Abstract
Amide proton NMR signals from the N-terminal domain of monomeric α-synuclein (αS) are lost when the sample temperature is raised from 10°C to 35°C at pH 7.4. Although the temperature-induced effects have been attributed to conformational exchange caused by an increase in α-helix structure, we show that the loss of signals is due to fast amide proton exchange. At low ionic strength, hydrogen exchange rates are faster for the N-terminal segment of αS than for the acidic C-terminal domain. When the salt concentration is raised to 300 mM, exchange rates increase throughout the protein and become similar for the N- and C-terminal domains. This indicates that the enhanced protection of amide protons from the C-terminal domain at low salt is electrostatic in nature. Cα chemical shift data point to <10% residual α-helix structure at 10°C and 35°C. Conformational exchange contributions to R2 are negligible at both temperatures. In contrast to the situation in vitro, the majority of amide protons are observed at 37°C in 1H-15N HSQC spectra of αS encapsulated within living Escherichia coli cells. Our finding that temperature effects on αS NMR spectra can be explained by hydrogen exchange obviates the need to invoke special cellular factors. The retention of signals is likely due to slowed hydrogen exchange caused by the lowered intracellular pH of high-density E. coli cultures. Taken together, our results emphasize that αS remains predominantly unfolded at physiological temperature and pH—an important conclusion for mechanistic models of the association of αS with membranes and fibrils.
Keywords: Parkinson's disease, intrinsically unfolded proteins, amyloid, exchange broadening, in-cell NMR, membrane proteins
α-Synuclein (αS) is a 14.5-kDa protein expressed predominantly at the pre-synaptic terminals of brain neurons (George 2002; Sung and Eliezer 2007). The physiological functions of αS are unknown, although synaptic vesicle recycling is thought to be a plausible role (George 2002; Cookson 2005; Moore et al. 2005; Fink 2006; Brown 2007). Genetic and pathology evidence implicates αS as the principal culprit in Parkinson's disease. The αS missense mutations A53T, A30P, and E46K, as well as a triplication of the wild-type gene, result in hereditary diseases that include Parkinsonism, together with more widespread symptoms (Goedert 2001; George 2002; Cookson 2005; Moore et al. 2005). In its fibrillar form, αS is the principal component of Lewy body deposits—the neuropathological hallmarks of Parkinson's disease (Goedert 2001; George 2002; Cookson 2005; Fink 2006). Lewy bodies are spherical inclusions of lipids and proteins that are particularly abundant in substantia nigra neurons of patients with Parkinson's disease but that are also found in other regions of the brain (Goedert 2001; Cookson 2005). Presently, the issue of whether the αS fibrils themselves or smaller aggregates are neurotoxic remains unresolved. The fibrils, which are insoluble, may be a byproduct of the disease, or may actually confer protection from alternative soluble aggregates (Lansbury and Lashuel 2006). Toxicity in Parkinson's as well as in other amyloid diseases could result from the disruptive effects of soluble aggregates on cell membranes and the resultant breach of cellular ion balance (Lashuel et al. 2002), although other mechanisms are possible (Eliezer 2006; Lansbury and Lashuel 2006). In addition to its role in Parkinson's disease, αS is found in a number of other Lewy body pathologies (George 2002; Cookson 2005), and a secreted extracellular 35-residue fragment of αS (El-Agnaf et al. 2003) occurs as the non-Aβ-component (NAC) of Alzheimer plaques (Weinreb et al. 1996).
The sequence of αS is organized into an amphipathic N-terminal domain (residues 1–100) that contains seven imperfect repeats of the KTKEGV motif, and a C-terminal domain (residues 101–140) that has a high proportion of acidic residues (Fernandez et al. 2004; Cookson 2005). The structure of monomeric αS is “intrinsically unfolded” but under different solution conditions different levels of structure are observed (Fernandez et al. 2004; Fink 2006). When combined with SDS micelles, the amphipathic N-terminal domain forms two long α-helices that lie on the surface of the micelle. The acidic C-terminal domain remains unstructured in the micelle-bound state (Eliezer et al. 2001; Chandra et al. 2003; Ulmer and Bax 2005; Ulmer et al. 2005).
Loss of 1H-15N HSQC cross peaks with a sequence pattern reminiscent of that seen when the protein folds into an α-helical structure on SDS micelles, was reported as the temperature of monomeric αS increased from 10°C to 35°C (McNulty et al. 2006a). The temperature effects on 1H-15N HSQC spectra of αS were reversible, and were attributed to conformational exchange between unfolded and α-helix structure, with the latter becoming stabilized in the N-terminal domain of monomeric αS at physiological temperatures (McNulty et al. 2006a). In contrast to the results in vitro, the majority of the expected 1H-15N HSQC correlations were detected at 35°C in spectra obtained for αS in live Escherichia coli cells. This observation was attributed to the stabilization of the unfolded state by the molecular crowding the protein experiences inside living cells (McNulty et al. 2006b). These conclusions have important kinetic and thermodynamic consequences for the mechanistic understanding of how αS interacts with membranes and self-assembles into fibrils. Preformed α-helical structure should facilitate association of αS with membranes, but would need to be disrupted to form the β-sheet structure characteristic of amyloids (Marsh et al. 2006; Martinez et al. 2007). Moreover, if cellular environment had a vital influence on modulating the conformation of αS, it would cast serious doubt on the significance of structural studies with purified αS in solution.
Here we show that the loss of 1H-15N HSQC correlations from αS at physiological temperature is due to fast exchange between amide protons and solvent. We show that at 15°C, where all peaks from αS can still be detected, exchange is occurring for all residues with rates between 2 and 20 s−1 and that exchange rates are faster for the N-terminal 100 residues than for the C-terminal 40 residues. Hydrogen exchange rates increase throughout the protein at 300 mM NaCl and become more similar for the N- and C-terminal domains, suggesting that hydrogen exchange protection of the C-terminal domain at low salt is of electrostatic origin. 1H-15N HSQC cross-peak intensities of monomeric αS at 35°C are highly pH dependent in the range between pH 7.4 and 6.4. The retention of 1H-15N correlations in spectra obtained for αS within E. coli is probably due to slower amide proton exchange rates inside the cells, where the pH is about 7.1 (Shimba et al. 2003) compared to the pH 7.4 values typically used for in vitro NMR studies of αS (McNulty et al. 2006b). Finally, backbone Cα chemical shift data obtained at pH 6.4, establish that the fraction of α-helix structure for monomeric αS is ∼5% at 10°C and increases to only ∼9% at 35°C. These results emphasize that αS exists in predominantly unfolded conformations near physiological temperatures and pH.
