Abstract
Objective
First-degree relatives (FDRs) of people diagnosed with colorectal cancer (CRC) have a two- to three-fold increased risk of developing the same disease. Tailored print interventions based on behavior change theories have demonstrated considerable promise in facilitating health-promoting behaviors. This study compared the impact of two mailed print interventions on CRC screening outcomes among FDRs.
Methods
This randomized trial compared effects of two mailed print interventions -- one tailored and one nontailored – on participation in CRC screening among FDRs of CRC survivors. Data collected via phone interviews from 140 FDRs at baseline, one week post-intervention, and three months post-intervention.
Results
At three months, both the tailored and nontailored interventions yielded modest but statistically insignificant increases in adherence to any CRC screening test (14% vs. 21%, respectively; p=0.30). While there were no main effects for tailored versus nontailored interventions, there were significant interactions that showed that the tailored print intervention had significantly greater effects on forward stage movement for CRC screening depending on stage of adoption at baseline, race, and objective CRC risk. Receipt of the tailored intervention was 2.5 times more likely to move baseline precontemplators and contemplators forward in stage of adoption for colonoscopy (95% CI=1.10–5.68) and was three times more likely to move Caucasians forward in stage of adoption for FOBT (95% CI=1.00–9.07). In addition, the tailored intervention was 7.7 times more likely to move people at average risk forward in stage of adoption for colonoscopy (95% CI=1.25–47.75).
Conclusion
The tailored print intervention was more effective at moving Caucasians, those in precontemplation and contemplation at baseline, and those at average risk forward in their stage of adoption for CRC screening.
Practice Implications
Both tailored and nontailored print interventions showed moderate effects for increasing CRC screening participation. Tailored print interventions may be more effective for certain subgroups.
1. Introduction
Colorectal cancer (CRC) is the most common cause of cancer deaths in non-smokers in the United States [1]. An estimated 153,750 people in the U.S. will be diagnosed and more than 52,000 will die from this disease in 2007. Some individuals being at greater risk than others [2]. Approximately 25% of new CRC cases occur in individuals who have a first-degree relative (FDR) with the disease [3]. Other than age, having a positive family history is the most common risk factor [4]. Whereas, without preventive action, a person at average risk has a 6% chance of developing CRC sometime during his or her lifetime, those with a family history of CRC have a two- to eight-fold increased risk of developing the disease [4–10].
When discovered early, CRC is highly treatable. The natural development of CRC from polyps provides the opportunity for cancer prevention through identification and removal of these cancer precursors [11, 12]. Endoscopic removal of precancerous polyps has been found to decrease CRC incidence by 75% to 90% [3]. Efforts to increase participation in screening among the general population can result in significant CRC mortality and morbidity reduction. Increasing screening among people at increased risk, including close relatives of colorectal cancer survivors, would yield even greater reductions in morbidity and mortality from this preventable disease.
Recent advances in our knowledge of CRC risk factors, including family history, have resulted in screening guidelines stratified by risk [13]. Colonoscopy is the only recommended screening test for individuals at increased risk for CRC, whereas guidelines for people at average risk include choices among fecal occult blood testing, sigmoidoscopy, barium enema, and colonoscopy. However, because published guidelines that stratify screening test options by risk are relatively new and continue to evolve, both patients and health care providers are often uncertain about which tests are appropriate for whom.
Tailored health communications have demonstrated potential to influence health behavior change and have been shown to increase smoking cessation, dietary change, and cancer screening more effectively than nontailored communications that do not take into account the characteristics of their intended audience [14, 15]. Although studies have compared tailored and nontailored communications to increase breast cancer screening, we know little about the relative efficacy of tailored versus nontailored interventions to promote CRC screening. Given the complexity of guidelines which recommend different screening tests depending on an individual’s risk factors, interventions that deliver tailored messages based on risk assessment are necessary if the goal is to increase participation in risk-appropriate screening.
Marcus and colleagues conducted one of the few randomized trials to examine the efficacy of tailored print materials to promote CRC screening among callers to the Cancer Information Service [16]. These investigators used a 4-group design and enrolled more than 4000 callers to the Cancer Information Service to test the effects of tailoring, multiple mailouts, and re-tailoring of print materials. Four types of mailed print interventions were compared: a single nontailored mailout of print materials (the control condition), a single tailored mailout of print materials, four tailored mailouts of print materials sent over 12 months, and four mailouts of print materials that were re-tailored based on new/updated information obtained from participants at 6 months. Although there was no difference in CRC screening at 14 months between the single tailored and single groups, compelling evidence for tailoring was found when subgroup analyses were conducted. Among younger participants aged 50–59 years, the single mailout of print materials outperformed the single mailing of nontailored materials.
Noar and colleagues recently conducted a meta-analysis of 57 studies testing tailored print behavior change interventions. The majority of health behaviors studied included smoking cessation, dietary change and mammography. Results in dictated that tailored print interventions showed a small sample size-weighted mean effect size of r=.074 (95% CI=.066–.082), significantly outperforming nontailored messages [17].
The purpose of this study was to compare the efficacy of a mailed tailored print intervention with a nontailored print intervention on CRC screening adherence and stage of adoption among first-degree relatives (FDRs) of CRC survivors. The following research questions were addressed:
Which mailed print intervention (tailored vs. non-tailored) is more efficacious for promoting CRC screening adherence and forward stage movement in FDRs of CRC survivors?
Which individual characteristics influence intervention efficacy among FDRs of CRC survivors?
