Abstract
Pathogenic Yersinia strains evade the innate immune responses of the host by producing effector proteins (Yersinia outer proteins [Yops]), which are directly injected into mammalian cells by a type III secretion system (TTSS). One of these effector proteins (YopT) disrupts the actin cytoskeleton of the host cell resulting in cell rounding. YopT is a cysteine protease that cleaves Rho proteins directly upstream of the post-translationally modified cysteine. Thereby, it releases the GTPases from the membrane leading to inactivation. Small GTPases are modified by isoprenylation of the cysteine of the CAAX box, cleavage of the –AAX tripeptide, and methylation of the cysteine. We have shown that isoprenylation and the endoproteolytic cleavage of the tripeptide of Rho GTPases are essential for YopT-induced cleavage, whereas carboxyl methylation is not required. In the present study, we post-translationally modified RhoA, Rac, Cdc42, and several mutants in vitro and characterized the YopT-induced cleavage with recombinant YopT. We show that farnesylated RhoA is a preferred substrate of YopT compared with the geranylgeranylated GTPase. Geranylgeranylated RhoA, however, is the preferred substrate for YopT-catalyzed cleavage with a threefold faster turnover rate over Rac and Cdc42. Moreover, our data indicate that the composition of the polybasic region of the GTPases defines the specificity and efficiency of the YopT-induced cleavage, and that a space between the polybasic stretch of amino acids at the C terminus and the CAAX box enhances the turnover rate of YopT-catalyzed cleavage.
Keywords: bacterial toxin, YopT, Rho GTPases, post-translational modification, polybasic region
Evasion of innate immune responses by pathogenic Yersinia strains Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica depends on a 70-kb virulence plasmid (pVY), which encodes a type III secretion system (TTSS), translocator and regulator proteins, and a set of six effector proteins (Yersinia outer proteins [Yops]) (Cornelis et al. 1998; Cornelis 2002). Upon contact with the host cell, the six Yops are translocated via the TTSS into the cytoplasm (Viboud and Bliska 2005). YopP (also called YopJ in Y. pseudotuberculosis) inhibits the MAPK and NFκB pathways by acetylation of MEK2, MEK6, and IKK (Mittal et al. 1996; Mukherjee et al. 2006). Although the cellular function of YopM is still unknown, the protein interacts with and activates two host cell kinases and is transported to the nucleus (McDonald et al. 2003). YopH dephosphorylates proteins of the focal adhesion complex (FAK, p130Cas, and Fyb) (Hamid et al. 1999; Black et al. 2000). YopE, YopO (YpkA in Y. pseudotuberculosis), and YopT target members of the Rho family of small GTPases, which control the actin cytoskeleton. YopE acts as a GAP for RhoA and Rac and induces their inactivation (von Pawel-Rammingen et al. 2000; Andor et al. 2001). YopO/YpkA is a multidomain protein with a GDI-like binding domain (Prehna et al. 2006) and an actin-binding domain (Dukuzumuremyi et al. 2000), and an N-terminal serine/threonine kinase domain, which inhibits Gαq signaling pathways by direct phosphorylation of Gαq (Navarro et al. 2007). YopT is a member of cysteine proteases with invariant Cys/His/Asp residues (Shao et al. 2002) that causes rounding of host cells by disruption of the actin cytoskeleton (Iriarte and Cornelis 1998). It cleaves Rho proteins directly upstream of the post-translationally modified cysteine of the small GTPases, thereby releasing the GTPases from the membrane, leading to inactivation of the GTPases (Shao et al. 2003). Small GTPases that harbor a C-terminal CAAX box (C, cysteine; A, aliphatic residue, X, any residue) are post-translationally modified (Zhang and Casey 1996). After isoprenylation of the cysteine by geranylgeranyl transferase type I (GGT-I), the –AAX tripeptide is cleaved by RCE1 (Ras converting enzyme 1) and the carboxyl group of the geranylgeranylated cysteine is methylated by IcMT (isoprenylcysteine carboxyl methyltransferase) (Otto et al. 1999; Winter-Vann and Casey 2005). Isoprenylation and endoproteolytic cleavage of the –AAX tripeptide of the Rho GTPases is essential for YopT-induced cleavage, whereas carboxyl methylation is not required (Shao et al. 2003; Fueller et al. 2006). In addition, the polybasic region of the GTPase appears to be necessary and sufficient for YopT-induced recognition and cleavage (Shao et al. 2003).
