Abstract
In the ventral tegmental area (VTA), progestins facilitate lordosis via rapid actions at membrane dopamine Type 1-like (D1) and/or GABAA receptors (GBRs), rather than via cognate, intracellular progestin receptors. Downstream signal transduction pathways involved in these effects were investigated using lordosis as a bioassay. If progestins’ actions at D1 and/or GBRs in the VTA require activation of G-proteins, adenylyl cyclase, cyclic AMP-dependent protein kinase A (PKA), phospholipase C (PLC), and/or PKC, then pharmacologically blocking these pathways would be expected to attenuate progestin-facilitated lordosis and its enhancement by D1 and GBR activity. Ovariectomized, estradiol-primed rats were infused first with vehicle or signal transduction inhibitor, and second with vehicle, a D1 or GBR agonist, and then with vehicle or progestins to the VTA. Rats were tested for lordosis following infusions. Results indicated that initiation of G-proteins, adenylyl cyclase, PKA, PLC, or PKC in the VTA is required for rapid effects of progestins through D1 and/or GBRs to facilitate lordosis. As well, progestins’ actions at n-methyl-d-aspartate receptors (NMDARs) may modulate activity at D1 and/or GBRs and mitogen activated protein kinase may be a common signaling pathway. Findings from a microarray study demonstrated that there was upregulation of genes associated with steroid metabolism, GBRs, D1, NMDARs and signal transduction factors in the midbrain VTA of naturally-receptive mated compared to non-mated rats. Thus, in the VTA, progestins have rapid membrane-mediated actions via D1, GBRs, NMDARs and their downstream signal transduction pathways.
Keywords: neurosteroids, midbrain, 5α-pregnan-3α-ol-20-one, allopregnanolone, ventral tegmental area
Introduction
Our research focuses on elucidating the effects and mechanisms of progestins for mediating reproductive and non-reproductive behavior. To investigate the former, progestins’ actions are manipulated in brain regions that are targets of progestins’ actions for sexual receptivity in rodents, such as the ventromedial hypothalamus (VMH) and ventral tegmental area (VTA). The resulting effects on lordosis, the stereotypic posture that female rodents assume to enable mating to occur (given appropriate hormonal and environmental stimulation), are then used as a bioassay of progestins’ actions. Results of lesions or progestin administration to the VMH and/or VTA of ovariectomized (ovx) rodents primed with 17β-estradiol (E2) indicate that progesterone (P4) has effects to initiate and modulate lordosis respectively in these regions [1–7]. In addition to different consequences of P4’s actions in these regions, there is emerging evidence that P4 has different mechanisms in the VMH and VTA to mediate lordosis.
P4’s actions in the VMH for lordosis
P4’s effects in the VMH to initiate lordosis can occur via traditional actions at intracellular progestin receptors (PRs) and induction of gene transcription. Classic intracellular PRs are highly expressed in the VMH of rodents and are upregulated by E2 and/or P4 coincident with lordosis [8–10]. Blocking actions at PRs in the VMH inhibits lordosis of naturally-receptive or ovx, hormone-primed rats [11–16]. Administration of PR ligands to ovx, E2-primed rats [15], but not PR knockout mice (PRKO), facilitates lordosis [17–18]. The latency for P4 to enhance lordosis when applied to the VMH of ovx, E2-primed rodents is typically several hours [6,19–20]. Moreover, free P4, but not actions of P4 relegated to cell membranes, enhance lordosis when applied to the VMH [6,19–20]. Thus, in the VMH, P4 may initiate lordosis in part via actions at cognate PRs.
P4’s actions in the VTA for lordosis
In the VTA, P4 mediates lordosis through non-classical (“non-genomic”), rapid actions at neuronal membranes. Evidence that intracellular PRs in this region are not required for P4’s actions are as follows. First, there are very few intracellular PRs in the VTA of adult rodents and those that are localized to the VTA are not E2-induced [9,13,21]. Second, actions of P4 at PRs in the VTA are not required for facilitation of lordosis. P4 can enhance lordosis when applied to the VTA of PRKO mice and the efficacy of progestins to facilitate lordosis when applied to the VTA is independent of their capacity to bind PRs [22,23]. Third, membrane actions of P4 in the VTA are sufficient to enhance lordosis. Free P4 and P4 bound to large macromolecules (P4:BSA, P4:HRP) have similar effects to rapidly enhance lordosis when applied to the midbrain VTA [6,19–20]. Fourth, P4 has rapid effects in the midbrain VTA to facilitate lordosis. Within 60 secs of application to the VTA, P4 increases cell firing, and P4 enhances lordosis within 5 mins [24]. These rapid effects may be faster than actions of steroids to bind cognate PRs and initiate gene transcription and/or translation [25]. Together, these data suggest that P4 has PR-independent, membrane-mediated, and rapid effects in the VTA to facilitate lordosis.