1.1. CRC Screening Guidelines
Current guidelines for CRC screening published by the American Cancer Society (ACS) are stratified by CRC risk [13]. Individuals are deemed to be at average risk for CRC in the absence of known risk factors other than age. For average risk persons, screening recommendations start at age 50 and include five options: 1) annual fecal occult blood or fecal immunochemical test; 2) flexible sigmoidoscopy every 5 years; 3) sigmoidoscopy every 5 years with annual fecal occult blood test; 4) colonoscopy every 10 years; or 5) double contrast barium enema every 5 years. Individuals are at increased risk if they have a significant family history of CRC or a personal history of CRC, adenomas, or inflammatory bowel disease.
Screening recommendations have been stratified based on the number of risk factors present, including family history. People who have only one FDR who had CRC after the age of 60 are considered to be at average risk and should be offered the same five screening options as people with no family history. In contrast, having one FDR with CRC diagnosed before age 60, or having 2 or more FDRs with CRC places people at increased risk for CRC. Appropriate screening for these at-risk individuals includes total examination of the colon via colonoscopy every 10 years starting at age 40 or ten years earlier than the age at diagnosis of the youngest affected relative, whichever is earlier [13].
1.2. Screening among Relatives of Colorectal Cancer Survivors
Research on screening behaviors of first-degree relatives of individuals with CRC has yielded conflicting results. In several early studies, although FDRs were more likely to agree to participate in screening, they were no more likely to complete the test than those without a family history [18, 19]. In a recent cross-sectional survey of siblings of people with colon cancer, 57% were found to be currently on schedule with some kind of screening, not necessarily a risk-appropriate test [20]. This rate was only slightly higher than that of the general population.
Another study indicated that although half (50.5%) of FDRs 50 years or older reported ever having had an FOBT, only 18.5% had had an FOBT within the past year [21]. Rates of screening with sigmoidoscopy were actually lower than those of the general population, with 30% of FDRs reporting that they had ever had a sigmoidoscopy and 21% having ever had a colonoscopy.
In a study of 83 unaffected twin sisters of CRC patients, lifetime screening rates were high, with 88% of twins having ever had an FOBT and 69% having ever had a flexible sigmoidoscopy [22]. Despite these high lifetime screening rates, however, failure to adhere to recommended intervals for repeat screening was evident. Twins reported having an FOBT only once every 3–4 years, and only 49% reported having had a sigmoidoscopy in the past 5 years.
A number of studies have indicated that relatives of cancer survivors may be more responsive to interventions to increase screening participation than persons without a family history. In one study, siblings of CRC cases (n=133) and control siblings were mailed a letter inviting their participation in a CRC screening program along with a sample FOBT card [23]. Letters sent to siblings informed them of their increased risk status. Telephone interviews were conducted three weeks later to measure outcomes. Although a greater proportion of siblings of CRC patients (52%) returned completed FOBT cards compared to control siblings (37.7%), participation rates in both groups were quite low.
Harris and Byles surveyed 225 FDRs of CRC patients to explore knowledge, attitudes, and screening participation [24]. Although there were high levels of awareness and positive attitudes toward screening, screening rates were low, with only 3 relatives having being screened in accordance with current screening guidelines. With screening participation broadly defined as any FOBT, sigmoidoscopy, or colonoscopy during the past six years, only 57% of participants had ever been screened.
2. Methods
We used the Health Belief Model and the Transtheoretical Model/Stages of Change theory to guide intervention development through identifying predictors and mediators of screening behavior. The HBM proposes that individuals will take action to prevent, screen for, or control a health condition if they: 1) consider themselves to be susceptible to or at risk for the condition; 2) believe the condition has serious consequences; 3) believe that a particular course of action will reduce either their susceptibility to or the severity of the condition; and 4) believe that the anticipated barriers (costs) of taking the action are outweighed by the benefits [25]. Self-efficacy, originally conceptualized by Bandura [26, 27], was added to the HBM to increase its explanatory power. Self-efficacy is defined as “the conviction that one can successfully execute a specific behavior required to produce the outcomes” [26].
The TTM describes behavior change as a dynamic process through which individuals move in a series of discrete phases or “stages of change” [28]. The five stages have been labeled: 1) Precontemplation, 2) Contemplation, 3) Preparation, 4) Action, and 5) Maintenance. Relapse can occur during any phase in this process and may or may not be followed by a resumption of progress through the stages. Precontemplators have no intention of engaging in the relevant behavior in the foreseeable future; they are either uninformed or underinformed about the consequences of their behavior, refuse to acknowledge their risk, or have decided for some other reason not to engage in the behavior. Contemplators have begun to consider engaging in the behavior of interest. Some people remain in contemplation for a long period of time because, although they are aware of the benefits (pros) of the behavior, they are also highly aware of the barriers (cons). The balance between pros and cons can keep people stuck in the contemplation stage [29]. Preparation is the stage at which people make the decision to engage in the behavior and is most commonly characterized by having a plan of action for the behavior change. Actors are those who have actually implemented the behavior and perform it at the level professionals agree is sufficient to reduce the risk. Maintenance is the stage during which the behavior is routine and is characterized by attempts to prevent relapse. The TTM has been used to guide effective mammography interventions [30, 31]. In this study, stages of change were defined independently for FOBT, sigmoidoscopy, and colonoscopy in this study as shown in Table 1.
Table 1.