In the present study, we characterized the YopT-induced cleavage of post-translationally modified RhoA, Rac, and Cdc42, and determined that the specificity and efficiency of the YopT-induced cleavage is defined by the polybasic region of the GTPases.
Results and Discussion
The Yersinia enterocolitica outer protein YopT cleaves Rho GTPases directly before the C-terminal post-translationally modified cysteine. To analyze the proteolytic activity of YopT on Rho GTPases in vitro, the three post-translational modification steps catalyzed by geranylgeranyl transferase-I (GGT-I) or farnesyl transferase (FT), the Ras converting enzyme 1 (RCE1), and the isoprenylated cysteine methyl transferase (IcMT) were established.
Activities of the single enzymes were comparable with previous data from the literature (Km GGT-I 4.1 μM; this study, 1.5 μM) (Horiuchi et al. 1991); (Km IcMT with the substrate N-Acetyl-S-farnesyl-L-Cysteine 8.2 μM; this study, 11.5 μM) (Buckner et al. 2002). The post-translational modification was finished with [14C]-SAM (AdoMet) to quantify the amount of completely modified GTPases. Radioactive labeling of the C-terminal cysteine enabled us to measure the YopT-catalyzed cleavage of the Rho GTPases.
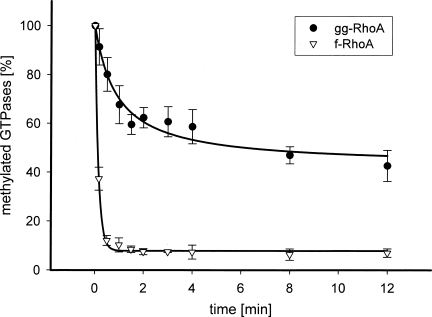
Recently, the authors and others have shown that isoprenylation and cleavage of the −AAX tripeptide is necessary for YopT-catalyzed cleavage of Rho GTPases (Shao et al. 2003; Sorg et al. 2003; Fueller et al. 2006). Therefore, we first analyzed whether the type of isoprene attached to the GTPase has any effect on toxin-induced cleavage. RhoA and RhoA(L193S), a CAAX mutant which is farnesylated, were either geranylgeranylated with GGT-I or farnesylated with FT, treated with RCE1, and labeled by IcMT in the presence of 14C-SAM. Subsequently, the GTPases were treated with YopT and their proteolytic cleavage measured in a time course. As shown in Figure 1, farnesylated RhoA is a preferred substrate of YopT over geranylgeranylated RhoA. The amount of completely modified GTPases was comparable (∼10%). Addition of a 10-fold amount of unmodified or exclusively isoprenylated GTPases had no influence on the velocity of YopT-catalyzed cleavage in these experiments, indicating that it is not the ratio between fully modified and not completely modified GTPases that determines the cleavage rate (data not shown).
Figure 1.
Farnesylated RhoA is the preferred substrate of YopT. RhoA wild type and RhoA(L193S) (a mutant that is farnesylated) was post-translationally modified and 14C-labeled. After determination of the concentration of radiolabeled, isoprenylated RhoA (2.1 μM ± 0.2), the proteolytic cleavage of farnesylated RhoA (∇, n = 3) and geranylgeranylated RhoA (●, n = 6) was initiated by addition of YopT in a 1:10 ratio (YopT: GTPase) at 37°C. The reaction was terminated by the addition of 5% SDS. The proteins were precipitated with 30% TCA and 14C-labeled proteins were quantified using a filter assay. Efficiency of YopT cleavage was illustrated as a decrease of radiolabeled protein in percent over time.