Role of the P4 metabolite and neurosteroid, 3α,5α-THP, for lordosis
Some of P4’s effects in the midbrain VTA to facilitate lordosis may be due in part to actions of 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP). P4 and 3α,5α-THP levels are high in the midbrain of naturally-receptive or hormone-primed rats. Levels of 3α,5α-THP, rather than P4, more positively correlate with the initiation of lordosis responses of naturally-receptive mice [22]. 3α,5α-THP is the most effective of all progestins at facilitating lordosis when applied to the VTA of E2-primed rodents [22]. The midbrain VTA is one of the brain regions with the highest levels of expression/activity of P4 metabolism enzymes, such as 5α reductase and 3α hydroxysteroid dehydrogenase, which convert P4 to dihydroprogesterone and subsequently 3α5α-THP [26]. Inhibitors of these enzyme can rapidly attenuate lordosis of naturally-receptive or ovx, hormone-primed rodents and reduce midbrain 3α5α-THP when administered systemically or to the VTA. Notably, these decrements in lordosis and 3α5α-THP levels produced by metabolism inhibitors can be reversed by subsequent infusions of 3α,5α-THP to the midbrain VTA [27–29]. Upregulating the activity of these P4 converting enzymes facilitates lordosis and increases 3α5α-THP levels [30]. Thus, P4’s metabolism to 3α5α-THP in the midbrain VTA is essential for lordosis.
Another source of 3α5α-THP in the midbrain VTA is biosynthesis in glial cells. Peripheral benzodiazepine receptors (PBRs) enhance the transport of cholesterol from the outer to the inner mitochondrial membrane forming pregnenolone, which is subsequently metabolized to P4 and 3α5α-THP [31]. This de novo synthesis of pregnenolone in the midbrain VTA occurs independent of peripheral gland secretion and in response to reproductively-relevant stimuli. For example, mating rapidly increases in 3α5α-THP in the midbrain VTA and this is observed among intact, naturally-receptive rodents, as well as ovx, and/or adrenalectomized E2-primed rodents [32–33]. Increasing or decreasing neurosteroidogenesis of 3α5α-THP in the VTA with infusions of PBR ligands, respectively, enhances and attenuates lordosis of hormone-primed or naturally-receptive rodents [34–35]. Inhibiting P450scc activity in the midbrain VTA produces decrements in 3α5α-THP and lordosis [34–35]. Thus, another source of 3α5α-THP in the midbrain VTA is from central biosynthesis.
GBRs as a target for 3α5α-THP’s actions in the VTA
γaminobutyric acid type (GABAA)/benzodiazepine receptor complexes (GBRs) may be a target for 3α5α-THP’s actions to facilitate lordosis (GBRs; [32–33]). 3α5α-THP is a potent, positive allosteric modulator of GBRs [36]. In nanomolar concentrations, 3α5α-THP potentiates the activity of GABA to increase the duration of chloride channel opening, and in higher concentrations, can exert these effects in the absence of GABA. GABAergic interneurons and GBRS have been localized to the VTA [37]. Progestins can enhance the function of GABAergic neurons by increasing the number, density, and/or affinity of GBRs [38–39]. In the midbrain VTA, levels of GABA are higher when 3α5α-THP levels are increased during behavioral estrous and/or mating [32–33]. As well, less GABA is needed to displace 50% of 3H muscimol in a competitive binding assay using midbrain VTA tissues from naturally-receptive rats that have high levels of 3α5α-THP in the midbrain VTA, as compared to diestrous, non-receptive rats [22,32–33]. Inhibiting GABA formation or actions at GBRs in the midbrain VTA attenuates progestin-facilitated lordosis of rodents [13–14]. Increasing GABA levels, by reducing breakdown of GABA, or enhancing the activity of GBRs, in the midbrain VTA enhances lordosis of rodents [13–14,22,32–33,40–41]. Progestins vary in their activity at GBRs and 3α5α-THP is the most potent endogenous progestin at enhancing GBRs function. The actions of progestins to facilitate lordosis when applied to the midbrain VTA, corresponds with their ability to enhance GBR function in the midbrain VTA [22]. Thus, 3α5α-THP may have actions in the midbrain VTA to facilitate lordosis through altering GABAergic function.
Dopamine receptors as a target for 3α5α-THP’s actions in the VTA
Dopaminergic neurons in the midbrain VTA may be another substrate through which 3α5α-THP has actions to facilitate lordosis. Dopamine cell bodies are localized to the midbrain VTA and activity of dopamine type 1-like (D1) receptors can regulate their function [43]. Progestins increase D1 density in the striatum and dopamine levels in the midbrain [24, 44]. D1 antagonists attenuate, and D1 agonists enhance, progestin-facilitated lordosis when administered to the VTA [45–46]. Thus, these data support a role of dopamine signaling for 3α,5α-THP-facilitated lordosis.