Results and Discussion
The effects of temperature on NMR spectra of αS are consistent with fast hydrogen exchange
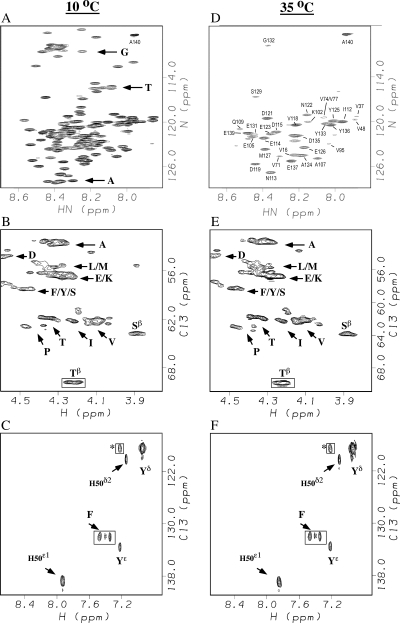
Figure 1 compares NMR spectra of αS at temperatures of 10°C and 35°C. The 1H-15N HSQC spectra are closely similar to published spectra of αS under the same conditions (Eliezer et al. 2001; McNulty et al. 2006a). The majority of residues in αS give detectable 1H-15N HSQC correlations at pH 7.4 and a temperature of 10°C (Fig. 1A). As the temperature is raised to 35°C resonances from the first 100 residues in the N-terminal domain are lost, while correlations from the last 40 residues in the acidic C-terminal domain survive (Fig. 1D) as previously reported (McNulty et al. 2006a). However, if we compare the Hα–Cα region (Fig. 1B,E) or the aromatic region (Fig. 1C,F) of constant-time (ct) 1H-13C-HSQC spectra of αS, the number, intensities, and positions of the correlations are nearly identical. His50, for example, is the only histidine in the αS sequence. The 1H-15N backbone amide correlation for His50 is lost between 10°C and 35°C. In the 1H-13C spectrum the Cε1 and Cδ2 aromatic ring correlations for His50, however, remain unchanged between 10°C and 35°C (Fig. 1C,F). Similar behavior is seen for a number of other amino acids from the N-terminal 100 residues, which have been proposed to have resonances broadened by conformational exchange (McNulty et al. 2006a). Considering the amino acid sequence of αS, the 2 Phe residues, all 10 Thr residues, 18 of 19 Val, 16 of 19 Ala, and 14 of 18 Gly residues are in the amphipathic N-terminal domain. These residue types account for a large fraction of the correlations lost in 1H-15N HSQC spectra of αS as the temperature is raised from 10°C to 35°C (Fig. 1A,D; Eliezer et al. 2001; McNulty et al. 2006a). Yet the corresponding regions of 1H-13C HSQC spectra remain largely unchanged over the same temperature range (Fig. 1B,C,E,F). These observations strongly suggest that the loss of peaks in 1H-15N HSQC spectra of αS with increasing temperature are due to fast exchange of amide protons with solvent (Dempsey 2001) rather than conformational exchange broadening (McNulty et al. 2006a). The 1H-13C HSQC spectra are unaffected since carbon-bound protons are not labile to solvent exchange.
Figure 1.
NMR spectra of αS at 10°C (A–C) and 35°C (D–F). The panels show 1H-15N HSQC spectra (A,D), portions of Hα–Cα selective ct-1H-13C HSQC spectra (B,E), and aromatic-selective ct-1H-13C HSQC spectra (C,F). Samples were in 20 mM sodium phosphate, pH 7.4. Boxed correlations have negative phases in the ct-1H-13C HSQC spectra. Residue type assignments are indicated by single-letter amino acid codes. Because of crowding in A we labeled only three regions of the spectrum that contain the majority of glycine, threonine, and alanine correlations, respectively. A complete set of sequence-specific assignments for the spectrum in A is provided in Supplemental Figure S1. In D, sequence-specific assignments are given for the subset of cross peaks that remain at 35°C. The assignments in D were obtained by following chemical shifts changes in 1H-15N HSQC spectra at 5° intervals between 35°C and 10°C. Most peaks that survive at 35°C are from slowly exchanging residues in the C-terminal domain (residues 100–140). Some weaker correlations are also seen for valines 16, 37, 48, 71, and 74 from the N-terminal domain, since the valines have slow intrinsic exchange rates. Superscripts in B, C, E, and F denote carbon types (e.g., Yδ are the delta carbons of the tyrosine ring). Labels without superscripts in panels B and E are Hα–Cα cross peaks.
Micelle effects on the αS NMR spectra are consistent with structuring of the N-terminal domain
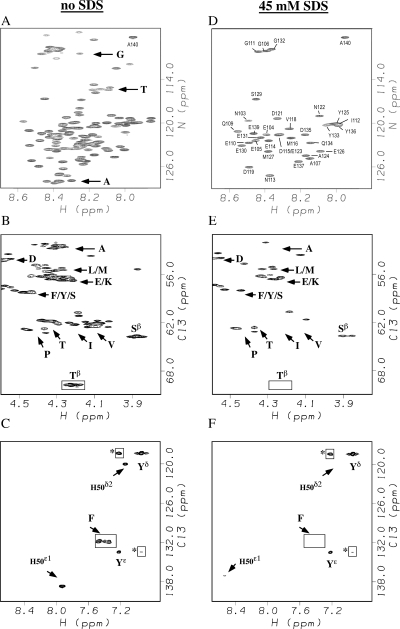
In contrast to the effects of temperature, addition of 45 mM SDS micelles leads to pronounced changes in both 1H-15N and 1H-13C HSQC signals of residues from the N-terminal amphipathic domain (Fig. 2). The domain containing the first ∼100 residues of αS adopts an α-helical structure in the presence of micelles or small unilamellar vesicles, whereas the C-terminal acidic domain comprised of the last ∼40 amino acids of αS, remains unstructured (Eliezer et al. 2001; Chandra et al. 2003; Ulmer and Bax 2005; Ulmer et al. 2005).
Figure 2.
NMR spectra of αS without (A–C) or with 45 mM SDS micelles (D–F). All spectra were obtained at 10°C, pH 7.4. The panels show 1H-15N HSQC spectra (A,D), Hα–Cα selective ct-1H-13C HSQC spectra (B,E), and aromatic-selective ct-1H-13C HSQC spectra (C,F). Boxed correlations have negative phases in the ct-1H-13C HSQC spectra. A complete set of sequence-specific assignments for the spectrum shown in A is provided in Supplemental Figure S1. Sequence-specific assignments in B are for all cross peaks that remain with 45 mM SDS.
In ct HSQC experiments, the 1H-13C correlations of carbons coupled to one aliphatic carbon will have a different phase than those coupled to two aliphatic carbons. The degenerate Hβ–Cβ correlations from the 10 Thr residues in αS are readily identifiable as an unresolved negative phase correlation as shown boxed in Figure 2B. All 10 Thr residues occur in the first 100 amino acids of the αS sequence. The correlation for the Thr residues disappear from both the 1H-15N HSQC and 1H-13C HSQC spectra of αS at a saturating concentration of the SDS micelles (Fig. 2D,E). Other correlations that can be uniquely assigned based on their “random coil” chemical shifts show similar behavior. The unique His50 and the two Phe residues in αS are located in the first 100 amino acids of the sequence. Their NMR signals are lost or strongly shifted in the presence of micelles (Fig. 2C,F). By contrast, the unresolved correlations from three Tyr residues at positions 125, 133, and 136 persist in the aromatic region of the 1H-13C HSQC and 1H-15N HSQC because of their location in the unstructured C-terminal tail of αS (Fig. 2F). The poorly resolved group of Ala and Val cross peaks (Fig. 2B) experience a marked reduction in intensity in the presence of micelles (Fig. 2E) consistent with the location of 16 of 19 Ala and 18 of 19 Val in the N-terminal domain of αS, which becomes structured in the presence of micelles. The regions of the spectrum corresponding to Glu and Asp show relatively small changes in intensity consistent with the location of a large fraction of these residues in the unstructured acidic C-terminal domain.