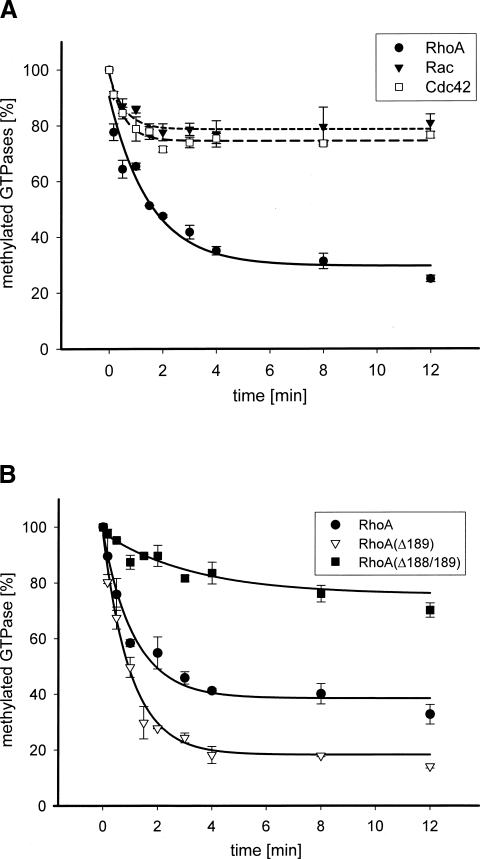
Besides the requirements for toxin-catalyzed cleavage of RhoA, it is still a matter of debate which Rho proteins are substrates for YopT. Therefore, we studied, whether the kinetics of YopT-induced cleavage of RhoA, Rac, and Cdc42 are different in our in vitro assay. As shown in Figure 2A, RhoA is the preferred substrate for YopT-catalyzed cleavage with a threefold faster turnover rate over Rac and Cdc42, which are cleaved with comparable kinetics (see Table 1). It has been shown that the polybasic region of RhoA is sufficient for recognition and cleavage by YopT (Shao et al. 2003). In contrast to Rac and Cdc42, RhoA contains two additional amino acids (SG) that separate the basic amino acids from the CAAX box (Table 1). To analyze whether these amino acids influence the YopT-catalyzed cleavage of RhoA, the deletion mutants RhoA(Δ189) and RhoA(Δ188/189) were generated, post-translationally modified, and cleaved by YopT. Cleavage of the mutant, RhoA(Δ188/189), with the CAAX box directly following the basic amino acids was slower than that of Rac or Cdc42 (Fig. 2B; Table 1), indicating that a space between the basic amino acids and the CAAX box is required for rapid GTPase cleavage. Unexpectedly, the deletion mutant RhoA(Δ189) was cleaved even faster than wild-type RhoA (Fig. 2B).
Figure 2.
(A) RhoA is the preferred substrate for YopT-catalyzed proteolysis. The GTPases RhoA, Rac, and Cdc42 were post-translationally modified and radiolabeled (1.6 μM ± 0.2). Following quantification of 14C-labeled RhoA (●, n = 6), Rac (▼, n = 6), and Cdc42 (□, n = 6) the proteolytic cleavage of the C-terminal cysteine was initiated by adding YopT in a 1:10 ratio (YopT: GTPase) at 37°C. At the indicated time the reaction was stopped by the addition of 5% SDS. Proteins were precipitated with 30% TCA. The radiolabeled GTPases were measured using a filter assay and scintillation counting. (B) Proteolytic processing of RhoA, RhoA(Δ189), and RhoA(Δ188/189) by YopT. RhoA and the deletion mutants RhoA(Δ189) and RhoA(Δ188/189) were post-translationally modified and then radiolabeled. After determination of the concentration of 14C-labeled RhoA (●, n = 6), RhoA(Δ189) (▽, n = 9), and RhoA(Δ188/189) (■, n = 3) (2.1 μM ± 0.2) the proteolytic processing of the GTPases was initiated by addition of YopT in 1:10 ratio (YopT: GTPase) at 37°C. The reaction was terminated by adding 5% SDS. The proteins were precipitated by the addition of 30% TCA. Radiolabeled GTPases were determined using a filter assay and a scintillation counter. The proteolytic activity of YopT was illustrated in percent of remaining 14C-labeled GTPases (t 0 = 100%).
Table 1.
C-terminal mutations of RhoA, Rac, and Cdc42 and the kinetics of YopT-induced cleavage of the isoprenylated cysteine.