The role of signal transduction cascades for progestins’ actions
It has been proposed that signal transduction may be a common important pathway for steroids’ actions [47]. D1 receptors are well-known metabotropic receptors [48]. Emerging evidence suggest that GBRs may also have actions involving signal transduction pathways. For example, 3α5α-THP’s effects to prolong the opening of GBR chloride channels in vitro can be inhibited with antagonists of G-proteins, protein kinase C (PKC), or cAMP-dependent protein kinase [49]. Further, systemic administration of P4 increases levels of cAMP in the prefrontal cortex in a 3α5α-THP- and GBR-dependent manner [32–33,50]. Thus, together these data suggest that activity of signal transduction factors may underlie \functioning of progestins at downstream targets, such as GBRs and D1
Signal transduction targets of 3α5α-THP, D1, and GBRs in the VTA
We hypothesized that if progestins’ actions at D1 and/or GBRs in the VTA [51] require activation of G-proteins, adenylyl cyclase, cAMP-dependent PKA, phospholipase C (PLC), and/or PKC, then pharmacologically inhibiting these factors in the VTA should attenuate lordosis. The primary approach that we have used to investigate this involves ovx Long Evans rats that are implanted with bilateral guide cannulae to the midbrain VTA. Rats are E2-primed (10 μg/0.2cc) and forty-four hours later are tested for their baseline lordosis responses with a sexually-experienced male rat and general motor behavior in an activity monitor. Rats then receive bilateral infusions of vehicle or inhibitors of signal transduction molecules, followed by infusions of vehicle, a GBR agonist (muscimol; 100 ng/μl), or a D1 agonist (SKF38393; 100 ng/μl). A half hour after each of these infusions, rats are tested for lordosis behavior. Rats are then bilaterally infused with vehicle or 100 ng/μl 3α5α-THP and are tested for lordosis 10 minutes later and lordosis and motor behavior 60 minutes later (See Figure 1). Drug and 3α5α-THP manipulations that were utilized did not alter general motor behavior, suggesting that drug infusions did not produce non-specific effects on behavior (data not shown). Furthermore, effects observed were specific to 3α5α-THP because manipulation to the VTA of rats primed with E2 alone did not alter lordosis. When all testing was complete, rats were perfused, and fixed brain tissues were frozen, sliced, and stained with cresyl violet so that implant location could be determined by light microscopy. Infusions outside of the VTA did not produce the same pattern of effects as did infusions to the VTA. Thus, only data from rats that received bilateral infusions to the VTA are considered in data analyses.
Figure 1.
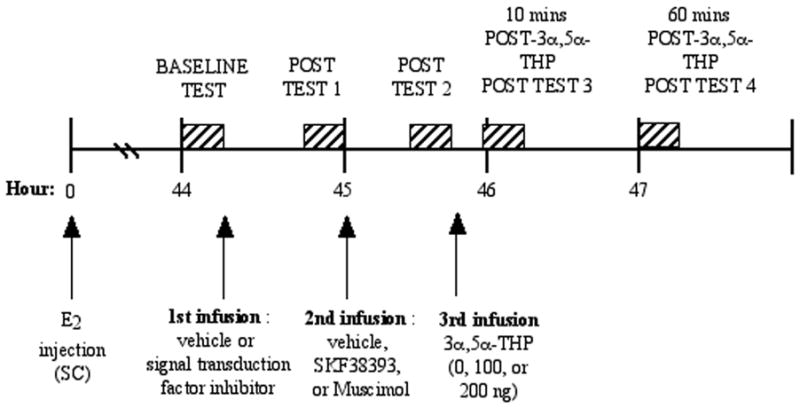
Timeline depicting when rats receive experimental manipulations and testing.
The results of this series of experiments supported our hypothesis (Table 1). In each experiment, compared to ovx, E2-primed control rats, infusions of 3α5α-THP increased lordosis and this was enhanced by infusions of muscimol or SKF38393 to the VTA. First, whether activation of G-proteins is necessary for progestin-facilitated lordosis and its enhancement by actions at GBRs and/or D1 in the VTA was investigated by administering a G-protein inhibitor (GDP-β;S; 50 μM/side). Blocking G-protein activity in the VTA attenuated effects of 3α5α-THP, muscimol and/or SKF38393 to enhance lordosis [52]. Second, whether progestins’ ability to enhance lordosis via actions at GBRs and/or D1 in the VTA is dependent upon activation of adenylyl cyclase and PKA was investigated by administering infusions of an adenylyl cyclase (2′,5′-dideoxyadenosine (DDA; 12 microM/side) or PKA (Rp-cAMP (100 ng/side) inhibitor to the VTA. Infusions of DDA or Rp-cAMP, but not vehicle, to the VTA attenuated 3α5α-THP-, muscimol-, and SKF38393- facilitated lordosis [53–54]. Third, whether progestins’ ability to enhance lordosis via actions at GBRs and/or D1 in the VTA is contingent upon activation of PLC and PKC was investigated by administering infusions of a PLC (U73122; 400 nM/side) or PKC (bisindolylmaleimide; 75 nM/side) inhibitor to the VTA. 3α5α-THP-, muscimol-, and/or SKF38393-facilitated lordosis was attenuated by infusions of a PLC or PKC inhibitor, but not vehicle, to the VTA [55]. Together, data from this series of experiments suggest that, in the VTA, 3α5α-THP’s actions for lordosis via D1 and/or GBRs require activity of G-proteins, adenylyl cyclase, PKA, PLC, and/or PKC.
Table 1.