We emphasize that the similarity between the appearance of 1H-15N HSQC spectra of free αS at 35°C (Fig. 1D) and micelle bound αS at 10°C (Fig. 2D) is superficial. As we show below, signals from the N-terminal 100 residues of monomeric αS at pH 7.4 and 35°C are lost due to fast hydrogen exchange. NMR signals of the acidic C-terminal domain persist because the negatively charged sequence of this region hinders hydrogen exchange at low salt concentrations. In micelle-bound αS, 1H-15N correlations from the first 100 residues are probably lost at 10°C due to the large correlation time of the protein–micelle complex. These resonances can be recovered at temperatures >25°C presumably due to faster tumbling of the protein–micelle complex (Eliezer et al. 2001; Chandra et al. 2003; Ulmer et al. 2005). Alternatively, conformational exchange between free and micelle-bound states could increase the R2 relaxation rates of the N-terminal domain of αS (Eliezer et al. 2001), or positional exchange between αS molecules experiencing different magnetic environments in their micelle-bound states could be responsible for broadening of resonances from the N-terminal domain (Ulmer et al. 2005). At the present time we cannot distinguish between these mechanisms. The C-terminal domain remains unstructured in the presence of micelles, and NMR signals from this region persist in 1H-15N HSQC spectra under conditions where the resonances from the N-terminal domain are lost. In addition to SDS micelles we obtained similar data with a second GM1 micelle system (Martinez et al. 2007), which are shown in Supplemental Figure S2.
Hydrogen exchange rates are slower for the acidic C-terminal domain of monomeric αS
To quantify amide exchange in αS monomers we obtained CLEANEX spectra (Hwang et al. 1998) of a pH 7.4 sample at a temperature of 15°C. Under these conditions the entire complement of backbone 1H-15N HSQC correlations could be observed. In CLEANEX experiments water is selectively excited, and the magnetization is transferred between solvent and amide protons during a spin-locked mixing period. Phase modulation during the mixing period is tailored to accomplish mutual cancellation of ROE and NOE contributions (Fejzo et al. 1990; Mori et al. 1996), so that the experiment is selective for transfer of magnetization through chemical exchange. The initial slope of the rise in peak intensity with increasing mixing time is used to obtain the hydrogen exchange rate of a given site (Hwang et al. 1998).
An unusual feature of the CLEANEX spectra of monomeric αS is that the entire 1H-15N spectrum of the protein can be seen at 15°C with a mixing time of 24 ms (not shown). This indicates that all amide protons undergo solvent exchange with rates between ∼0.1 and 100 s−1 (Koide et al. 1995; Hwang et al. 1998; Dempsey 2001). At shorter mixing times, most of the peaks from the N terminus have larger intensities in the CLEANEX spectrum than those from the C terminus, indicating faster exchange rates for the former (Supplemental Figs. S3 and S4). The observation that exchange is prevalent at a temperature of 15°C, strongly suggests that the mechanism of cross-peak loss in the 1H-15N HSQC spectrum at 35°C involves solvent exchange rather than conformational exchange, as amide protons move from the slow into the intermediate exchange regime (Shojania and O'Neil 2006) with increasing temperature.
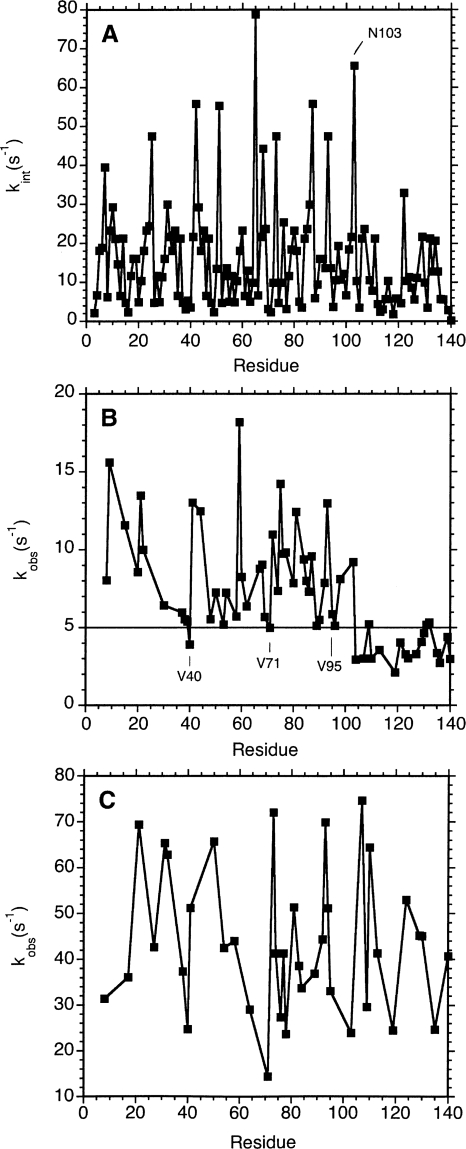
That more 1H-15N HSQC correlations from the C-terminal domain survive at 35°C requires an explanation of why amide protons exchange faster from the first 100 amino acids of αS. Unstructured proteins manifest differences in exchange rates at individual sites, which depend on the sequence of the protein and solution conditions, particularly the sample pH and temperature. These “intrinsic” exchange rates can be calculated using parameters derived from model peptides. Figure 3A shows intrinsic exchange rates calculated for the αS sequence at a pH of 7.4 and a temperature of 15°C. The mean exchange rate for residues 3–103 from the N-terminal domain is 17 s−1, while that for residues 104–140 from the C-terminal domain is 10 s−1. The difference in the means is statistically significant, with ρ = 0.01. Although intrinsic exchange rates are faster for residues from the N-terminal domain, some of the residues in this region have rates as slow as those from the C terminus (Fig. 3A).
Figure 3.
Amide hydrogen exchange rates of αS. (A) Theoretically predicted intrinsic exchange rates (Bai et al. 1993) for αS at pH 7.4 and a temperature of 15°C. The rates were calculated with the SPHERE server (Zhang 1995). (B) Experimentally determined amide proton exchange rates from CLEANEX spectroscopy (Hwang et al. 1998) using an αS sample at low salt (20 mM sodium phosphate). (C) Hydrogen exchange rates as in B, but for a sample containing 20 mM phosphate and 300 mM NaCl. Only well-resolved HN correlations were included in the analysis. Uncertainties in exchange rates were on the order of ∼20% of the values for the low salt sample, and 40% for the high salt sample. Rates and uncertainties are given in Supplemental Table 1.
Figure 3B shows experimentally determined hydrogen exchange rates from CLEANEX experiments. The average of the experimental rates for residues 3–103 from the N-terminal domain is 8.6 s−1. The average exchange rate for residues 104–140 from the C-terminal domain is 3.7 s−1. The difference in the means is statistically significant with ρ < 0.0001. First, we note that the agreement between the experimental and intrinsic rates is poor, with a correlation coefficient R = 0.37. The magnitudes of the differences between experimental and predicted values are typically twofold, and in one case eightfold. Comparable two- to threefold differences between predicted and measured exchange rates have been seen for unstructured peptides and proteins using a number of different methods, so that the discrepancies probably reflect the limited accuracy of modeling exchange rates in unfolded states using data derived from model peptides (Buck et al. 1994; Koide et al. 1995; Mori et al. 1997). For folded proteins, two- to threefold uncertainties in intrinsic rates will have a negligible effect for applications such as calculating protection factors (Englander et al. 1997; Krishna et al. 2004), since the presence of structure can slow exchange rates by eight orders of magnitude or more (Pedersen et al. 1991). For unfolded proteins where exchange rates typically differ <10-fold along the sequence, uncertainties in the predicted intrinsic exchange rates will have a larger impact (Buck et al. 1994; Koide et al. 1995; Mori et al. 1997). Compared to the intrinsic rates (Fig. 3A) the experimental rates for αS (Fig. 3B) show less scatter along the sequence, almost like a smoothed version of the intrinsic rate data.
