenuation of IKK activity in the knockout cells (Supplemental Fig. 4B).
Overall, our study reveals distinct roles for the two highly inducible negative feedback regulators IκBα and A20 (Fig. 4C, top). Whereas the inducibility of IκBα determines the duration of the first phase of NF-κB activity (Fig. 4C, bottom), A20 determines the temporal dose response of the TNF-NF-κB signaling pathway by controlling primarily the duration of the second phase of NF-κB activity. Interestingly, we found that the concentration, not the rate of synthesis or inducibility of the A20 protein, determines the duration of the second phase (Fig. 4C, bottom). The concentration of A20 protein within the cell can be modulated not only by TNF itself but also by multiple inflammatory stimuli (Fig. 4C, top), thus conferring a rheostat function. At high levels of expression in “primed” cells, the A20 rheostat can dampen the amplitude of the first phase of NF-κB activity (but never the duration), suggesting a role in establishing inflammatory tolerance.
Discussion
As the molecular connectivity within signaling networks has increasingly become a focus of biomedical research, a surprising number of inducible negative regulators have been identified; these are usually categorized as negative feedback regulators. However, remarkably few have been examined to determine what functional roles their inducible expression may play; indeed, little is known about whether inducible expression can even allow for distinct functional roles. Our analysis demonstrates distinct functions for IκBα and A20, whose expression is driven by similarly inducible promoters. In the case of IκBα, negative feedback is required for function; in other words, no value of constitutive IκBα expression parameters will provide the degree of NF-κB activation and post-induction repression that NF-κB-responsive expression of IκBα allows for. In contrast, there is a range of constitutive A20 expression values that can functionally replace A20 negative feedback. Hence, we distinguish between an obligate (IκBα) and a nonobligate (A20) feedback regulator. Indeed, the A20 regulatory mechanism may not fit a narrower definition of a negative feedback regulator.
Instead, the inducibility of A20 expression functions to tune a rheostat that controls cellular signaling responsiveness. This is demonstrated most clearly by the fact that A20 mediates signaling cross-talk between inflammatory stimuli when they are administered sequentially. However, A20’s rheostat function is not limited to cross-talk between IL-1 and TNF, as its promoter is inducible by all NF-κB-inducing stimuli tested so far. It provides short-term cellular memory by transiently “tolerizing” (i.e., reducing the sensitivity of) the TNF signaling pathway. Whereas the dynamics of IκBα inducibility, but not the actual protein concentration, critically define NF-κB activity, the A20 protein concentration determines its attenuation function, regardless of whether the protein level was the result of inducible or constitutive expression. This distinction between the negative regulators may explain how subtle misregulation of A20 protein levels have been implicated in a range of physiological and pathological processes, including atherosclerosis (Wolfrum et al. 2007), T-cell responsiveness (Stilo et al. 2008), the homeostasis of signaling by pathogen-sensing receptors (Turer et al. 2008) and of commensual bacteria (Hitotsumatsu et al. 2008), and suppression of autoreactive immune responses (Song et al. 2008).
What might be the molecular basis for the differential functionality? There are differences in network connectivity (model topology) and rate constants (parameter values) that may be relevant to consider. Although both IκBα and A20 are rapidly and highly inducible at the level of mRNA transcripts (which show a similarly short half-life), producing the larger A20 protein takes more time. More importantly, a significantly longer protein half-life allows for not only a gradual build-up of the A20 protein, but also a memory function that we revealed in cross-talk or priming experiments. Whereas the obligate negative feedback regulator IκBα functions as a stochiometric binder of the NF-κB activator, the nonobligate feedback attenuator A20 reaches back many more reactions into the pathway, making its effect more temporally diffuse at the NF-κB level. In addition, A20 possesses an enzymatic function, which further slows its total functional effect. These conclusions are insensitive to alterations of parameter values within the ranges set by experimental constraints (Supplemental Material). In fact, model topology aspects mirror prior theoretical considerations pertaining to metabolic networks (Dibrov et al. 1982). We suggest that theoretical modeling work may prove useful in distinguishing between different categories of negative feedback regulators in signaling. In addition, our combined computational and experimental strategy may be applied to other signaling systems to characterize the functional diversity of negative feedback regulators.