Summary of results of experiments in ovx, E2-primed rats administered vehicle or a signal transduction inhibitor to the midbrain VTA followed by infusions of vehicle, a GBR agonist (muscimol), or D1 agonist (SKF38393) before infusions to the VTA of 100 ng 3α,5α-THP and lordosis testing. Lordosis data are represented as a % change from E2-administered rats administered vehicle only to the midbrain VTA (100%).
| Signal transductiontarget/inhibitor infused | Infusions to the VTA | ||||||
| Signal transduction inhibitor | − | − | − | + | + | + | |
| 3α,5 α-THP infusion | + | + | + | + | + | + | |
| Muscimol | − | + | − | − | + | − | |
| SKF38393 | − | − | + | − | − | + | |
| G-proteins/GDP-β-S | 375 ± 12* | 500 ± 10** | 487 + 10** | 75 ± 7^ | 77 ± 5^ | 70 ± 12^ | |
| Adenylyl Cyclase/DDA | 370 ± 10* | 487 ± 13** | 487 ± 9** | 100 ± 3^ | 102 ± 5^ | 65 ± 5^ | |
| PKA/Rp-cAMP | 375 ± 10* | 460 ± 12** | 400 ± 7** | 100 ± 2^ | 125 ± 7^ | 110 ± 5^ | |
| PLC/U73122 | 323 ± 12* | 450 ± 12** | 453 ± 10** | 91 ± 8^ | 85 ± 5^ | 82 ± 5^ | |
| PKC/bisindolymalemide | 300 ± 8* | 400 ± 12** | 398 ± 9** | 85 ± 3^ | 110 ± 7^ | 95 ± 4^ | |
indicates significant increase from E2-administered control rats.
indicates significant increase from E2-administered, 3α,5α-THP -infused rats.
indicates significant decrease from rats that received infusions of vehicle and 3α,5α-THP and/or muscimol or SKF38393.
Other downstream targets of progestins in the VTA- NMDARs and MAPK
An important consideration to make in interpreting these results is the circuitry of the midbrain VTA involving GABAergic, dopaminergic, and glutamatergic cells (See Figure 2). GABAergic terminals, which have D1, synapse on dopaminergic cell bodies that contain GBRs and NMDARs [36]. Increased GABA input onto GBRs that are located on GABAergic interneurons in the VTA reduces the inhibitory actions of these cells on dopamine-containing cell bodies and increases somatodendritic dopamine release [56], which may underlie enhancements in lordosis. In the VTA, excitation of D1 receptors on GABAergic afferents enhances GABA release [57]. Indeed, inhibiting GBRs attenuates progestin-facilitated lordosis and its enhancement by a D1 agonist [58]. In addition to actions involving GBRs and D1, NMDARs in the VTA may also be involved in progestins’ actions in the VTA. GABAergic terminals, which have D1, synapse on dopaminergic cell bodies that contain GBRs and NMDARs [37,59]. 3α5α-THP reduces glutamate’s excitatory effects [60] and increases glutamate levels in the midbrain VTA [32]. Infusions of a NMDAR antagonist, MK-801, to the VTA, enhance 3α5α-THP-facilitated lordosis of rodents [61]. Thus, progestins’ actions, in the VTA, involving GBRs, D1, and/or NMDARs may facilitate lordosis by reducing tonic inhibition of dopamine neurons.
Figure 2.
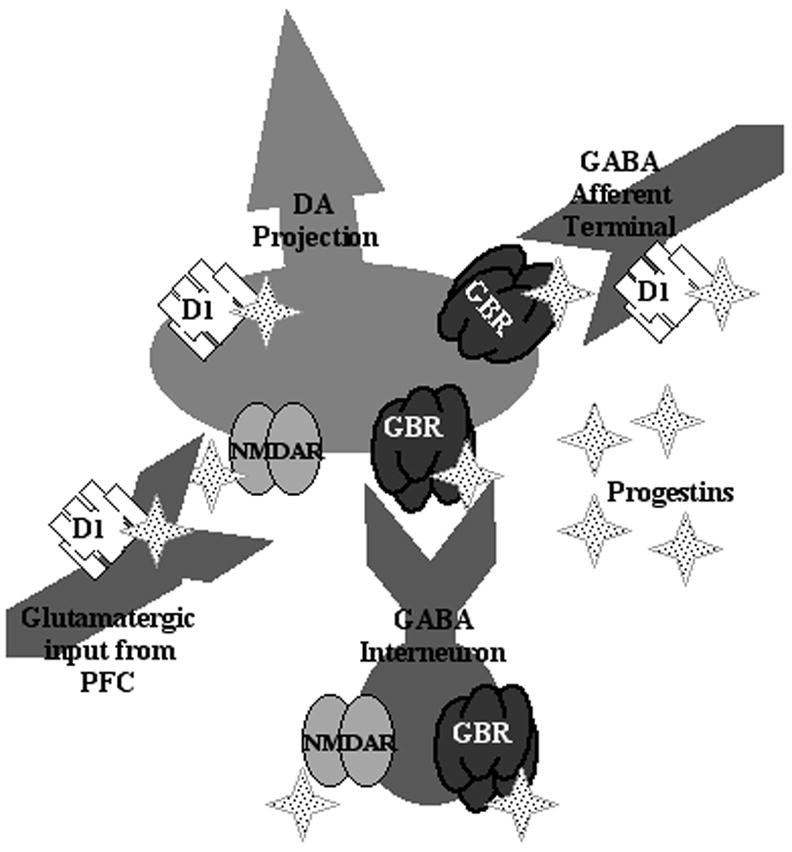
Circuitry of the mibrain VTA for putative effects of progestins, such as 3α,5α-THP, at dopaminergic, GABAergic and glutamatergic substrates.