While the distinction between the N- and C-terminal domains is clearer in the experimental data at low salt (Fig. 3B) than in the theoretical intrinsic rates, some exceptions to the trend are still observed. Residues V40, V71, A89, V95, and K96 show small CLEANEX exchange rates similar to residues from the C terminus (Fig. 3B). These residues have some of the lowest intrinsic rates in the protein. Moreover, 1H-15N HSQC correlations for valines 16, 37, 48, 71, and 74 from the N-terminal domain persist at low contours in spectra of monomeric αS at 35°C, pH 7.4 (Fig. 1D) reflecting the slow intrinsic exchange rates of valine residues (Bai et al. 1993). These cross peaks from the N-terminal domain are entirely absent in spectra of micelle-bound αS at 10°C (Fig. 2D), consistent with the different line-broadening mechanisms for the two cases.
Besides hydrogen exchange, a second factor may contribute to lower 1H-15N cross-peak intensities from residues in the N-terminal domain of monomeric αS. At 15°C signals from the N terminus have smaller intensities than those from the C terminus in standard 1H-15N HSQC spectra of αS. If the strongest cross peak in the protein is assigned an arbitrary intensity of 1.00, the cross peaks from the N-terminal residues 3–103 have an average intensity of 0.29, and those from the C terminus 0.56. The difference between the means is statistically significant with ρ < 0.0001. 15N relaxation measurements do not suggest any obvious differences in the R2 relaxation rates between the N- and C-terminal domains (Bussell Jr. and Eliezer 2001). Rather, the N-terminal 100 residues of αS have a conspicuously large number of Thr (10), Lys (15), and Ser (3) residues due to the KTKEGV repeats in this region. By contrast, the C-terminal 40 amino acids have only one serine residue at position 129. The three types of residues have hydroxyl and amine side chains that exchange very rapidly (k ∼ 105 s−1) with solvent. Even though we are using an experiment that employs pulsed-field gradients to suppress the solvent signal, some of the solvent dephasing could be transferred to the side chains of Thr, Lys, and Ser through hydrogen exchange, and then to nearby amide protons through NOEs (Grzesiek and Bax 1993; Mori et al. 1995) causing a selective reduction in the signals of 1H-15N HSQC cross peaks from residues in the N-terminal domain. These effects coupled with the faster backbone amide proton exchange rates could account for the selective loss of cross peaks from the N-terminal domain of αS in 1H-15N HSQC spectra recorded at pH 7.4 and 35°C.
Effects of salt on hydrogen exchange rates
The differences in hydrogen exchange rates between the N- and C-terminal domains prompted us to examine if the highly negative sequence of the C-terminal domain could be responsible for slowing hydrogen exchange. Electrostatic interactions have relatively shallow (1/r) distance dependences, and can affect sites well beyond immediate neighbors in the amino acid sequence. The high concentration of negative charges in the C-terminal domain could affect exchange rates by (1) changing the effective pH in the vicinity of the protein compared to that in the bulk solution, and (2) screening the protein from the reactive H3O+ and OH− ions that catalyze hydrogen exchange (Kim and Baldwin 1982; Matthew and Richards 1983). The long-range nature of electrostatic interactions, even in the absence of residual structure could thus be responsible for the “smoothed” appearance of the experimental rate profile (Fig. 3B) compared to the predicted intrinsic rates (Fig. 3A). Note that the SPHERE program does not account for ionic strength in the calculations of intrinsic rates.
To test the hypothesis that the negatively charged amino acid sequence of the C-terminal domain affects its hydrogen exchange properties, we compared 1H-15N HSQC data at low salt (20 mM sodium phosphate) with data for samples containing 150 mM and 300 mM NaCl. With increasing salt, peaks disappear from 1H-15N HSQC spectra at lower temperatures. At 300 mM salt the majority of peaks in the 1H-15N HSQC spectrum are missing by about 25°C compared to 35°C for the low salt sample. This qualitatively suggests that hydrogen exchange rates increase with increasing salt at pH 7.4, and cautions that NMR spectra of αS are sensitive to salt in addition to pH and temperature. For samples containing 300 mM NaCl there was less of a distinction between cross-peak intensities from the N- and C-terminal domains at high temperatures. This suggests hydrogen exchange rates from the two domains become more similar when the excess of negative charges on the C-terminal domain is screened at high salt.
To obtain quantitative information we collected CLEANEX spectra on an αS sample at 15°C in the presence of 300 mM NaCl (Fig. 3C). At high salt there is a marked increase in hydrogen exchange rates throughout the sequence (the average rate is 44 s−1). The rates of the N- and C-terminal domains become similar (Fig. 3C), suggesting that exchange protection of the C-terminal domain at low ionic strength is due to the high proportion of negative charges in this segment. Chemical shifts are preserved between high and low salt, suggesting that salt screens charges in αS but does not induce structural transitions.
The influence of pH on 1H-15N cross-peak intensities
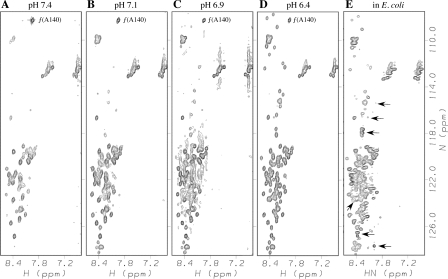
Figure 4A–D shows the effects of pH on 1H-15N HSQC spectra of αS. Above the pH ∼3 minimum for hydrogen exchange, intrinsic exchange rates are expected to increase 10-fold with every pH unit (Dempsey 2001). Lowering the solution pH from 7.4 to 6.4 is sufficient to recover the entire spectrum of αS at a temperature of 35°C. The reappearance of peaks is consistent with a move of amide protons back from the intermediate to the slow NMR exchange regime, as the pH is lowered and hydrogen exchange rates decrease. These changes are reversible, and the order with which peaks reappear at lower pH is the same as when the temperature is decreased from 35°C to 10°C at pH 7.4. Further evidence that the loss of peaks in 1H-15N HSQC spectra of αS at 35°C and pH 7.4 is due to hydrogen exchange is that the 1H-15N HSQC correlations are lost in the same order when the sample pH is raised from 7.4 to 9.0 at the lower temperature of 10°C (data not shown). The pH dependence of 1H-15N HSQC spectra of αS also led others to suggest that the loss of amide signals at high temperature is more likely to result from hydrogen exchange than a conformational transition (Marsh et al. 2006).
Figure 4.
Recovery of αS 1H-15N correlations with decreasing pH (A–D) and αS in live E. coli cells (E). Spectra in A–D are of purified αS in 20 mM phosphate buffer at a temperature of 35°C and the indicated pH values. The spectrum in E is of αS within living E. coli cells and was obtained at a temperature of 37°C. The cross peak of Ala140 denoted by “f(A140)” is aliased in the 15N dimension in A–D, but is outside of the region shown in E. Arrows in E indicate cross peaks from unknown E. coli metabolites (Serber et al. 2001). While these peaks fall within the random coil region of the 1H-15N HSQC spectrum, their intensities are usually smaller than the αS signals.
NMR spectra of αS within living E. coli cells
In contrast to the spectra of the protein in vitro, most 1H-15N HSQC correlations survive at 35°C in spectra of αS encapsulated within living E. coli cells (Fig. 4E), as previously reported (McNulty et al. 2006b). This observation was attributed to the unfolding of the postulated α-helix structure stabilized at high temperature, by intracellular molecular crowding in E. coli. As we have shown, the changes in the NMR spectrum of αS observed at pH 7.4 and 35°C are due to accelerated solvent exchange rather than a folding transition (Figs. 1, 3). It is therefore unnecessary to invoke special cellular factors to explain the unfolded conformation of αS that is observed in living E. coli cells (Fig. 4E).