TNF-induced NF-κB dynamics are encoded not only by IκBα and A20, but also by an IKK autorepression mechanism that provides powerful negative feedback on a faster scale than mechanisms involving de novo gene expression. Although our model recapitulates the observed temporal IKK activity profile, it does not describe the actual regulatory mechanism(s) because further molecular characterization is required. Indeed, recent work suggests that the association of the essential IKK scaffold subunit NEMO with catalytic subunits IKK1 and IKK2 is regulated via phosphorylation (Hayden and Ghosh 2008). Similarly, the mechanism by which K63-linked ubiquitin chains activate IKK, the involvement of A20 in their removal (M.P. Boldin and D. Baltimore, unpubl.), and whether and how TAK1/Tab2/3 is involved in IKK control remains to be characterized in more detail. New mechanistic insights should lead to a revision of our mathematical model, in turn enabling an investigation of their role in determining NF-κB dynamics. The iterative strategy of combined experimental and modeling work promises to result in amply validated and sufficiently detailed models that may function as standalone discovery tools.
Materials and methods
Experimental studies
Immortalized MEFs were prepared and cultured as described previously (Lee et al. 2000; Hoffmann et al. 2002). a20−/− MEFs were reconstituted with retroviral vectors pBABE.A20.puro (created by cloning a human A20 ORF into pBABE-puro) and fIL8.A20.puro, which was generated by inserting an inducible A20 cassette (A20 ORF under the control of the IL-8 promoter), followed by a PuroR expression module into a FUGW vector (Lois et al. 2002). iκbα−/− MEFs were reconstituted with pBABE.IκBα.puro or empty vector controls, and were a kind gift from Erika Mathes. Cells were stimulated with recombinant murine TNFα (Roche Diagnostics) or murine IL-1β (EMD Biosciences). p65 (sc-372), actin (sc-1615), and mSin3A (sc-994) antibodies were generously provided by Santa Cruz Biotechnologies and A20 (IMG-161) antibody was obtained from Imgenex. EMSA, immunoprecipitation kinase assays, RNase protection assays (RPAs), and immunoblotting were done as described (Hoffmann et al. 2002; Werner et al. 2005; Kearns et al. 2006).
In silico studies
A computational model was constructed to describe key reactions linking extracellular TNF ligand to NF-κB nuclear localization. This model includes a reaction set based on the previously published models of the NF-κB–IκB signaling module (Hoffmann et al. 2002; Werner et al. 2005; Kearns et al. 2006; O’Dea et al. 2007), as well as reactions that describe the upstream TNFR–IKK signaling module (see the Supplemental Material for details). The model was written in and analyzed with Mathworks MATLAB version R2008a, and the files are available upon request.
Acknowledgments
We acknowledge J. Hasty, B. Kholodenko, J. Faeder, and A. Levchenko for insightful discussions. This study was supported by National Institutes of Health R01 grant GM72024. S.L.W. is supported by the NSF Graduate Research Fellowship, and J.D.K. is supported by an American Heart Association Predoctoral Fellowship.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1680708.