We have begun further investigation of the downstream targets, such as mitogen activated protein kinase (MAPK), of progestins at these neurotransmitter substrates. MAPK is the primary intracellular signaling cascade that is activated by NMDAR phosphorylation [62]. In this study, ovx, E2-primed rats were administered P4 systemically (100 or 250 μg; SC) and then were infused with a MAPK (MEK-ERK) inhibitor, PD98059 (3 μg/side; dissolved in 10% DMSO) or vehicle, bilaterally to the VTA. As expected, P4 dose-dependently increased lordosis. However, compared to vehicle infusions, infusions of PD98059 to the VTA significantly decreased lordosis of rats administered 100 or 250, but not 0 μg, P4 (See Figure 3). Together, these data suggest that MAPK may an important downstream regulator of progestins’ actions in the midbrain VTA for lordosis.
Figure 3.
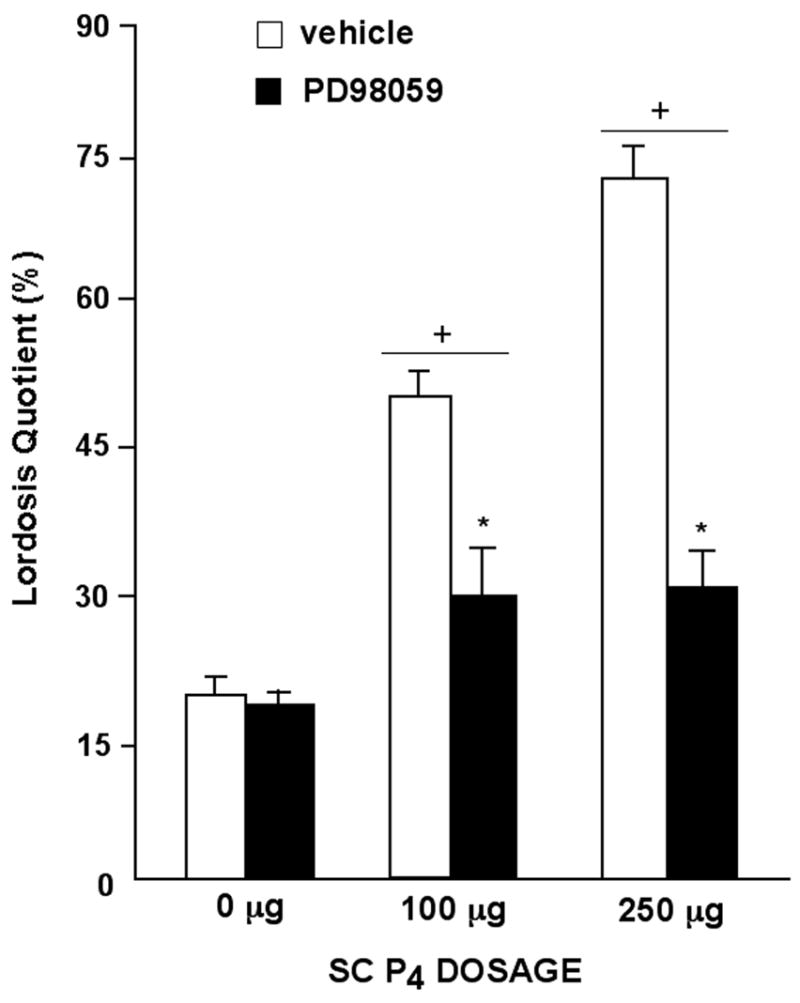
Lordosis quotients (mean ± sem) of ovariectomized, E2-primed rats administered SC injections of P4 (0μg, n=10; 100μg, n=14, or 250 μg, n=13) and vehicle or P4 (0 μg, n=11; 100 μg, n=13, or 250 μg, n=9) and a MEK/ERK inhibitor to the midbrain VTA (PD98059). In brief, rats were pre-tested 44 hours after E2-priming for normative lordosis and motor behavior responses in an activity monitor. No differences were observed for motor behavior. As such data are not shown. Immediately after this pre-test, rats were infused with DMSO vehicle or PD98059 to the VTA then injected with 0, 100, or 250 μg progesterone thirty minutes later. Three and a half hours following P4 administration, rats were tested for lordosis and motor behavior. + indicates a significant increase compared to 0 μg P4. * indicates a significant decrease compared to vehicle infusions to the VTA.
Genes upregulated in the VTA of mated vs. non-mated rats
In the experiments described thus far, lordosis has been used as a behavioral endpoint to investigate the mechanisms for progestins’ effects. One criticism of these experiments could be that pharmacological manipulations had overriding effects on physiologically-relevant processes. Although we did not observe evidence of this, we have used a microarray approach to ascertain genes in the midbrain VTA that are altered by mating in naturally-receptive rats that do not have central manipulations. As such, intact female rats that were naturally-receptive were mated or were just placed in a mating chamber without mating. Immediately thereafter, midbrain VTA tissues were collected and stored and subsequently sent to one of two microarray core facilities (Center for Functional Genomics, Albany, NY; University of California- Los Angeles, Los Angeles, CA) to be analyzed for changes in gene expression. Results of changes in gene expression, described below, reflect those that were a minimum two-fold change and were corroborated between the two core facilities.