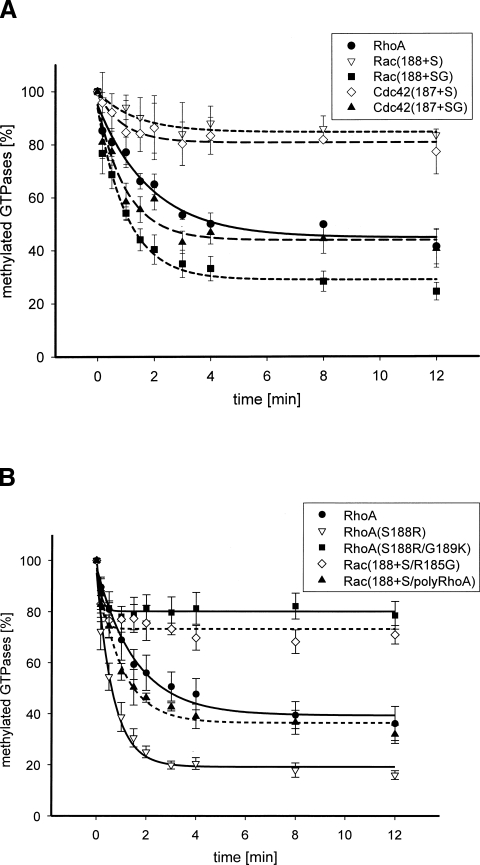
In a reciprocal experiment, the influence of the additional amino acids (SG) on YopT-catalyzed cleavage of Rac and Cdc42 were analyzed. Insertion of serine or serine plus glycine into Rac (Rac[188+S], Rac[188+SG]) or Cdc42 (Cdc42[187+S], Cdc42[187+SG]) in front of the CAAX box should enhance the catalytic activity of YopT cleavage. On the other hand, mutation of serine and glycine in RhoA to basic amino acids (S188R/G189K) should remove the space between the polybasic region and the isoprenylated cysteine without changing the length of the RhoA C terminus. We performed the post-translational modification of the mutant proteins and subsequent cleavage by YopT. As shown in Figure 3A, insertion of serine in front of the CAAX box only slightly enhanced the velocity of the YopT-catalyzed cleavage of Rac or Cdc42, whereas insertion of serine plus glycine enhanced the velocity of YopT-induced cleavage of the mutants by sixfold (Fig. 3B; Table 1). Mutation of serine and glycine of RhoA to basic amino acids (RhoA[S188R/G189K]) reduced the turnover rate to <1 min−1, comparable to the cleavage of RhoA(Δ188/189).
Figure 3.
(A) Proteolytic cleavage of RhoA, Rac(188+S), Rac(188+SG), Cdc42(187+S), and Cdc42(187+SG) by YopT. RhoA and the insertion mutants Rac(188+S), Rac(188+SG), Cdc42(187+S), and Cdc42(187+SG) were post-translationally modified and radiolabeled with 14C-SAM. After determination of the concentration of radiolabeled GTPases (2.5 μM ± 0.1) the proteolytic cleavage of RhoA (●, n = 3), Rac(188+S) (▽, n = 3), GST-Rac(188+SG) (■, n = 3), Cdc42(187+S) (◇, n = 3), and GST-Cdc42(187+SG) (▲, n = 3) was initiated by addition of YopT in a ratio of 1:10 (YopT: GTPase) at 37°C. The reaction was stopped by the addition of 5% SDS and the proteins were precipitated with 30% TCA. Radiolabeled GTPases were determined using a filter assay. The efficiency of YopT cleavage was demonstrated by the amount of remaining radiolabeled GTPases in percent (t 0 = 100%). (B) Proteolytic cleavage of RhoA, RhoA(S188R), RhoA(S188R/G189K), Rac(188+S/R185G), and Rac(188+S/polyRhoA) by YopT. GST-RhoA, GST-RhoA(S188R), GST-RhoA(S188R/G189K), GST-Rac(188+S/R185G), and GST-Rac(188+S/polyRhoA), were post-translationally modified and radiolabeled. After determination of the concentration of radiolabeled GTPases (2.5 μM ± 0.3) the proteolytic cleavage of GST-RhoA (●, n = 3), GST-RhoA(S188R) (▽, n = 3), GST-RhoA(S188R/G189K) (■, n = 3), GST-Rac(188+S/R185G) (◇, n = 3), and GST-Rac(188+S/polyRhoA) (▲, n = 3), was initiated by addition of YopT in a ratio of 1:10 (YopT: GTPase) at 37°C. The reaction was terminated by adding 5% SDS. The proteins were precipitated by the addition of 30% TCA. Radiolabeled GTPases were determined using a filter assay and a scintillation counter. The proteolytic activity of YopT was illustrated by the remaining 14C-labeled GTPases in percent (t 0 = 100%).
The data indicate that it is not the variation in length of the C-terminal peptide of RhoA but rather the space between the basic amino acids and the CAAX box that determines the difference in kinetics of Rho GTPase cleaved by YopT. Since the basic amino acids contact the inner surface of the membrane, a linker between this interaction site and the isoprenylated cysteine, which is also inserted into the membrane, may allow for increased flexibility of the cleavage site. However, it was not consistent for each Rho GTPase whether more rapid cleavage occurred with one or two amino acids between the polybasic region and the CAAX box. Without a spacer the velocity of the YopT-catalyzed cleavage ranged from 0.8 min−1 to 1.4 min−1, with the highest rate occurring for Rac1. However, all proteins with an insertion of serine plus glycine were cleaved with a velocity of 4.8–7.6 min−1.