Stage definitions by screening test
| Precontemplation | Contemplation | Preparation | Action | |
|---|---|---|---|---|
| Fecal Occult Blood Test | Never had OR had one more than 12 months ago AND does not intend to have one in next 6 months. | Never had OR had one more than 12 months ago AND intends to have one in next 6 months. | Never had OR had one more than 12 months ago and has an FOBT kit. | Had one since baseline. |
| Sigmoidoscopy | Never had OR had one more than 5 years ago AND does not intend to have one in next 6 months. | Never had OR had one more than 5 years ago AND intends to have one in next 6 months. | Never had OR had one more than 5 years ago and has an appointment to have one. | Had one since baseline. |
| Colonoscopy | Never had OR had one more than 10 years ago AND does not intend to have one in next 6 months. | Never had OR had one more than 10 years ago AND intends to have one in next 6 months. | Never had OR had one more than 10 years ago and has an appointment to have one. | Had one since baseline. |
2.1. Research Design
A two-group randomized design was used to determine if a tailored print intervention was more effective than a nontailored print brochure at increasing CRC screening or advancing stage of CRC screening among FDRs. The sample for this analysis was drawn from the larger population of people who were screening eligible, that is those who were either: 1) 50 years of age and older; or 2) between 40 and 50 years but at increased risk for CRC. Follow-up telephone interviews were conducted at one week post-intervention (process evaluation) and at 3 months post-intervention delivery (outcomes).
2.2. Interventions: Tailored and Non-tailored
The tailored print intervention was customized for each participant. An extensive library of potential messages was developed, and tailoring algorithms were created to select individual messages from the library. Messages were selected based on baseline telephone interview data and assembled into an intervention booklet that was unique to each participant. Theoretical constructs from the HBM and the TTM that guided intervention development included perceived susceptibility (risk), perceived benefits, perceived barriers, and self-efficacy related to each screening test. Stage of change for each screening test was also assessed during the baseline interview. David Farrell, MPH, and colleagues from People Designs, Inc. wrote the computer software which executed the tailoring algorithms, assisted with tailored message development, and designed the layout and graphics for the 10-page, 8½ ×11 inch booklets.
Demographic variables and CRC risk factors, including age, family history, and presence of other comorbid conditions that affect CRC risk (personal history of polyps or inflammatory bowel disease), were also collected at baseline and integrated into the tailored intervention. Risk factors were used to determine each participant’s objective CRC risk and to generate a tailored risk profile and the appropriate screening test recommendation. Assessments of individual barriers to each screening test were also conducted through the baseline interview. Messages designed to help participants overcome their top three barriers were included in the tailored intervention. Variables that were used for tailoring and sample messages are listed in Table 2.
Table 2.
Tailoring Variables and Sample Tailored Messages
| Tailoring Variables | Sample Tailored Messages |
|---|---|
| Stage of Change | You may not have thought much about having your colon examined. As a close relative of someone who has been touched by colon or rectal cancer, screening is especially important for you. This booklet will tell you how colon cancer develops. Then, we will give you some information about your personal risk for getting this disease. Finally, we will review some things that might concern you about colon testing, so you can get ready. |
| Perceived Risk | Many people are not aware of the risk factors that increase their chances of getting colon cancer. The major factors that increase a person’s risk are: 1) Being over 40 years old (if you have a relative who had colon cancer) 2) Having had colon cancer or colon polyps in the past 3) Having an inflammatory bowel disease such as ulcerative colitis (ko-ly-tis) or Crohn’s (kronz) disease 4) Having one or more relatives who had colon cancer. |
| Objective Risk Factors | First, let’s review the factors that increase your personal chance of getting colon cancer. Then we’ll talk about what you can do to lower your chance of getting it. In addition to getting older, you’ve had 1 close relative who had cancer of the colon or rectum before they were 60 years old. This means your chances of getting colon or rectal cancer are higher than those of most people your age. Your Risk Factors • Being ____ (exact age) • Having a family member who had colon or rectal cancer at a young age What Can You Do To Lower Your Risk? Begin screening now, if you haven’t already. The American Cancer Society suggests having your entire colon checked by colonoscopy every 5 years, starting at age 40, or when you are ten years younger than your relative’s age when the cancer was found, whichever comes first. Talk with your doctor about getting screened soon. |
| Perceived Barriers to CRC Screening | Even though having a colonoscopy is very important, it’s natural to have concerns about having one. Don’t let those concerns keep you from taking this life-saving step. You told us you were most concerned about…(top three endorsed barriers are addressed). Having a colonoscopy is painful. People are often concerned about feeling pain or discomfort during a colonoscopy. Because you are given medicine to help you relax, it’s likely you’ll sleep through the test and feel no pain at all. If you do feel any pain, tell the doctor and you can be given more medication. Be sure to talk with the nurse or doctor about your concern before the test. Your doctor never told you to have a colonoscopy. Sometimes doctors get so busy taking care of patients when they’re sick, that they don’t take enough time to talk about things you can do to stay healthy…like having a colonoscopy. That’s why we developed this booklet for you, to help you discuss these colon tests with your doctor the next time you see him or her. You and your doctor will be glad you did! |
| Perceived Self-efficacy | The best way for you to have your colon examined is with a COLONOSCOPY. A colonoscopy (koh-lun-os-kup-ee) is a colon screening test that allows a doctor to look inside the entire length of your colon. During a colonoscopy, the doctor inserts a long, flexible tube with a light into your rectum to examine your entire colon for any unusual growths. Right before the test, they give you some medicine to help you relax. Most people fall asleep during the test. The test takes between 30 minutes and an hour, depending on whether there are any growths or polyps that need to be removed. Afterward, you wait for the relaxing medicine to wear off, and someone needs to drive you home. You will not be able to drive or work for the rest of that day because it takes several hours for the medicine to wear off. (Graphic included) One very important part of having a colonoscopy is preparing for the test the day before. Your colon needs to be completely empty so the doctor can see the inside clearly. To clean out your colon, you will need to do three things on the day before your colonoscopy: 1) stop eating solid foods (graphic) 2) stop taking certain medicines, (graphic) 3) drink a special laxative to clean out your colon (Fleet Phosphosoda graphic) As you can imagine, you will spend some time in the bathroom. But this is a very important step in having a colonoscopy. You can do it! Talk with your doctor now about having a colonoscopy. |
The nontailored print intervention used for comparative purposes was the American Cancer Society (ACS) brochure titled Colon Testing Can Save Your Life (2003). This brochure contained information about CRC, symptoms, risk factors, and current screening guidelines, with information on screening tests provided in a tabular format along with cost, advantages, disadvantages, and limitations of each test described. The brochure encourages readers to discuss CRC screening with their health care provider. Consistent with the tailored print intervention, participants assigned to this comparison group received the mailed ACS brochure within one month of baseline data collection. Additional details and samples of the tailored and nontailored interventions are included in the appendices.