In naturally-receptive mated rats, genes that were upregulated in the midbrain VTA were those that our pharmacological studies have demonstrated are involved in progestins’ actions for lordosis (Figure 4). Mating induces 3α5α-THP biosynthesis [63–66] and, of ~50 genes that were upregulated in the midbrain VTA of mated vs. non-mated rats, many were those involved in steroid metabolism. Of the upregulated genes in the midbrain VTA of mated vs. non-mated rats, many were related to GABA, dopamine, and glutamate function and signal transduction. Genes involved in cell proliferation and cell death and housekeeping genes were also upregulated in the midbrain VTA of mated vs. non-mated rats. Thus, these findings suggest that natural actions of progestins in the midbrain VTA\associated with mating may involve GBRs, D1, NMDARs, and signal transduction pathways.
Figure 4.
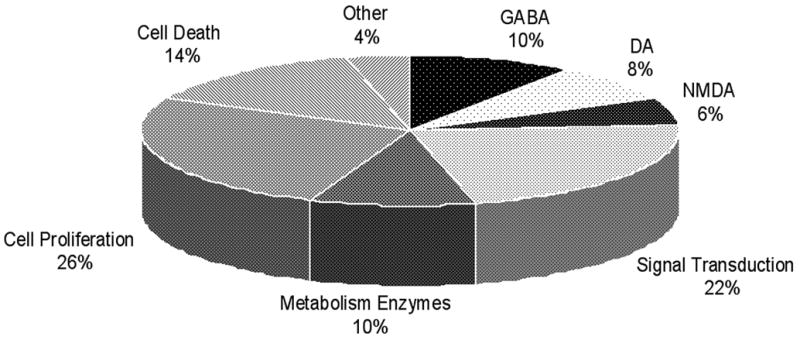
Microarray results of the distribution of upregulated genes in the midbrain VTA of naturally-receptive mated vs. non-mated rats. Converging evidence from data collected from both laboratories in which the microarray analyses were done demonstrate that 48 genes were upregulated at least 2-fold in the midbrain of naturally-receptive, mated (n=6) compared to non-mated (n=6) rats at both sites. Rats were mated with a sexually-experienced male rat in what is known as a pseudo-naturalistic environment, i.e. a chamber in which she can pace or control the timing of sexual contacts made with the male. Non-mated controls were exposed to the empty testing chamber. Collected midbrain tissues were analyzed using an Affymetrix platform with a Rat Genome 230 2.0 Array.
Conclusions
Steroids produce diverse functional effects and mechanisms, which can present a challenge when trying to elucidate steroids’ actions. The approach we utilize of using lordosis, a behavior that is reliably modulated by progestins, as a bioassay has revealed many features of progestins’ actions that are clearly physiologically-relevant and involve rapid signaling. Altering 3α5α-THP, GBRS, and/or D1 receptors and their downstream signal transduction factors (G-proteins, cAMP/adenylyl cyclase, PKA, PLC, and PLC) in the VTA alter lordosis. In addition to these pharmacological manipulations, behavioral manipulations (i.e. mating) alters expression of genes associated with progestin metabolism enzymes, GBRs, D1, NMDARs and their downstream signal transduction pathways. These data demonstrate that non-classical actions of progestins in the midbrain VTA to facilitate lordosis involve GBRS, D1, receptors (and NMDARs, MAPK) and their downstream signal transduction factors. Moreover, these studies have revealed the complexity of progestin actions in the midbrain VTA, which are functionally-relevant and meaningful. Ongoing studies in our laboratory are investigating these mechanisms in the VTA as well as brain areas, such as the hippocampus, using the same type of approach for learning and memory, affective behavior, and neurodegeneration.