Further support for the idea that molecular crowding affects conformational transitions in αS came from 1H-15N HSQC spectra of the protein in the presence of 300 mg/mL concentrations of the crowding agent BSA (bovine serum albumin), which was reported to cause the recovery of most of the amide proton signals lost from αS at 35°C (McNulty et al. 2006b). In our hands, we were unable to reproduce this result. We started with a 250 μM solution of αS buffered at pH 7.4 by 20 mM sodium phosphate. At 35°C the NMR signals of amide protons from the N-terminal domain were lost. To this sample we added 320 mg/mL of BSA (ACROS catalog number 61191-0010, Lot # B0719135), which caused the sample pH to drop to 5.7. BSA is an acidic protein with a pI of 5.4, and a net excess of ∼18 negative charges at pH 7.4. The addition of 5 mM BSA appears to overwhelm the buffering capacity of 20 mM phosphate. At pH 5.7 most of the missing resonances from αS were recovered, however, when we adjusted the sample pH back to 7.4 with NaOH, we only detected strong amide proton signals from the C-terminal domain of αS in the presence of 5 mM BSA.
The recovery of αS signals when the protein is studied in E. coli is also likely to be a pH effect. Whereas the in vitro experiments on purified αS were done at pH 7.4 (McNulty et al. 2006b), it has been shown that the intracellular pH of E. coli cells observed under the high-density conditions of NMR studies is closer to pH 7.1 ± 0.1 (Shimba et al. 2003). As shown in Figure 4, a large number of the αS signals can be recovered in vitro simply by lowering the solution pH from 7.4 to 7.1. The full spectrum, however, is not recovered until ∼pH 6.4. Although there is no apparent signal sequence, αS expressed in E. coli is mostly found in the periplasm rather than the cytosol (McNulty et al. 2006b; Ren et al. 2007). As recently shown (Wilks and Slonczewski 2007), the pH of the E. coli periplasm approximates the pH of the growth medium which is expected to drop for bacterial cultures grown at high densities such as for NMR experiments (Goldberg et al. 1994; Calik et al. 2004; Serber et al. 2006). Intracellular electrolytes and osmolytes are additional factors that could affect hydrogen exchange rates for αS observed from within living E. coli.
Secondary chemical shifts indicate only small amounts of residual α-helix structure at 35°C
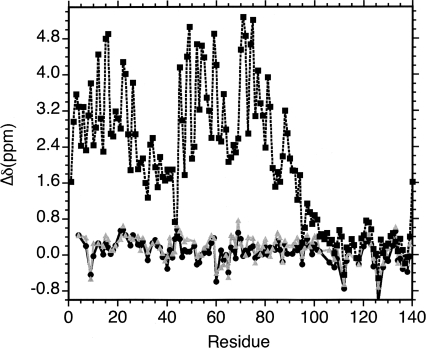
To obtain a quantitative estimate of the increase in residual α-helix structure with temperature we obtained 3D HN(CO)CA data for αS at pH 6.4, where amide protons can be observed at both 10°C and 35°C (Fig. 4D). 13Cα chemical shifts are particularly sensitive indicators of the amount and type of secondary structure in folded and unfolded proteins (Alexandrescu et al. 1994; Wishart and Sykes 1994; Jaravine et al. 2001; Steinmetz et al. 2007). Figure 5 shows differences between 13Cα chemical shifts of αS and random coil values obtained from model peptides (Wishart et al. 1995a). The secondary chemical shift differences for free αS are on the order of ∼0.3 ppm, compared to ∼3.5 ppm when αS is bound to SDS micelles (Chandra et al. 2003). Assuming that the effects of micelles are averaged over the large number of residues for which data are available, we can take the Cα chemical shifts of micelle-bound αS to represent the fully helical folded state. Based on the high-resolution NMR structure (Ulmer et al. 2005), residues Val3–Val37 and Lys45–Thr92 form two long α-helices when αS is bound to SDS micelles. Restricting our analysis to these regions, we can estimate the fraction of α-helix from
Figure 5.
Differences in Cα chemical shifts from random coil values (Wishart et al. 1995a). Black circles, monomeric αS at pH 6.4 and a temperature of 10°C; gray triangles, monomeric αS at pH 6.4 and a temperature of 35°C; squares with dotted lines, micelle-bound αS (Chandra et al. 2003).
to be between 4% and 6% at 10°C, and between 8% and 10% at 35°C. These estimates are entirely consistent with circular dichroism data where θ222 is about −23,000 for αS in the presence of micelles (Eliezer et al. 2001), −1500 for the free protein at 10°C, and –2300 for free αS at 35°C (Uversky et al. 2001). The change in the fraction of α-helix between 10°C and 35°C corresponds to a ΔΔG of only about −0.24 kcal/mol and a van't Hoff ΔH of about +1.1 kcal/mol.
Depending on the kinetics of interconversion between conformational states even a small population of α-helix could give rise to large exchange broadening contributions (Alexandrescu et al. 1996; Kempf and Loria 2004). To test for dynamics in the micro- to millisecond exchange regime we measured 15N R2ex terms for monomeric αS at pH 5.9 at both 10°C and 35°C. The experiments were done at pH 5.9 to minimize solvent exchange contributions, and in order to see all of the amide protons of αS at the higher temperature. R2ex terms were obtained as the difference in apparent 15N R2 values measured with CPMG spin-echo intervals δ of 20 ms and 0.625 ms (Kempf and Loria 2004). The results are given in Supplemental Figure S5 and show that R2ex contributions are negligibly small at both temperatures, within an experimental uncertainty of 1 to 2 Hz. To cause broadening of peaks beyond detection the R2ex terms would probably have to be one or two orders of magnitude larger than what we observed. Typical of highly unfolded proteins αS appears to be dynamically flexible on nano- and sub-nanosecond timescales but lacks the slower micro- to millisecond motions that often accompany the formation of nascent folded structure (Alexandrescu et al. 1996).
Conclusions
We have shown that the loss of amide proton signals from the N-terminal domain of αS near physiological temperature and pH is caused by fast solvent exchange rather than conformational exchange. Although the effects of temperature and micelles on 1H-15N HSQC spectra of αS are superficially similar, the mechanisms by which NMR peaks from the C-terminal domain are retained under the two sets of conditions is very different. With micelles, the C-terminal domain remains unstructured and gives rise to detectable NMR signals due to electrostatic repulsion between its negatively charged residues and the negatively charged surface of the micelle. With free αS at low ionic strength, the negatively charged sequence of the C-terminal domain hinders solvent exchange, so that amide protons from this region are preferentially observed at higher temperatures. Cα chemical shifts indicate that the amount of residual α-helix structure in monomeric αS remains below 10% between 10°C and 35°C. We therefore conclude that αS remains predominately unstructured at physiological temperature and pH, and that changes in temperatures between 10°C and 35°C have negligible effects on the conformational properties of αS monomers. Since αS remains unfolded at physiological temperatures, there is no need to invoke cellular factors to explain the unfolded conformation observed for αS encapsulated within living E. coli cells. In fact, the properties of purified αS in solution and in living E. coli cells would appear to be very close given the similarity of 1H and 15N chemical shifts. Differences in peak intensities between 1H-15N HSQC spectra in cells and in solution are most likely related to differences in pH between the two types of environments, and the large impact of small differences in pH on amide proton solvent exchange.