In contrast, insertion of serine into Rac (Rac[188+S] or Cdc42 (Cdc42[187+S]) only slightly enhanced the turnover rate of YopT-induced cleavage. It has been suggested in earlier studies that the C-terminal part of Rho GTPases is sufficient for YopT-catalyzed cleavage (Shao et al. 2003). For this cleavage to occur binding of YopT to membrane-bound Rho–GTPases must be possible, although the basic amino acids are in close contact with the inner plasma membrane. In this respect, recent studies showed that the Rac effector PRK1 binds to the polybasic region of membrane-bound Rac, and that this interaction has no influence on membrane anchoring of the complex (Modha et al. 2008). It has been proposed that the polybasic region serves as a plasma membrane targeting signal for a number of small G proteins (Hancock et al. 1991). Not only did the spacer between the polybasic region and the CAAX box vary between the GTPases and thereby influence the cleavage of YopT, but the composition of the polybasic region of the Rho GTPases also defined the cleavage by YopT. To test this we made the polybasic domain of Rac(188+S) more Rho-like by mutation of arginine 185 of Rac(188+S) to glycine (Rac[188+S/R185G]), which discontinues the stretch of basic amino acids and thereby reduces the affinity to the plasma membrane. The introduction of glycine into Rac(188+S) just slightly enhanced the turnover rate of YopT-catalyzed GTPase cleavage (from 1.5–2.5 min−1). The exchange of the complete polybasic region of RhoA into Rac(188+S), Rac(188+S/polyRhoA), led to a comparable turnover rate of cleavage as measured for RhoA(Δ189) (see Table 1). The data indicate that the composition of the polybasic region and the length of space between basic residues and the isoprenylated cysteine influence the kinetics of YopT-catalyzed cleavage of Rho GTPases. This explains why RhoA is a preferred substrate for YopT compared with Rac and Cdc42.
Besides our studies on the influence of the very C terminus of the Rho GTPases on YopT-catalyzed cleavage, the most dramatic effect was found for the cleavage of farnesylated versus geranylgeranylated RhoA with an enhancement of the turnover rate by a factor of 10. In a model of YopT-catalyzed cleavage, Shao and coworkers proposed an initial substrate binding and subsequent shift of the isoprenyl group from the membrane to a presumed hydrophobic binding pocket of the protease before cleavage (Shao et al. 2003). Farnesylation of GTPases, which gives them intrinsically less hydrophobicity than geranylgeranylation, could lead to a weaker contact with the membrane and better accessibility for YopT (Wright and Philips 2006).
With the in vitro post-translational modification of Rho GTPases we have demonstrated that the composition of the polybasic region of Rho GTPases determines the efficiency of the cleavage of Rho GTPases by the Yersinia enterocolitica outer protein YopT. From our data we conclude that YopT would prefer a farnesylated Rho–GTPase with a lysine in position P3 and an arginine in position P7. Additionally, an ideal substrate for YopT would contain a space of one or two small amino acids between the polybasic domain and the isoprenylated cysteine.
Materials and Methods
Materials
Oligonucleotides were obtained from Apara Bioscience. The QuikChange Kit was from Stratagene; TOPO cloning was performed with the TOPO TA Cloning Kit from Invitrogen. Glutathione-Sepharose 4B was from Amersham Bioscience Europe. Thrombin, geranylgeranyl pyrophosphate, and farnesyl pyrophosphate were from Sigma. The BaculoGold Transfections Kit was obtained from Pharmingen. [14C]-SAM was purchased from Perkin-Elmer.