References
- Aggarwal B.B., Shishodia S., Ashikawa K., Bharti A.C. The role of TNF and its family members in inflammation and cancer: Lessons from gene deletion. Curr. Drug Targets Inflamm. Allergy. 2002;1:327–341. doi: 10.2174/1568010023344571. [DOI] [PubMed] [Google Scholar]
- Banner D.W., D’Arcy A., Janes W., Gentz R., Schoenfeld H.J., Broger C., Loetscher H., Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF β complex: Implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Beg A.A., Sha W.C., Bronson R.T., Baltimore D. Constitutive NF-κ B activation, enhanced granulopoiesis, and neonatal lethality in I κ B α-deficient mice. Genes & Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- Beutler B.A., Milsark I.W., Cerami A. Cachectin/tumor necrosis factor: Production, distribution, and metabolic fate in vivo. J. Immunol. 1985;135:3972–3977. [PubMed] [Google Scholar]
- Boone D.L., Turer E.E., Lee E.G., Ahmad R.C., Wheeler M.T., Tsui C., Hurley P., Chien M., Chai S., Hitotsumatsu O., et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Cabannes E., Khan G., Aillet F., Jarrett R.F., Hay R.T. Mutations in the IkBa gene in Hodgkin’s disease suggest a tumour suppressor role for IκBα. Oncogene. 1999;18:3063–3070. doi: 10.1038/sj.onc.1202893. [DOI] [PubMed] [Google Scholar]
- Cheong R., Bergmann A., Werner S.L., Regal J., Hoffmann A., Levchenko A. Transient IκB kinase activity mediates temporal NF-κB dynamics in response to a wide range of tumor necrosis factor-α doses. J. Biol. Chem. 2006;281:2945–2950. doi: 10.1074/jbc.M510085200. [DOI] [PubMed] [Google Scholar]
- Delhase M., Hayakawa M., Chen Y., Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- Dibrov B.F., Zhabotinsky A.M., Kholodenko B.N. Dynamic stability of steady states and static stabilization in unbranched metabolic pathways. J. Math. Biol. 1982;15:51–63. doi: 10.1007/BF00275788. [DOI] [PubMed] [Google Scholar]
- Dixit V.M., Green S., Sarma V., Holzman L.B., Wolf F.W., O’Rourke K., Ward P.A., Prochownik E.V., Marks R.M. Tumor necrosis factor-α induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J. Biol. Chem. 1990;265:2973–2978. [PubMed] [Google Scholar]
- Ghosh S., Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109 (Suppl.):S81–S96. doi: 10.1016/S0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Grell M., Wajant H., Zimmermann G., Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc. Natl. Acad. Sci. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L.H., Hopfield J.J., Leibler S., Murray A.W. From molecular to modular cell biology. Nature. 1999;402 (Suppl.):C47–C52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hitotsumatsu O., Ahmad R.C., Tavares R., Wang M., Philpott D., Turer E.E., Lee B.L., Shiffin N., Advincula R., Malynn B.A., et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A., Baltimore D. Circuitry of NF-kB signaling. Immunol. Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann A., Levchenko A., Scott M.L., Baltimore D. The IκB–NF-κB signaling module: Temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- Hoffmann A., Leung T.H., Baltimore D. Genetic analysis of NF-κB/Rel transcription factors defines functional specificities. EMBO J. 2003;22:5530–5539. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A., Adamik B., Hawari F.I., Ma G., Rouhani F.N., Zhang J., Levine S.J. Extracellular TNFR1 release requires the calcium-dependent formation of a nucleobindin 2–ARTS-1 complex. J. Biol. Chem. 2006;281:6860–6873. doi: 10.1074/jbc.M509397200. [DOI] [PubMed] [Google Scholar]
- Kaufman D.R., Choi Y. Signaling by tumor necrosis factor receptors: Pathways, paradigms and targets for therapeutic modulation. Int. Rev. Immunol. 1999;18:405–427. doi: 10.3109/08830189909088491. [DOI] [PubMed] [Google Scholar]
- Kearns J.D., Basak S., Werner S., Huang C., Hoffmann A. IκBε provides negative feedback to control the dynamics of NF-kB signaling and gene expression. J. Cell Biol. 2006;173:659–664. doi: 10.1083/jcb.200510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko B.N. Cell-signalling dynamics in time and space. Nat. Rev. Mol. Cell Biol. 2006;7:165–176. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement J.F., Rice N.R., Car B.D., Abbondanzo S.J., Powers G.D., Bhatt P.H., Chen C.H., Rosen C.A., Stewart C.L. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol. Cell. Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappmann D., Emmerich F., Kordes U., Scharschmidt E., Dorken B., Scheidereit C. Molecular mechanisms of constitutive NF-κB/Rel activation in Hodgkin/Reed-Sternberg cells. Oncogene. 1999;18:943–953. doi: 10.1038/sj.onc.1202351. [DOI] [PubMed] [Google Scholar]