2.3. Recruitment
After obtaining approval from the Institutional Review Boards and from treating physicians, colorectal cancer survivors were contacted to refer FDRs. Survivors were identified through cancer registries and by in-person contact in oncology clinics. Letters and study brochures were sent to all approved/referred survivors to introduce the study. One week after letters were sent, trained recruiters called survivors who did not decline to be contacted to explain the study and determine interest. Survivors who verbally consented to participate were asked to: 1) return the signed written consent form; 2) provide family history information; and 3) refer their FDRs who were over age 40 to the study. One hundred forty survivors agreed to participate, returned consent forms, and provided FDR referrals.
Eligibility criteria for FDRs included being: 1) at least 40 years old; 2) able to read English; 3) without a personal history of colorectal cancer; and 4) non-adherent with American Cancer Society (ACS) screening guidelines. Non-adherence to ACS guidelines was defined as not having had either: 1) an FOBT in the past year and a flexible sigmoidoscopy in the past 5 years; or 2) a colonoscopy in the past 10 years. FDRs were recruited using the same mail and phone follow-up procedures described above. After FDRs provided verbal consent, baseline interviews were conducted by trained interviewers. Follow-up phone interviews to assess outcomes were conducted approximately 3 months after the baseline interview (+ or − one week). Participants received a $20 gift certificate following completion of the follow-up interview.
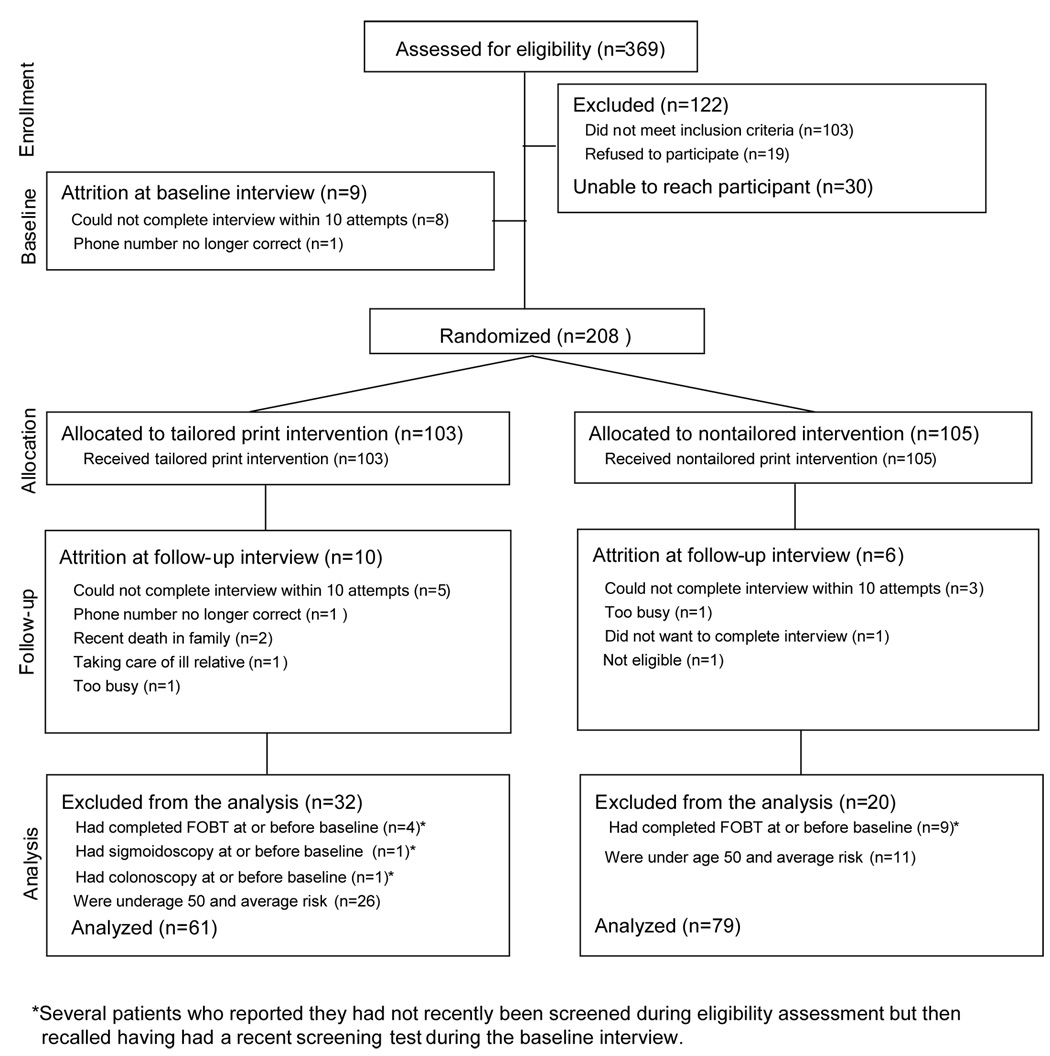
Subject enrollment, attrition and retention rates are summarized in Figure 1. A total of 217 FDRs were enrolled, although nine participants were not able to be reached to complete the baseline interview. Computerized randomization occurred after the baseline interview was completed, resulting in 103 subjects in the tailored print intervention group and 105 in the nontailored group. Ten participants could not be reached at three months and three refused to complete the follow-up interview.