Constructs of GTPase mutants
GTPase mutants were constructed by site-directed mutagenesis with the QuikChange Kit, according to the manufacturer's instructions, using pGEX–2T–RhoA, pGEX–2T–Rac, or pGEX–2T–Cdc42 as a template, and the following primers (and corresponding antisense primers): RhoA(S188K) (5′-GGGAAGAAAAAACGTGGTTGCCTTGTCTTG-3′), RhoA(S188K/G189R) (5′-GGGAAGAAAAAACGTAAATGCCTTGTCTTGTG-3′), Rac(188+S) (5′-GGAAGAGAAAATCATGCCTGCTGTTGTAA-3′), Rac(188+S/R185G) (5′-CCTCCCGTGAAGAAGGGGAAGAGAAAATCATGC-3′), Rac(188+S/polyRhoA) (5′-CCGCCTCCCGTGAGGAGGGGGAAGAAAAAATCATGCCTG-3′), Rac(188+SG) (5′-GGAAGAGAAAATCAGGATGCCTGCTGTTG-3′), Cdc42(187+S) (5′-GAGCCGCAGGTCATGCGTCCTGCTATG-3′), Cdc42(187+S/polyRhoA) (5′-CCTCCAGAACCGAGGAGGGGCAAAAAGAAATCATGCGTCCTG-3′), and Cdc42(187+SG) (5′-GCCGCAGGTCAGGATGCGTCCTGCTAT-3′). RhoA deletions mutations were generated by PCR using a T1 thermocycler from Biometra and Taq polymerase from Invitrogen with the following primers that created a BamHI cleavage site at the 5′-end and an EcoRI cleavage site at the 3′-end: RhoA(Δ189)-f (5′-TAGCATGGCCTTTGCAGGG-3′), RhoA(Δ189)-r (5′-GAATTCCTCACAAGACCAGGCAAGATTTTTTCTTCTTCCCACGTCTAGC-3′), RhoA(Δ188/189)-f (5′-TAGCATGGCCTTTGCAGGG-3′), and RhoA(Δ188/189)-r (5′-GAATTCCTCACAAGACCAGGCAAGATTTTTTCTTCTTCCCACGTCTAGC-3′). The amplified and purified PCR product was used for TOPO cloning and subsequently cloned into the pGEX vector and transformed into Esherichia coli TG1 cells. All mutations were confirmed by DNA sequencing. DNA was sequenced using an ABI PRISM 310 genetic analyzer from Perkin-Elmer.
Expression and purification of recombinant proteins
YopT was expressed as a GST fusion protein in E. coli TG1 and purified as described previously (Fueller et al. 2006). The GST component, the fusion protein, was cleaved by incubation with thrombin (3.25 NIH units/mL) in 50 mM TEA (pH 7.5), 150 mM NaCl, and 2.5 mM CaCl2 for 30 min at room temperature. Thrombin was removed with benzamidine beads (Amersham Pharmacia Biotech). Plasmids for the α- (pGATEV) and β-(pET30) subunit of the geranylgeranyl transferase (GGT) and the farnesyl transferase (FT), respectively, were kindly provided by Dr. K. Alexandrov (Dortmund, Germany). Proteins were produced as a heterodimer by transformation of both plasmids into E. coli BL21. Protein expression was induced at an optical density of 0.8 with isopropyl-β-D-thiogalactopyranoside (IPTG, 0.8 mM final concentration) at 37°C. After 5 h cells were harvested and lysed by sonication in lysis-buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, 1 mM phenylmethylsulfonylfluoride [PMSF], and 5 mM dithiothreitol [DTT]). The GST-tagged α-subunit together with the β-subunit was purified by affinity chromatography with glutathione-sepharose (Amersham Bioscience). Purification steps and thrombin cleavage of YopT and GST–GGT were monitored by SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Production of recombinant proteins in Sf9 cells
The plasmids for ΔRCE1 (pFASTBAC1–ΔRCE1) and IcMT (pVL1393–IcMT) were kindly provided by Dr. P. Casey and Dr. P. Gierschick, respectively. Recombinant baculoviruses were produced using the BaculoGold Transfection Kit according to the manufacturer's instructions. The recombinant proteins were produced as described previously (Otto et al. 1999). Subconfluent Sf9 cells (1 × 106 cells/mL) were infected with recombinant baculovirus at multiplicities of infection ranging from 5–10. Cells were harvested 72 h after infection, washed two times with PBS, and resuspended in 50 mM Tris–HCl (pH 7.7) for cells producing RCE1 or in 5 mM NaH2PO4 (pH 7.0) and 5 mM EDTA for cells expressing IcMT. After resuspension, cells were disrupted by a French press (SLM Instruments) and centrifuged at 500g for 15 min to remove cellular debris and nuclei. Membranes were pelleted at 100,000g for 1 h. Membranes from cells expressing RCE1 were resuspended in 50 mM Tris–HCl (pH 7.7) and membranes from cells expressing IcMT were resuspended in 5 mM NaH2PO4 (pH 7.0) with 5 mM EDTA. The final protein concentration of the membrane solution (10–20 mg/mL) was determined by BCA-Assay. Membrane aliquots were flash frozen in liquid nitrogen and stored at −80°C.