Acknowledgments
Studies were supported by grants from the National Science Foundation (IBN03-16083) and National Institute of Mental Health (MH06769801). The authors want to acknowledge the assistance of Dr. Sridar Chittur and Jason Paris with the microarray experiment, Dr. Sandra Petralia for the design of Figure 2 and technical assistance provided, and Dr. Madeline Rhodes, Kanako Sumida, Jana Vanderslice-Barr, and Stephanie Youmans for their technical assistance with these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herndon JG., Jr Effects of midbrain lesions on female sexual behavior in the rat. Physiol Behav. 1976;17:143–8. doi: 10.1016/0031-9384(76)90281-x. [DOI] [PubMed] [Google Scholar]
- 2.Malsbury CW, Kow LM, Pfaff DW. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in remale golden hamsters. Physiol Behav. 1977;19:223–37. doi: 10.1016/0031-9384(77)90331-6. [DOI] [PubMed] [Google Scholar]
- 3.Mathews D, Greene SB, Hollingsworth EM. VMN lesion deficits in lordosis: partial reversal with pergolide mesylate. Physiol Behav. 1983;31:745–8. doi: 10.1016/0031-9384(83)90269-x. [DOI] [PubMed] [Google Scholar]
- 4.Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–24. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- 5.Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrinology. 1983;113:797–804. doi: 10.1210/endo-113-2-797. [DOI] [PubMed] [Google Scholar]
- 6.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–91. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 7.Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- 8.Ahdieh HB, Brown TJ, Wade GN, Blaustein JD. Hypothalamic nuclear progestin receptors and the duration of sexual receptivity in ovariectomized and ovariectomized-hysterectomized rats. Physiol Behav. 1986;36:211–5. doi: 10.1016/0031-9384(86)90005-3. [DOI] [PubMed] [Google Scholar]
- 9.Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann N Y Acad Sci. 2003;1007:238–50. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 10.Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–12. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- 11.Brown TJ, Moore MJ, Blaustein JD. Maintenance of progesterone-facilitated sexual behavior in female rats requires continued hypothalamic protein synthesis and nuclear progestin receptor occupation. Endocrinology. 1987;121:298–304. doi: 10.1210/endo-121-1-298. [DOI] [PubMed] [Google Scholar]
- 12.Mani SK, Blaustein JD, Allen JM, Law SW, O’Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;135:1409–14. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 13.Frye CA, Vongher JM. GABA(A), D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Murphy RE, Platek SM. Anti-sense oligonucleotides, for progestin receptors in the VMH and glutamic acid decarboxylase in the VTA, attenuate progesterone-induced lordosis in hamsters and rats. Behav Brain Res. 2000;115:55–64. doi: 10.1016/s0166-4328(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 15.Etgen AM, Barfield RJ. Antagonism of female sexual behavior with intracerebral implants of antiprogestin RU 38486: correlation with binding to neural progestin receptors. Endocrinology. 1986;119:1610–7. doi: 10.1210/endo-119-4-1610. [DOI] [PubMed] [Google Scholar]
- 16.Vathy IU, Etgen AM, Barfield RJ. Actions of RU 38486 on progesterone facilitation and sequential inhibition of rat estrous behavior: correlation with neural progestin receptor levels. Horm Behav. 1989;23:43–56. doi: 10.1016/0018-506x(89)90073-1. [DOI] [PubMed] [Google Scholar]
- 17.Frye CA, Vongher JM. Progesterone has rapid and membrane effects in the facilitation of female mouse sexual behavior. Brain Res. 1999;815:259–69. doi: 10.1016/s0006-8993(98)01132-9. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA, Vongher JM. Progesterone and 3α,5α-THP enhance sexual receptivity in mice. Behav Neurosci. 2001;115:1118–28. [PubMed] [Google Scholar]
- 19.Frye CA, Mermelstein PG, DeBold JF. Evidence for a non-genomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Res. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- 20.Frye CA, DeBold JF. P-3-BSA, but not P-11-BSA, implants in the VTA rapidly facilitate receptivity in hamsters after progesterone priming to the VMH. Behav Brain Res. 1993;53:167–75. doi: 10.1016/s0166-4328(05)80276-1. [DOI] [PubMed] [Google Scholar]
- 21.Munn AR, 3rd, Sar M, Stumpf WE. Topographic distribution of progestin target cells in hamster brain and pituitary after injection of [3H]R5020. Brain Res. 1983;274:1–10. doi: 10.1016/0006-8993(83)90515-2. [DOI] [PubMed] [Google Scholar]
- 22.Frye CA, Vongher JM. Progestins’ rapid facilitation of lordosis when applied to the ventral tegmentum corresponds to efficacy at enhancing GABA(A)receptor activity. J Neuroendocrinol. 1999;11:829–37. doi: 10.1046/j.1365-2826.1999.00367.x. [DOI] [PubMed] [Google Scholar]
- 23.Pleim ET, DeBold JF. The relative effectiveness of progestins for facilitation and inhibition of sexual receptivity in hamsters. Physiol Behav. 1984;32:743–7. doi: 10.1016/0031-9384(84)90188-4. [DOI] [PubMed] [Google Scholar]
- 24.Frye CA, Bayon LE, Vongher JM. Intravenous progesterone elicits a more rapid induction of lordosis in rats than does SKF38393. Psychobiology. 2000;28:99–109. [Google Scholar]
- 25.Pfaff DW, McEwen BS. Actions of estrogens and progestins on nerve cells. Science. 1983;219:808–14. doi: 10.1126/science.6297008. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–8. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- 27.Frye CA, Vongher JM. Ventral tegmental area infusions of inhibitors of the biosynthesis and metabolism of 3α,5α-THP attenuate lordosis of hormone-primed and behavioural oestrous rats and hamsters. J Neuroendocrinol. 2001;13:1076–86. doi: 10.1046/j.1365-2826.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 28.Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- 29.Petralia SM, Jahagirdar V, Frye CA. Inhibiting biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioural oestrus. J Neuroendocrinol. 2005;17:545–52. doi: 10.1111/j.1365-2826.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- 30.Frye CA, Petralia SM, Rhodes ME, Stein B. Fluoxetine may influence lordosis of rats through effects on midbrain 3α,5α-THP concentrations. Ann N Y Acad Sci. 2003;1007:37–41. doi: 10.1196/annals.1286.004. [DOI] [PubMed] [Google Scholar]