Materials and Methods
Materials
SDS (electrophoresis purity reagent) was from Bio-Rad. GM1 ganglioside (from ovine brain) was from Avanti Polar Lipids.
Expression of αS was carried out using the pT7-7/asyn-WT vector (Conway et al. 2000) transformed into E. coli BL21(DE3)pLysS cells. The vector was a gift from the Lansbury laboratory and encodes the gene for the 140-residue human αS gene under the control of a T7 promoter. Cells were grown at 37°C in M9 minimal medium supplemented with 1 g/L 15N ammonium chloride and/or 3 g/L 13C-glucose, 100 μg/mL ampicillin to select for the ampicillin resistance marker, and 37 μg/mL chloramphenicol. When the cells reached an OD600 of 0.6, expression was induced with 1 mM IPTG, and cells were incubated for an additional 4 h at 37°C. Purification was based on a literature protocol (McNulty et al. 2006a). Purified αS was dialyzed extensively against 20 mM phosphate buffer (pH 7.4), and stored at –80°C in 250-μL aliquots of ∼0.25 mM protein. Alternatively, the aliquots can be stored as freeze-dried powder, since lyophilization has no effect on the 1H-15N HSQC fingerprint of αS. The protein was determined to be in excess of 90% pure by 15%-tricine gel electrophoresis. The final yield of purified protein was about 50 mg/L of E. coli culture.
NMR spectroscopy
NMR data were obtained on Varian 600 MHz instruments equipped with cryogenic probes. DSS was used as an internal 1H chemical shift reference, and as an indirect reference for 13C and 15N chemical shifts (Wishart et al. 1995b). Partial published 1H-15N HSQC (Eliezer et al. 2001), and 15N, 13Cα, 13Cβ, and 13C′ assignments (Bermel et al. 2006) were extended to obtain complete 1HN, 15N, 13Cα, 13Cβ, and 13C′ assignments at pH 7.4 and 10°C using 3D HNCACB, HNCA, HN(CO)CA, and HNCO experiments. 1H-15N HSQC assignments obtained under this initial set of conditions were translated to spectra at different temperatures or pH values by monitoring the incremental changes in chemical shifts with changing solution conditions in series of 1H-15N HSQC spectra.
NMR spectra were run on samples with 0.25 to 0.5 mM concentrations of αS. 2D 1H-15N HSQC spectra were typically obtained with 1H × 15N spectral widths of 6000 × 1370 Hz defined by 1024 × 128 complex points. The cross peak for the C-terminal residue, A140, was aliased in the 15N dimension (see Fig. 1A) to optimize resolution. For 13C, 15N double-labeled samples, 13C decoupling was used during the t1 period of 1H-15N HSQC experiments, as this results in a noticeable reduction in 15N linewidths of the intrinsically unfolded αS monomers. 1H-13C HSQC experiments were run in constant-time mode, with 1H × 13C spectral widths of 6000 × 4500 Hz defined by 1024 × 121 complex points. One set of experiments was selective for Cα–Hα correlations, the second set for aromatic correlations. Total acquisition times were ∼10–20 min per HSQC experiment.
The critical micelle concentrations of GM1 (Basu and Glew 1985) and SDS (Fuguet et al. 2005) are ∼20 μM and 3.3 mM, respectively. Above these concentrations, the lipids spontaneously form micelles. Stock solutions of the micelles were added to 250 μM samples of αS until no further changes were observed in 1H-15N HSQC spectra. This occurred at concentrations of 3.5 mM GM1 and 45 mM SDS.
Hydrogen exchange data for αS at pH 7.4 and a temperature of 15°C were measured with phase-modulated clean chemical exchange (CLEANEX-PM) experiments using the Fast-HSQC detection scheme (Hwang et al. 1998), as implemented in the Varian Biopack. Control experiment to determine reference peak intensities I 0, and six CLEANEX experiments with mixing times τm of 4, 8, 12, 16, 20, and 24 ms for 20 mM phosphate, or 12, 16, 24, 32, 40, and 48 ms for 300 mM NaCl were acquired to determine amide proton exchange rates. Data were analyzed using nonlinear least-squares fits of the equation:
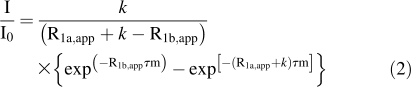 |
where R1a,app is an apparent relaxation rate that has both R1 and R2 contributions and depends on the details of the CLEANEX experiments (Hwang et al. 1998), R1b,app is an apparent relaxation rate for the water signal (0.6 s−1), and k is the amide proton exchange rate. The terms k and (R1a,app + k) were the free variables in the least squares fits. Intrinsic exchange rates were calculated with the SPHERE server (Zhang 1995), using default parameters for exchange in H2O.
To assess how much α-helical structure is present in αS from Cα chemical shifts, 3D HN(CO)CA experiments were recorded at pH 6.4, and sample temperatures of 10°C and 35°C. The experiments were obtained with spectral widths of 6000 (1H) × 3618 (13C) × 1370 (15N) Hz digitized into 1024 × 100 × 32 complex points. To estimate the amount of α-helix according to Equation 1, random coil chemical shifts were taken from the literature, and the published chemical shifts (Chandra et al. 2003) of micelle-bound αS (BMRB accession code 5744) were taken to represent the folded state with fully populated α-helix structure.
NMR experiments with living E. coli cells
A starter culture of BL21(DE3)pLysS E. coli cells harboring the pT7-7/asyn-WT plasmid was grown overnight at 37°C in 1 mL of Luria Broth (LB) supplemented with 100 μg/mL ampicillin and 34 μg/mL chloramphenicol. A 200 μL aliquot of this starter culture was used to inoculate 50 mL of 15N-labeled MOPS minimal media modified to contain components of the auto-induce protocol (Studier 2005): 0.5% glycerol, 0.05% glucose, 0.2% lactose, 2 mM MgSO4, 50 mM Na2HPO4, and 50 mM KH2PO4. The 50-mL culture was incubated overnight at a temperature of 37°C with a shaking speed of 250 rpm to express 15N-labeled αS.
A 500-μL aliquot of the culture expressing 15N-labeled αS, with 10% (v/v) D2O for the magnet lock, was placed in a 535-pp NMR tube from Wilmad for an initial optimization of the NMR shims (Serber et al. 2006). The remainder of the culture was collected by centrifugation at 1200g for 20 min at 4°C. The supernatant was decanted into a separate container, and 125 μL of the supernatant was used to gently re-suspend the pelleted cells to a homogeneous slurry with a final volume of ∼600 μL. The cell slurry was taken up in an NMR tube, and 10% (v/v) D2O was added for the deuterium lock. 1H−15N HSQC data of αS in live E. coli cells were acquired with spectral widths of 6500 (1H) × 1560 (15N) Hz and data sizes of 1024 × 100 complex points. Eight scans were averaged per free induction decay, for a total acquisition time of 33 min.
Following NMR experiments, 100 μL of the sample were used to streak LB-agar plates containing 100 μg/mL ampicillin and 34 μg/mL chloramphenicol at cell sample dilutions of 103, 104, 105, 106, 107, 108, and 109. The plates were incubated overnight at 37°C. The presence of individual colonies on plates prepared from the diluted samples verified cell viability (McNulty et al. 2006b; Serber et al. 2006). The remainder of the live-cell NMR sample was spun in an Eppendorf 5415C centrifuge for 20 min at 1200g. The cell pellet was subjected to SDS-PAGE to verify overexpression of the αS gene. The supernatant from the spin were analyzed by 1H-15N HSQC spectroscopy to verify that there were no NMR signals from αS that could have leaked outside the cells (McNulty et al. 2006b; Serber et al. 2006).