Prenylation of Rho GTPases
RhoA, Rac, Cdc42, and the corresponding mutants were expressed in E. coli and purified, and used directly as GST fusion proteins or cleaved with thrombin to remove GST (other GTPases). GTPases (25 μM) were isoprenylated by incubation with 0.2 μM GST–GGT or GST–FT, respectively, in a total volume of 10 μL in the presence of 100 μM GGPP or FPP in 0.1 M HEPES (pH 7.4), 5 mM MgCl2, 5 mM DTT, and 1 mM GDP for 30 min at 37°C. The amount of isoprenylated GTPases was determined by scintillation counting using 3H-labeled GGPP or FPP.
Endoproteolytic removal of the –AAX tripeptide and methylation of GTPases
After isoprenylation of the GTPases, cleavage of the –AAX tripeptide was initiated by addition of 16 μg of membranes containing the recombinant Ras converting enzyme (RCE1) in 0.1 M HEPES (pH 7.4), 5 mM MgCl2, and 5 mM DTT for 30 min at 37°C. Subsequently, the prenylated and cleaved proteins were methylated by addition of 4 μg of membranes containing the recombinant methyltransferase in a total volume of 20 μL reaction buffer (20 μM [14C]-SAM (AdoMet) in 0.1 M HEPES (pH 7.4), 5 mM MgCl2, 5 mM DTT, 1 mM PMSF, 300 μM TPCK, and 2 mM phenanthroline) for 30 min at 37°C. Addition of protease inhibitors prevented protease activity by RCE1 as well as other proteases present in the membranes.
Proteolytic cleavage of post-translationally modified GTPases by YopT
The 14C-methylated proteins were quantified using a filter assay. Precipitated proteins were spotted on a PVDF membrane and washed two times with 2 mL ethanol. Bound 14C-methylated proteins were determined in a scintillation counter. Following determination of the amount of fully modified GTPases, cleavage of the methylated C-terminal cysteine of GTPases was initiated by the addition of YopT in a 1:10 ratio (YopT:GTPase). The reaction was terminated by denaturing proteins with 0.5 mL of 4% SDS for 15 min at room temperature and precipitation of the proteins by addition of 0.5 mL 30% TCA for 15 min at room temperature.
Acknowledgments
This study was supported by the DFG, SPP 1150. We thank Dr. K. Alexandrov (Dortmund, Germany) for the isoprenyl transferases, Dr. P. Casey (Durham, North Carolina) for the RCE1, Dr. P. Gierschick (Ulm, Germany) for the IcMT, and J. Dumbach for excellent technical assistance.
Footnotes
Reprint requests to: Gudula Schmidt, Institute for Experimental and Clinical Pharmacology and Toxicology, Albert-Ludwigs-University of Freiburg, Albert-Strasse 25, 79104 Freiburg, Germany; e-mail: Gudula.Schmidt@pharmakol.uni-freiburg.de; fax: 49-761-203-5311.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.035386.108.
References
- Andor, A., Essler, M., Roggenkamp, A., Heesemann, J., Aepfelbacher, M. YopE of Yersinia, a GAP for Rho-GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell. Microbiol. 2001;3:301–310. doi: 10.1046/j.1462-5822.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- Black, D.S., Marie-Cardine, A., Schraven, B., Bliska, J.B. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signaling complex in macrophages. Cell. Microbiol. 2000;2:401–414. doi: 10.1046/j.1462-5822.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- Buckner, F.S., Kateete, D.P., Lubega, G.W., Van Voorhis, W.C., Yokoyama, K. Trypanosoma brucei prenylated-protein carboxyl methyltransferase prefers farnesylated substrates. Biochem. J. 2002;367:809–816. doi: 10.1042/BJ20020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis, G.R. The Yersinia Ysc-Yop “type III” weaponry. Nat. Rev. Mol. Cell Biol. 2002;3:742–752. doi: 10.1038/nrm932. [DOI] [PubMed] [Google Scholar]
- Cornelis, G.R., Boland, A., Boyd, A.P., Geuijen, C., Iriarte, M., Neyt, C., Sory, M.-P., Stainier, I. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukuzumuremyi, J.-M., Rosqvist, R., Hallberg, B., Akerström, B., Wolf-Watz, H., Schesser, K. The Yersinia protein kinase A is a host factor inducible RhoA/Rac-binding virulence factor. J. Biol. Chem. 2000;275:35281–35290. doi: 10.1074/jbc.M003009200. [DOI] [PubMed] [Google Scholar]