- Lee E.G., Boone D.L., Chai S., Libby S.L., Chien M., Lodolce J.P., Ma A. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C., Hong E.J., Pease S., Brown E.J., Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Mercurio F., Zhu H., Murray B.W., Shevchenko A., Bennett B.L., Li J., Young D.B., Barbosa M., Mann M., Manning A., et al. IKK-1 and IKK-2: Cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Monaco C., Paleolog E. Nuclear factor κB: A potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc. Res. 2004;61:671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Nelson D.E., Ihekwaba A.E., Elliott M., Johnson J.R., Gibney C.A., Foreman B.E., Nelson G., See V., Horton C.A., Spiller D.G., et al. Oscillations in NF-κB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- O’Dea E.L., Barken D., Peralta R.Q., Tran K.T., Werner S.L., Kearns J.D., Levchenko A., Hoffmann A. A homeostatic model of IκB metabolism to control constitutive NF-κB activity. Mol. Syst. Biol. 2007;3:111. doi: 10.1038/msb4100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani S., Pantano S., Natoli G. Two waves of nuclear factor κB recruitment to target promoters. J. Exp. Med. 2001;193:1351–1359. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Brachert W., Tchikov V., Neumeyer J., Jakob M., Winoto-Morbach S., Held-Feindt J., Heinrich M., Merkel O., Ehrenschwender M., Adam D., et al. Compartmentalization of TNF receptor 1 signaling: Internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Scott M.L., Fujita T., Liou H.C., Nolan G.P., Baltimore D. The p65 subunit of NF-κ B regulates I κ B by two distinct mechanisms. Genes & Dev. 1993;7:1266–1276. doi: 10.1101/gad.7.7a.1266. [DOI] [PubMed] [Google Scholar]
- Song H.Y., Rothe M., Goeddel D.V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc. Natl. Acad. Sci. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.T., Evel-Kabler K., Shen L., Rollins L., Huang X.F., Chen S.Y. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat. Med. 2008;14:258–265. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelling J., Sauer U., Szallasi Z., Doyle F.J., Doyle J. Robustness of cellular functions. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Stilo R., Varricchio E., Liguoro D., Leonardi A., Vito P. A20 is a negative regulator of BCL10- and CARMA3-mediated activation of NF-κB. J. Cell Sci. 2008;121:1165–1171. doi: 10.1242/jcs.021105. [DOI] [PubMed] [Google Scholar]
- Tian B., Nowak D.E., Brasier A.R. A TNF-induced gene expression program under oscillatory NF-κB control. BMC Genomics. 2005;6:137. doi: 10.1186/1471-2164-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer E.E., Tavares R.M., Mortier E., Hitotsumatsu O., Advincula R., Lee B., Shifrin N., Malynn B.A., Ma A. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J. Exp. Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Varfolomeev E.E., Malinin N.L., Goltsev Y.V., Kovalenko A.V., Boldin M.P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- Wang C., Deng L., Hong M., Akkaraju G.R., Inoue J., Chen Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kuriyama H., Sone H., Neda H., Yamauchi N., Maeda M., Niitsu Y. Continuous internalization of tumor necrosis factor receptors in a human myosarcoma cell line. J. Biol. Chem. 1988;263:10262–10266. [PubMed] [Google Scholar]
- Werner S.L., Barken D., Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- Wertz I.E., O’Rourke K.M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D.L., et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Wolfrum S., Teupser D., Tan M., Chen K.Y., Breslow J.L. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-κB target genes. Proc. Natl. Acad. Sci. 2007;104:18601–18606. doi: 10.1073/pnas.0709011104. [DOI] [PMC free article] [PubMed] [Google Scholar]