Figure 1.
Participant Enrollment, Attrition and Retention
2.4. Measures and Data Analyses
We assessed screening adherence and movement across stages of adoption for screening as the primary outcomes of interest in this study. Screening adherence was measured in 5 different ways: 1) adherence to any test was a dichotomous variable (Yes/No) defined as having had any of the three screening tests (FOBT, sigmoidoscopy, or colonoscopy) since baseline; 2) adherence to the risk-appropriate test was a dichotomous variable defined as having had the screening test appropriate for an individual’s risk since baseline; and 3) adherence to each test (FOBT, sigmoidoscopy, and colonoscopy) separately.
Movement across stages of adoption was assessed by examining change in stage from baseline to follow-up. Specifically, at baseline we measured three possible stages (precontemplation, contemplation, and preparation). At follow-up, there were four possible stages (precontemplation, contemplation, preparation, and action). Thus, there were six possible movements in stage (−2 stages, −1 stage, no movement, +1 stage, +2 stages, +3 stages.
Univariate analyses were conducted for all continuous variables to examine their underlying distribution. To compare intervention groups (tailored vs. non-tailored) on categorical sample characteristics and outcomes (adherence and stage movement), Pearson chisquare tests were used. If 20% of expected cell counts were less than 5, two-sided Fisher's exact tests were used. Intervention effects on adherence and stage movement, adjusting for baseline characteristics, were assessed with logistic regression models.
Screening adherence was determined for each individual screening method, for adherence to any test, and adherence to the right test. In bivariate analyses, stage movement was assessed at the most detailed level (all seven categories), and also at the two-category level described next. For multivariate analyses, consistent with our previous analyses, stage movement was categorized into two groups (forward movement vs. no movement or backward movement) to avoid sparseness in outcome categories.
3. Results
3.1. Sample Description
Of 208 subjects who enrolled and completed baseline interviews, 177 completed the three month follow-up interview. However, because the purpose of the present study was to analyze efficacy of the interventions to promote screening, the 37 people who were younger than 50 and at average risk for CRC were excluded from analyses. This left a total of 140 participants − 61 (44%) in the tailored intervention group and 79 (56%) in the nontailored group.
As shown in Table 3, participants in both groups were similar on all demographic variables. Mean age was 54 years for the tailored group and 53 years for the nontailored group, and mean number of years of education was near 15 (i.e., three years of college) for both groups. Participants in both groups were predominantly female, Caucasian, employed with a medium-tohigh income, insured, and married or living with a partner. The majority of participants were categorized as at increased risk for CRC due to the strength of their family or personal history. We analyzed data for questions 1 and 2 adjusting for potential covariates including race, age, marital status, and employment. Point estimates for the odds ratios were unchanged, therefore, the unadjusted results are reported here.
Table 3.