Electronic supplemental material
The Supplemental material consists of NMR assignments for αS, summary of the effects of GM1 micelles on 1H-15N and 1H-13C HSQC spectra of αS, representative CLEANEX data, conformational exchange contributions to R2 at 10°C and 35°C (five figures), and a table of backbone amide solvent exchange rates at pH 7.4 and a temperature of 15°C.
Acknowledgments
We thank Professor Peter Lansbury and Dr. Michael J. Volles (Harvard Medical School) for the pT7-7/asyn-WT expression vector used in this work. This work was supported by a Roger C. Duvoisin, MD, research grant from the American Parkinson Disease Association to A.T.A., a Parkinson Disease Foundation summer fellowship to E.W., and a summer graduate stipend from the Richard C. Crain Jr. Memorial Fellowship Fund to C.O.S. NMR instrumentation was funded in part by grant 1S10RR016760 from the NIH-NCRR.
Footnotes
Supplemental material: see www.proteinscience.org
Reprint requests to: Andrei Alexandrescu, Department of Molecular and Cell Biology, University of Connecticut, 91 North Eagleville Road, U-3125, Storrs, CT 06269-3125, USA; e-mail: andrei@uconn.edu; fax: (860) 486-4331.
Abbreviations: αS, α-synuclein; ct, constant time; CLEANEX, clean chemical exchange; DSS, 2,2-Dimethyl-2-silapentane-5-sulfonic acid; HSQC, heteronuclear single quantum coherence spectroscopy; GM1, monosialotetrahexosylganglioside; R2, transverse relaxation rate (1/T2); R2ex, chemical exchange contribution to R2; SDS, sodium dodecyl sulfate.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.033803.107.
References
- Alexandrescu, A.T., Abeygunawardana, C., Shortle, D. Structure and dynamics of a denatured 131-residue fragment of staphylococcal nuclease: A heteronuclear NMR study. Biochemistry. 1994;33:1063–1072. doi: 10.1021/bi00171a004. [DOI] [PubMed] [Google Scholar]
- Alexandrescu, A.T., Jahnke, W., Wiltscheck, R., Blommers, M.J. Accretion of structure in staphylococcal nuclease: A 15N NMR relaxation study. J. Mol. Biol. 1996;260:570–587. doi: 10.1006/jmbi.1996.0422. [DOI] [PubMed] [Google Scholar]
- Bai, Y., Milne, J.S., Mayne, L., Englander, S.W. Primary structure effects on peptide group hydrogen exchange. Proteins. 1993;17:75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, A., Glew, R.H. Characterization of the activation of rat liver β-glucosidase by sialosylgangliotetraosylceramide. J. Biol. Chem. 1985;260:13067–13073. [PubMed] [Google Scholar]
- Bermel, W., Bertini, I., Felli, I.C., Lee, Y.M., Luchinat, C., Pierattelli, R. Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J. Am. Chem. Soc. 2006;128:3918–3919. doi: 10.1021/ja0582206. [DOI] [PubMed] [Google Scholar]
- Brown, D.R. Interactions between metals and α-synuclein–function or artefact? FEBS J. 2007;274:3766–3774. doi: 10.1111/j.1742-4658.2007.05917.x. [DOI] [PubMed] [Google Scholar]
- Buck, M., Radford, S.E., Dobson, C.M. Amide hydrogen exchange in a highly denatured state. Hen egg-white lysozyme in urea. J. Mol. Biol. 1994;237:247–254. doi: 10.1006/jmbi.1994.1228. [DOI] [PubMed] [Google Scholar]
- Bussell R., Jr, Eliezer, D. Residual structure and dynamics in Parkinson's disease-associated mutants of β-synuclein. J. Biol. Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- Calik, P., Yilgor, P., Ayhan, P., Demir, A.S. Oxygen transfer effects on recombinant benzaldehyde lyase production. Chem. Eng. Sci. 2004;59:5075–5083. [Google Scholar]
- Chandra, S., Chen, X., Rizo, J., Jahn, R., Sudhof, T.C. A broken α-helix in folded α-synuclein. J. Biol. Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- Conway, K.A., Lee, S.J., Rochet, J.C., Ding, T.T., Williamson, R.E., Lansbury P.T., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: Implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson, M.R. The biochemistry of Parkinson's disease. Annu. Rev. Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- Dempsey, C.E. Hydrogen exchange in peptides and proteins using NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2001;39:135–170. [Google Scholar]
- El-Agnaf, O.M., Salem, S.A., Paleologou, K.E., Cooper, L.J., Fullwood, N.J., Gibson, M.J., Curran, M.D., Court, J.A., Mann, D.M., Ikeda, S., et al. α-Synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- Eliezer, D. Amyloid ion channels: A porous argument or a thin excuse? J. Gen. Physiol. 2006;128:631–633. doi: 10.1085/jgp.200609689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliezer, D., Kutluay, E., Bussell R., Jr, Browne, G. Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- Englander, S.W., Mayne, L., Bai, Y., Sosnick, T.R. Hydrogen exchange: The modern legacy of Linderstrom-Lang. Protein Sci. 1997;6:1101–1109. doi: 10.1002/pro.5560060517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejzo, J., Westler, W.M., Macura, S., Markley, J.L. Elimination of cross-relaxation effects from two-dimensional chemical-exchange spectra of macromolecules. J. Am. Chem. Soc. 1990;112:2574–2577. [Google Scholar]
- Fernandez, C.O., Hoyer, W., Zweckstetter, M., Jares-Erijman, E.A., Subramaniam, V., Griesinger, C., Jovin, T.M. NMR of α-synuclein–polyamine complexes elucidates the mechanism and kinetics of induced aggregation. EMBO J. 2004;23:2039–2046. doi: 10.1038/sj.emboj.7600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, A.L. The aggregation and fibrillation of α-synuclein. Acc. Chem. Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- Fuguet, E., Rafols, C., Rosés, M., Bosch, E. Critical micelle concentration of surfactants in aqueous buffered and unbuffered systems. Anal. Chim. Acta. 2005;548:95–100. [Google Scholar]
- George, J.M. The synucleins. Genome Biol. 2002;3:reviews3002.1–reviews3002.6. doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert, M. α-Synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Goldberg, J.J., Bramley, A.J., Sjogren, R.E., Pankey, J.W. Effects of temperature and oxygen tension on growth of Escherichia coli in milk. J. Dairy Sci. 1994;77:3338–3346. doi: 10.3168/jds.S0022-0302(94)77275-1. [DOI] [PubMed] [Google Scholar]
- Grzesiek, S., Bax, A. The importance of not saturating H2O in protein NMR. Application to sensitivity enhancement and NOE measurements. J. Am. Chem. Soc. 1993;115:12593–12594. [Google Scholar]
- Hwang, T.L., van Zijl, P.C., Mori, S. Accurate quantitation of water-amide proton exchange rates using the phase-modulated CLEAN chemical EXchange (CLEANEX-PM) approach with a Fast-HSQC (FHSQC) detection scheme. J. Biomol. NMR. 1998;11:221–226. doi: 10.1023/a:1008276004875. [DOI] [PubMed] [Google Scholar]