- Fueller, F., Bergo, M.O., Young, S.G., Aktories, K., Schmidt, G. Endoproteolytic processing of RhoA by Rce1 is required for the cleavage of RhoA by Yersinia enterocolitica outer Protein T. Infect. Immun. 2006;74:1712–1717. doi: 10.1128/IAI.74.3.1712-1717.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid, N., Gustavsson, A., Andersson, K., McGee, K., Persson, C., Rudd, C.E., Fallman, M. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb. Pathog. 1999;27:231–242. doi: 10.1006/mpat.1999.0301. [DOI] [PubMed] [Google Scholar]
- Hancock, J.F., Cadwallader, K., Paterson, H., Marshall, C.J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi, H., Kawata, M., Katayama, M., Yoshida, Y., Musha, T., Ando, S., Takai, Y. A novel prenyltransferase for a small BGTP-binding protein having a C-terminal Cys-Ala-Cys structure. J. Biol. Chem. 1991;266:16981–16984. [PubMed] [Google Scholar]
- Iriarte, M., Cornelis, G.R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- McDonald, C., Vacratsis, P.O., Bliska, J.B., Dixon, J.E. The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 2003;278:18514–18523. doi: 10.1074/jbc.M301226200. [DOI] [PubMed] [Google Scholar]
- Mittal, R., Erickson, J.W., Cerione, R.A. Uncoupling of GTP binding from target stimulation by a single mutation in the transducin α subunit. Science. 1996;271:1413–1416. doi: 10.1126/science.271.5254.1413. [DOI] [PubMed] [Google Scholar]
- Modha, R., Campbell, L.J., Nietlispach, D., Buhecha, H.R., Owen, D., Mott, H.R. The Rac1 polybasic region is required for interaction with its effector PRK1. J. Biol. Chem. 2008;283:1492–1500. doi: 10.1074/jbc.M706760200. [DOI] [PubMed] [Google Scholar]
- Mukherjee, S., Keitany, G., Li, Y., Wang, Y., Ball, H.L., Goldsmith, E.J., Orth, K. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- Navarro, L., Koller, A., Nordfelth, R., Wolf-Watz, H., Taylor, S., Dixon, J.E. Identification of a molecular target for the Yersinia protein kinase A. Mol. Cell. 2007;26:465–477. doi: 10.1016/j.molcel.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Otto, J.C., Kim, E., Young, S.G., Casey, P.J. Cloning and characterization of a mammalian prenyl protein-specific protease. J. Biol. Chem. 1999;274:8379–8382. doi: 10.1074/jbc.274.13.8379. [DOI] [PubMed] [Google Scholar]
- Prehna, G., Ivanov, M.I., Bliska, J.B., Stebbins, C.E. Yersinia virulence depends on mimicry of host Rho-family nucleotide dissociation inhibitors. Cell. 2006;126:869–880. doi: 10.1016/j.cell.2006.06.056. [DOI] [PubMed] [Google Scholar]
- Shao, F., Merritt, P.M., Bao, Z., Innes, R.W., Dixon, J.E. A Yersinia effector and a Pseudomonas avirulence protein define a family of cycteine proteases functioning in bacterial pathogenesis. Cell. 2002;109:575–588. doi: 10.1016/s0092-8674(02)00766-3. [DOI] [PubMed] [Google Scholar]
- Shao, F., Vacratsis, P.O., Bao, Z., Bowers, K.E., Fierke, C.A., Dixon, J.E. Biochemical characterization of the Yersinia YopT protease: Cleavage site and recognition elements in Rho GTPases. Proc. Natl. Acad. Sci. 2003;100:904–909. doi: 10.1073/pnas.252770599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg, I., Hoffmann, C., Dumbach, J., Aktories, K., Schmidt, G. The C terminus of YopT is crucial for activity and the N terminus is crucial for substrate binding. Infect. Immun. 2003;71:4623–4632. doi: 10.1128/IAI.71.8.4623-4632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud, G.I., Bliska, J.B. Yersinia outer proteins: Role in modulation of host cell signalling responses and pathogenesis. Annu. Rev. Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- von Pawel-Rammingen, U., Telepnev, M.V., Schmidt, G., Aktories, K., Wolf-Watz, H., Rosqvist, R. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: A mechanism for disruption of actin microfilament structure. Mol. Microbiol. 2000;36:737–748. doi: 10.1046/j.1365-2958.2000.01898.x. [DOI] [PubMed] [Google Scholar]
- Winter-Vann, A.M., Casey, P.J. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat. Rev. Cancer. 2005;5:405–412. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- Wright, L.P., Philips, M.R. Thematic review series: Lipid post-translational modifications. CAAX modification and membrane targeting of Ras. J. Lipid Res. 2006;47:883–891. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- Zhang, F.L., Casey, P.J. Protein prenylation: Molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]