Sample Characteristics
| Nontailored Intervention | Tailored Intervention | Total | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P | |
| Gender: Female | 54 | 68.4 | 42 | 68.9 | 96 | 68.6 | 0.95 |
| Male | 25 | 31.6 | 19 | 31.1 | 44 | 31.4 | |
| Age: 50 years and older | 43 | 54.4 | 39 | 63.9 | 82 | 58.6 | 0.26 |
| Less than 50 years old | 36 | 45.6 | 22 | 36.1 | 58 | 41.4 | |
| Race: Caucasian | 65 | 82.3 | 43 | 70.5 | 108 | 77.1 | 0.23 |
| African American | 11 | 13.9 | 14 | 22.9 | 25 | 17.9 | |
| Other | 3 | 3.8 | 4 | 6.6 | 7 | 5.0 | |
| Education: Less than high school | 8 | 10.1 | 3 | 4.9 | 11 | 7.9 | 0.70 |
| High school graduate | 23 | 29.1 | 17 | 27.9 | 40 | 28.6 | |
| Voc school/some college | 20 | 25.3 | 19 | 31.1 | 39 | 27.9 | |
| College graduate | 11 | 13.9 | 11 | 18.0 | 22 | 15.7 | |
| Graduate school | 17 | 21.5 | 11 | 18.0 | 28 | 20.0 | |
| Employment: Not employed | 15 | 19.0 | 17 | 27.9 | 32 | 22.9 | 0.21 |
| Currently employed | 64 | 81.0 | 44 | 72.1 | 108 | 77.1 | |
| Income: Less than $30,000 | 18 | 24.7 | 17 | 28.8 | 35 | 26.5 | 0.76 |
| $30,000 – $75,000 | 26 | 35.6 | 22 | 37.3 | 48 | 36.4 | |
| More than $75,000 | 29 | 39.7 | 20 | 33.9 | 49 | 37.1 | |
| Marital Status: Not partnered | 17 | 21.5 | 17 | 27.9 | 34 | 24.3 | 0.38 |
| Married/living with partner | 62 | 78.5 | 44 | 72.1 | 106 | 75.7 | |
| Health insurance: No | 6 | 7.6 | 4 | 6.6 | 10 | 7.1 | 0.81 |
| Yes | 73 | 92.4 | 57 | 93.4 | 130 | 92.9 | |
| FOBT stage (at baseline) | |||||||
| Precontemplation | 66 | 83.5 | 49 | 80.3 | 115 | 82.1 | 0.53 |
| Contemplation | 10 | 12.7 | 11 | 18.0 | 21 | 15.0 | |
| Preparation | 3 | 3.8 | 1 | 1.6 | 4 | 2.9 | |
| Colonoscopy stage (at baseline) | |||||||
| Precontemplation | 47 | 59.5 | 37 | 60.7 | 84 | 60.0 | 0.94 |
| Contemplation | 27 | 34.2 | 21 | 34.4 | 48 | 34.3 | |
| Preparation | 5 | 6.3 | 3 | 4.9 | 8 | 5.7 | |
| Colorectal Cancer (CRC) Objective Risk | |||||||
| Average risk | 21 | 26.6 | 14 | 22.9 | 35 | 25.0 | 0.62 |
| Increased Risk | 58 | 73.4 | 47 | 77.1 | 105 | 75.0 | |
| FDRs diagnosed with CRC: | |||||||
| Had 1 FDR aged < 60 when diagnosed | 41 | 53.9 | 31 | 50.8 | 72 | 52.5 | 0.86 |
| Had 1 FDR aged ≥ 60 when diagnosed | 25 | 32.9 | 20 | 32.8 | 45 | 32.9 | |
| Had 2 or more FDRs | 10 | 13.2 | 10 | 16.4 | 20 | 14.6 | |
| Had colon or rectal polyps: No | 77 | 97.5 | 58 | 95.1 | 135 | 96.4 | |
| Yes | 2 | 2.5 | 3 | 4.9 | 5 | 3.6 | |
| Had inflammatory bowel disease: No | 78 | 98.7 | 60 | 98.4 | 138 | 98.6 | 1.00 |
| Yes | 1 | 1.3 | 1 | 1.6 | 2 | 1.4 | |
| Had hereditary CRC: FAP No | 77 | 97.5 | 59 | 96.7 | 136 | 97.1 | 1.00 |
| Yes | 2 | 2.5 | 2 | 3.3 | 4 | 2.9 | |
| Had hereditary CRC: HNPCC No | 79 | 100.0 | 61 | 100.0 | 140 | 100.0 | NA |
| Yes | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
3.2 Process Evaluation: Readership, Relevance, and Satisfaction with Print Interventions
Readership, relevance and satisfaction with both the tailored and nontailored print interventions were examined with a subsample of 123 participants using Pearson chi-square tests of general association (See Table 4). When 20% or more of the cells of the contingency table had expected counts less than 5, the two-sided Fisher's exact test for the overall cross-classification table was used. Approximately 88% of participants in both intervention groups had read the materials completely and approximately 95% had saved the information. Participants in both groups universally agreed (100%) that the information was easy to understand and visually appealing. Over 95% of subjects reported the information was interesting and that they would recommend this information to others. More than 80% of participants who received both print interventions had or were planning to discuss the information with their doctors. A higher percentage of subjects who received the tailored print intervention had discussed the information with their families compared to the nontailored brochure (61 % vs. 49%, respectively), however this difference was not significant (p=.20).
Table 4.