- Jaravine, V.A., Alexandrescu, A.T., Grzesiek, S. Observation of the closing of individual hydrogen bonds during TFE-induced helix formation in a peptide. Protein Sci. 2001;10:943–950. doi: 10.1110/ps.48501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf, J.G., Loria, J.P. Measurement of intermediate exchange phenomena. Methods Mol. Biol. 2004;278:185–231. doi: 10.1385/1-59259-809-9:185. [DOI] [PubMed] [Google Scholar]
- Kim, P.S., Baldwin, R.L. Influence of charge on the rate of amide proton exchange. Biochemistry. 1982;21:1–5. doi: 10.1021/bi00530a001. [DOI] [PubMed] [Google Scholar]
- Koide, S., Jahnke, W., Wright, P.E. Measurement of intrinsic exchange rates of amide protons in a 15N-labeled peptide. J. Biomol. NMR. 1995;6:306–312. doi: 10.1007/BF00197811. [DOI] [PubMed] [Google Scholar]
- Krishna, M.M., Hoang, L., Lin, Y., Englander, S.W. Hydrogen exchange methods to study protein folding. Methods. 2004;34:51–64. doi: 10.1016/j.ymeth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Lansbury, P.T., Lashuel, H.A. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- Lashuel, H.A., Hartley, D., Petre, B.M., Walz, T., Lansbury P.T., Jr Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- Marsh, J.A., Singh, V.K., Jia, Z., Forman-Kay, J.D. Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: Implications for fibrillation. Protein Sci. 2006;15:2795–2804. doi: 10.1110/ps.062465306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, Z., Zhu, M., Han, S., Fink, A.L. GM1 specifically interacts with α-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- Matthew, J.B., Richards, F.M. The pH dependence of hydrogen exchange in proteins. J. Biol. Chem. 1983;258:3039–3044. [PubMed] [Google Scholar]
- McNulty, B.C., Tripathy, A., Young, G.B., Charlton, L.M., Orans, J., Pielak, G.J. Temperature-induced reversible conformational change in the first 100 residues of α-synuclein. Protein Sci. 2006a;15:602–608. doi: 10.1110/ps.051867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty, B.C., Young, G.B., Pielak, G.J. Macromolecular crowding in the Escherichia coli periplasm maintains α-synuclein disorder. J. Mol. Biol. 2006b;355:893–897. doi: 10.1016/j.jmb.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Moore, D.J., West, A.B., Dawson, V.L., Dawson, T.M. Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Mori, S., Abeygunawardana, C., Johnson, M.O., van Zijl, P.C. Improved sensitivity of HSQC spectra of exchanging protons at short interscan delays using a new fast HSQC (FHSQC) detection scheme that avoids water saturation. J. Magn. Reson. B. 1995;108:94–98. doi: 10.1006/jmrb.1995.1109. [DOI] [PubMed] [Google Scholar]
- Mori, S., Berg, J.M., van Zijl, P.C. Separation of intramolecular NOE and exchange peaks in water exchange spectroscopy using spin-echo filters. J. Biomol. NMR. 1996;7:77–82. doi: 10.1007/BF00190459. [DOI] [PubMed] [Google Scholar]
- Mori, S., van Zijl, P.C., Shortle, D. Measurement of water-amide proton exchange rates in the denatured state of staphylococcal nuclease by a magnetization transfer technique. Proteins. 1997;28:325–332. [PubMed] [Google Scholar]
- Pedersen, T.G., Sigurskjold, B.W., Andersen, K.V., Kjaer, M., Poulsen, F.M., Dobson, C.M., Redfield, C. A nuclear magnetic resonance study of the hydrogen-exchange behaviour of lysozyme in crystals and solution. J. Mol. Biol. 1991;218:413–426. doi: 10.1016/0022-2836(91)90722-i. [DOI] [PubMed] [Google Scholar]
- Ren, G., Wang, X., Hao, S., Hu, H., Wang, C.C. Translocation of α-synuclein expressed in Escherichia coli . J. Bacteriol. 2007;189:2777–2786. doi: 10.1128/JB.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serber, Z., Ledwidge, R., Miller, S.M., Dotsch, V. Evaluation of parameters critical to observing proteins inside living Escherichia coli by in-cell NMR spectroscopy. J. Am. Chem. Soc. 2001;123:8895–8901. doi: 10.1021/ja0112846. [DOI] [PubMed] [Google Scholar]
- Serber, Z., Selenko, P., Hansel, R., Reckel, S., Lohr, F., Ferrell J.E., Jr, Wagner, G., Dotsch, V. Investigating macromolecules inside cultured and injected cells by in-cell NMR spectroscopy. Nat. Protoc. 2006;1:2701–2709. doi: 10.1038/nprot.2006.181. [DOI] [PubMed] [Google Scholar]
- Shimba, N., Serber, Z., Ledwidge, R., Miller, S.M., Craik, C.S., Dotsch, V. Quantitative identification of the protonation state of histidines in vitro and in vivo. Biochemistry. 2003;42:9227–9234. doi: 10.1021/bi0344679. [DOI] [PubMed] [Google Scholar]
- Shojania, S., O'Neil, J.D. HIV-1 Tat is a natively unfolded protein: The solution conformation and dynamics of reduced HIV-1 Tat-(1-72) by NMR spectroscopy. J. Biol. Chem. 2006;281:8347–8356. doi: 10.1074/jbc.M510748200. [DOI] [PubMed] [Google Scholar]
- Steinmetz, M.O., Jelesarov, I., Matousek, W.M., Honnappa, S., Jahnke, W., Missimer, J.H., Frank, S., Alexandrescu, A.T., Kammerer, R.A. Molecular basis of coiled-coil formation. Proc. Natl. Acad. Sci. 2007;104:7062–7067. doi: 10.1073/pnas.0700321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Sung, Y.H., Eliezer, D. Residual structure, backbone dynamics, and interactions within the synuclein family. J. Mol. Biol. 2007;372:689–707. doi: 10.1016/j.jmb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer, T.S., Bax, A. Comparison of structure and dynamics of micelle-bound human α-synuclein and Parkinson disease variants. J. Biol. Chem. 2005;280:43179–43187. doi: 10.1074/jbc.M507624200. [DOI] [PubMed] [Google Scholar]
- Ulmer, T.S., Bax, A., Cole, N.B., Nussbaum, R.L. Structure and dynamics of micelle-bound human α-synuclein. J. Biol. Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- Uversky, V.N., Li, J., Fink, A.L. Evidence for a partially folded intermediate in α-synuclein fibril formation. J. Biol. Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- Weinreb, P.H., Zhen, W., Poon, A.W., Conway, K.A., Lansbury P.T., Jr NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- Wilks, J.C., Slonczewski, J.L. pH Of the cytoplasm and periplasm of Escherichia coli: Rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 2007;189:5601–5607. doi: 10.1128/JB.00615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart, D.S., Sykes, B.D. Chemical shifts as a tool for structure determination. Methods Enzymol. 1994;239:363–392. doi: 10.1016/s0076-6879(94)39014-2. [DOI] [PubMed] [Google Scholar]
- Wishart, D.S., Bigam, C.G., Holm, A., Hodges, R.S., Sykes, B.D. 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J. Biomol. NMR. 1995a;5:67–81. doi: 10.1007/BF00227471. [DOI] [PubMed] [Google Scholar]
- Wishart, D.S., Bigam, C.G., Yao, J., Abildgaard, F., Dyson, H.J., Oldfield, E., Markley, J.L., Sykes, B.D. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR. 1995b;6:135–140. doi: 10.1007/BF00211777. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.-Z. University of Pennsylvania; 1995. “Protein and peptide structure and interactions studied by hydrogen exchange and NMR”. Ph.D. thesis. [Google Scholar]