- 31.Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 32.Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001;40:226–33. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- 33.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–22. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 34.Frye CA, Petralia SM. Mitochondrial benzodiazepine receptors in the ventral tegmental area modulate sexual behaviour of cycling or hormone-primed hamsters. J Neuroendocrinol. 2003;15:677–86. doi: 10.1046/j.1365-2826.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- 35.Frye CA, Petralia SM. Lordosis of rats is modified by neurosteroidogenic effects of membrane benzodiazepine receptors in the ventral tegmental area. Neuroendocrinology. 2003;77:71–82. doi: 10.1159/000068338. [DOI] [PubMed] [Google Scholar]
- 36.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 37.Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- 38.Mascó D, Weigel R, Carrer HF. Gamma aminobutyric acid mediates ventromedial hypothalamic mechanisms controlling the execution of lordotic responses in the female rat. Behav Brain Res. 1986;19:153–62. doi: 10.1016/0166-4328(86)90013-6. [DOI] [PubMed] [Google Scholar]
- 39.Wilson MA. Influences of gender, gonadectomy, and estrous cycle on GABA/BZ receptors and benzodiazepine responses in rats. Brain Res Bull. 1992;29:165–72. doi: 10.1016/0361-9230(92)90022-p. [DOI] [PubMed] [Google Scholar]
- 40.Frye CA, DeBold JF. Muscimol facilitates sexual receptivity in hamsters when infused into the ventral tegmentum. Pharmacol Biochem Behav. 1992;42:879–87. doi: 10.1016/0091-3057(92)90044-g. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Mermelstein PG, DeBold JF. Bicuculline infused into the hamster ventral tegmentum inhibits, while sodium valproate facilitates, sexual receptivity. Pharmacol Biochem Behav. 1993;46:1–8. doi: 10.1016/0091-3057(93)90308-g. [DOI] [PubMed] [Google Scholar]
- 42.Frye CA. Inhibition of 5α-reductase enzyme or GABA(A) receptors in the VMH and the VTA attenuates progesterone-induced sexual behavior in rats and hamsters. J Endocrinol Invest. 2001;24:399–407. [PubMed] [Google Scholar]
- 43.Stoof JC, Kebabian JW. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984;35:2281–96. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- 44.Lévesque D, Di Paolo T. Effect of the rat estrous cycle at ovariectomy on striatal D-1 dopamine receptors. Brain Res Bull. 1990;24:281–4. doi: 10.1016/0361-9230(90)90216-m. [DOI] [PubMed] [Google Scholar]
- 45.Frye CA, Walf AA, Sumida K. Progestins’ actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors. Pharmacol Biochem Behav. 2004;78:405–18. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Sumida K, Walf AA, Frye CA. Progestin-facilitated lordosis of hamsters may involve dopamine-like type 1 receptors in the ventral tegmental area. Behav Brain Res. 2005;161:1–7. doi: 10.1016/j.bbr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res. 1998;92:169–80. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 48.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–4. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 49.Fáncsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–75. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–19. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frye CA, Walf AA, Petralia SM. Progestins’ effects on sexual behaviour of female rats and hamsters involving D1 and GABA(A) receptors in the ventral tegmental area may be G-protein-dependent. Behav Brain Res. 2006;172:286–93. doi: 10.1016/j.bbr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Frye CA, Walf AA, Petralia SM. In the ventral tegmental area, progestins have actions at D1 receptors for lordosis of hamsters and rats that involve GABAA receptors. Horm Behav. 2006;50:332–7. doi: 10.1016/j.yhbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Frye CA, Walf AA, Petralia SM. Progestin facilitation of lordosis in rodents involves adenylyl cyclase activity in the ventral tegmental area. Horm Behav. 2006;50:237–44. doi: 10.1016/j.yhbeh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Petralia SM, Walf AA, Frye CA. In the ventral tegmental area, progestins’ membrane-mediated actions for lordosis of hamsters and rats involve protein kinase A. Neuroendocrinology. 2006;84:405–14. doi: 10.1159/000100510. [DOI] [PubMed] [Google Scholar]
- 55.Frye CA, Walf AA. In the ventral tegmental area, the membrane-mediated actions of progestins for lordosis of hormone-primed hamsters involve phospholipase C and protein kinase C. J Neuroendocrinol. 2007;19:717–24. doi: 10.1111/j.1365-2826.2007.01580.x. [DOI] [PubMed] [Google Scholar]
- 56.Churchill L, Dilts RP, Kalivas PW. Autoradiographic localization of gamma-aminobutyric acid A receptors within the ventral tegmental area. Neurochem Res. 1992;17:101–6. doi: 10.1007/BF00966870. [DOI] [PubMed] [Google Scholar]
- 57.Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–88. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–14. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willick ML, Kokkinidis L. The effects of ventral tegmental administration of GABAA, GABAB and NMDA receptor agonists on medial forebrain bundle self-stimulation. Behav Brain Res. 1995;70:31–6. doi: 10.1016/0166-4328(94)00181-e. [DOI] [PubMed] [Google Scholar]
- 60.Park-Chung M, Wu FS, Farb DH. 3α-Hydroxy-5α-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons. Mol Pharmacol. 1994;46:146–50. [PubMed] [Google Scholar]
- 61.Petralia SM, DeBold JF, Frye CA. MK-801 infusions to the ventral tegmental area and ventromedial hypothalamus produce opposite effects on lordosis of hormone-primed rats. Pharmacol Biochem Behav. 2007;86:377–85. doi: 10.1016/j.pbb.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- 63.Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female . Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.08.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5alpha-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–74. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–75. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 66.Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83:336–47. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]