Comparison of Process Variables for Tailored and Nontailored Print Interventions
| Variable | Nontailored Brochure (n=62) | Tailored Intervention (n=61) | Total (n=123) | ||||
|---|---|---|---|---|---|---|---|
| N Agree | % Agree | N Agree | % Agree | N Agree | % Agree | P value* | |
| I read the information completely | 56 | 87.5 | 54 | 88.5 | 110 | 88.0 | .86 |
| Contained new info about CRC screening | 34 | 54.0 | 31 | 50.8 | 65 | 52.4 | .73 |
| Info made me think about changing anything | 39 | 60.9 | 35 | 57.4 | 74 | 59.2 | .69 |
| I would recommend information to others | 61 | 98.4 | 60 | 98.4 | 121 | 98.4 | 1.00 |
| Information was interesting | 61 | 96.8 | 60 | 98.4 | 121 | 97.6 | 1.00 |
| Information was easy to understand | 63 | 100 | 61 | 100 | 124 | 100 | |
| Information was written especially for me | 45 | 71.4 | 55 | 91.7 | 100 | 81.3 | .004 |
| I liked the way the information looked | 62 | 100 | 61 | 100 | 123 | 100 | |
| I saved the information | 59 | 93.7 | 58 | 96.7 | 117 | 95.1 | .68 |
| I discussed the information with my family | 31 | 49.2 | 37 | 60.7 | 68 | 54.8 | .20 |
| I discussed the information with my doctor | 8 | 12.9 | 4 | 6.6 | 12 | 9.8 | .36 |
| I plan to discuss the info with my doctor | 46 | 76.7 | 47 | 77.0 | 93 | 76.9 | .96 |
| How much of the info applied to me: All | 14 | 22.2 | 21 | 35.6 | 35 | 28.7 | .08 |
| Most | 27 | 42.9 | 26 | 44.1 | 53 | 43.4 | |
| Some | 22 | 34.9 | 11 | 18.6 | 33 | 27.0 | |
| None | 0 | 0.0 | 1 | 1.7 | 1 | 0.8 | |
| Info was: More than you would have liked | 6 | 9.7 | 3 | 5.1 | 9 | 7.4 | .70 |
| Just the right amount | 46 | 74.2 | 45 | 76.3 | 91 | 75.2 | |
| Less than you would have liked | 10 | 16.1 | 11 | 18.6 | 21 | 17.4 | |
| Information was: Very Helpful | 39 | 61.9 | 42 | 68.9 | 81 | 65.3 | .19 |
| Somewhat helpful | 24 | 38.1 | 17 | 27.9 | 41 | 33.1 | |
| Not very helpful | 0 | 0.0 | 2 | 3.3 | 2 | 1.6 | |
| Changed my opinion about chances of getting CRC | 24 | 38.7 | 24 | 40.7 | 48 | 39.7 | .82 |
| Changed my opinion about doing a FOBT | 26 | 41.9 | 24 | 39.3 | 50 | 40.7 | .77 |
| Changed my opinion about having a sigmoidoscopy | 25 | 41.7 | 18 | 31.0 | 43 | 36.4 | .23 |
| Changed my opinion about having a colonoscopy | 25 | 40.3 | 30 | 49.2 | 55 | 44.7 | .32 |
| Changed my opinion about talking with my family about testing | 35 | 55.6 | 33 | 55.0 | 68 | 55.3 | .95 |
| Changed my opinion about talking with my doctor about testing | 42 | 66.7 | 24 | 39.3 | 66 | 53.2 | .002 |
Pearson chi-square for dichotomous variables, and Mantel chi-square for ordinal variables.
One significant group difference favoring the tailored intervention was observed on one item – the information was written especially for me (p=.002). A higher proportion of the tailored group (92%) agreed or strongly agreed that the information was written especially for them, compared to 71% in the nontailored group. Participants who received the tailored intervention perceived the material to be more personally relevant to them. Interestingly, a significantly higher proportion of the nontailored group (67%) compared to the tailored group (39%) reported that the materials had changed their opinion about talking with their doctor about CRC screening (p=.002).
3.3. Research Question 1
Which print intervention (tailored vs. non-tailored) is more efficacious for promoting CRC screening and forward stage movement among FDRs of CRC survivors?
As shown in Table 5, there were no significant differences between groups on overall adherence and stage movement. In terms of absolute percentages, three-month adherence to any screening test (FOBT, sigmoidoscopy or colonoscopy) was slightly lower among recipients of the tailored intervention compared to the nontailored intervention; 14% versus 21%, respectively (p=.30). Rates for adherence to the risk-appropriate test and for each individual test were similar between groups. Rates for adherence to the specific tests (FOBT and colonoscopy) must be interpreted together in that participation in colonoscopy negates the need for participation in FOBT.
Table 5.
Adherence and Stage Movement by Group at 3-month Follow-up
| Nontailored Intervention (n=79) | Tailored Intervention (n=61) | Total (n=140) | P value* | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Adherent to any test | 16 | 21.3 | 8 | 14.3 | 24 | 18.3 | 0.30 |
| Adherent to risk-appropriate test | 13 | 17.3 | 8 | 14.3 | 21 | 16.0 | 0.64 |
| Adherent to FOBT | 5 | 6.7 | 2 | 3.6 | 7 | 5.3 | 0.44 |
| Adherent to Colonoscopy | 11 | 14.5 | 8 | 14.3 | 19 | 14.4 | 0.98 |
| FOBT stage movement | 0.65 | ||||||
| −2 stages | 1 | 1.3 | 0 | 0.0 | 1 | 0.8 | |
| −1 stage | 4 | 5.3 | 4 | 7.1 | 8 | 6.1 | |
| No movement | 61 | 81.3 | 42 | 75.0 | 103 | 78.6 | |
| +1 stage | 4 | 5.3 | 7 | 12.5 | 11 | 8.4 | |
| +2 stage | 4 | 5.3 | 2 | 3.6 | 6 | 4.6 | |
| +3 stage | 1 | 1.3 | 1 | 1.8 | 2 | 1.5 | |
| FOBT forward stage movement | 9 | 12.0 | 10 | 17.9 | 19 | 14.5 | 0.35 |
| Colonoscopy stage movement | 0.21 | ||||||
| −2 stages | 0 | 0.0 | 1 | 1.8 | 1 | 0.8 | |
| −1 stage | 7 | 9.2 | 3 | 5.4 | 10 | 7.6 | |
| No movement | 51 | 67.1 | 32 | 57.1 | 83 | 62.9 | |
| +1 stage | 10 | 13.2 | 13 | 23.2 | 23 | 17.4 | |
| +2 stage | 8 | 10.5 | 5 | 8.9 | 13 | 9.8 | |
| +3 stage | 0 | 0.0 | 2 | 3.6 | 2 | 1.5 | |
| Colonoscopy forward stage movement | 18 | 23.7 | 20 | 35.7 | 38 | 28.8 | 0.13 |
two-sided Pearson chi-square test or Fisher’s exact test
Although adherence rates at three months were modest in both groups, rates for forward stage movement were slightly higher (See Table 5). Forward stage movement for FOBT was 18% among tailored intervention recipients versus 12% who received the nontailored brochure (p=.35). Forward stage movement for colonoscopy was 12% higher in the tailored intervention group; 36% versus 24% in the nontailored group (p=0.13).
3.4. Research Question 2
Which individual characteristics influence intervention efficacy in FDRs of CRC survivors?
To examine whether the intervention was more or less effective among subgroups, we tested interactions between intervention group and participant characteristics using logistic regression. As shown in Table 6, several significant interactions indicated that the intervention effect differed by race, objective CRC risk, and baseline stage of adoption for colonoscopy. The interaction between income and intervention effect was marginally significant (p=.06).
Table 6.
Interactions between individual characteristics and intervention efficacy
| Nontailored Intervention | Tailored Intervention | Total | Tailored intervention vs. Nontailored intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | P | Odds Ratio | 95% Confidence Limits | |
| FOBT Forward Stage Movement | |||||||||
| Race | |||||||||
| Caucasian | 6 | 9.7 | 10 | 24.4 | 16 | 15.5 | 0.04 | 3.01 | (1.00, 9.07) |
| Non-Caucasian | 3 | 23.1 | 0 | 0.0 | 3 | 10.7 | 0.09 | --- | --- |
| Income | |||||||||
| Less than $75,000 | 7 | 16.3 | 4 | 11.8 | 11 | 14.3 | 0.57 | 0.69 | (0.15, 2.00) |
| More than $75,000 | 2 | 7.4 | 6 | 30.0 | 8 | 17.0 | 0.06 | 5.36 | (0.95,30.2) |
| FOBT Baseline Stage | |||||||||
| Precontemplators | 5 | 8.1 | 9 | 19.6 | 14 | 13.0 | 0.08 | 2.77 | (0.86, 8.93) |
| Contemplators | 3 | 30.0 | 1 | 11.0 | 4 | 21.1 | 0.31 | 0.29 | (0.02, 3.48) |
| Preparation | 1 | 33.3 | 0 | 0.0 | 1 | 25.0 | 1.00 | --- | --- |
| Colonoscopy Forward Stage Movement | |||||||||
| Colonoscopy Baseline Stage | |||||||||
| Precontemplators | 7 | 16.6 | 11 | 62.4 | 18 | 22.8 | 0.08 | 2.60 | (0.88,7.64) |
| Contemplators | 6 | 23.1 | 8 | 42.1 | 14 | 31.1 | 0.17 | 2.42 | (0.67,8.80) |
| Preparation | 5 | 100.0 | 1 | 33.3 | 6 | 75.0 | 0.11 | --- | --- |
| Precontemplators+Contemplators | 13 | 18.3 | 19 | 35.9 | 32 | 25.8 | 0.03 | 2.49 | (1.10, 5.68) |
| Objective Risk | |||||||||
| Average risk | 2 | 10.0 | 6 | 46.2 | 8 | 24.2 | 0.04 | 7.71 | (1.25, 47.7) |
| High risk | 16 | 28.6 | 14 | 32.6 | 30 | 30.3 | 0.67 | 1.21 | (0.51, 2.86) |
Among Caucasians, forward stage movement for FOBT was significantly greater in the tailored group (24%) compared to the nontailored print group (10%). The tailored intervention was 3.01 times more likely to move Caucasians forward in stage of adoption for FOBT than the nontailored intervention (95% CI=1.00–9.07). For non-Caucasians, the intervention effect was not significant.
When those in precontemplation and contemplation at baseline were combined, forward stage movement for colonoscopy was significantly greater in the tailored group compared to the nontailored group (36% vs 18%, respectively). The tailored intervention was 2.49 times more likely to move this subgroup forward in stage compared to the nontailored intervention (95% CI=1.10–5.68).
The tailored intervention was 7.71 times more likely to move people with average risk for CRC forward in stage of adoption for colonoscopy (95% CI=1.25–47.75). In contrast, tailored and nontailored interventions were equally effective at moving people with increased CRC risk forward in stage of adoption for colonoscopy.
Among those at the highest household income level (> $75, 000), forward movement for FOBT was marginally significantly greater (p=.06) among those in the tailored intervention group compared to the nontailored group (30% vs. 7%, respectively). The tailored intervention was 5.36 times more likely to move participants with higher incomes forward in stage for FOBT than the nontailored intervention (95% CI= 0.95–30.2). For those with incomes less than $75,000, the intervention effect was not significant.
4. Discussion and Conclusion
4.1. Discussion
Although the difference in adherence to any CRC screening test between the tailored and the nontailored intervention groups was not significant overall, adherence rates increased by 14% and 21%, respectively, indicating a modest effect size for both interventions. While cost-effectiveness was not examined in this feasibility trial, the nontailored American Cancer Society brochure represents a moderately effective, inexpensive “minimal prompt” intervention to promote CRC screening among FDRs.
Our results are similar to those of Marcus and colleagues who found no significant differences in CRC screening rates overall between CIS callers who received a single tailored mailout of print materials and a single nontailored mailout (Marcus, et. al., 2005). One-time interventions, such as the ones tested here, may have limited efficacy in moving people to action for CRC screening. Our results lend support to the applicability of the TTM in the context of CRC screening. Motivating healthy people, even those at increased risk for CRC, to participate in screening may require multiple contacts and conversations. Findings from other studies have shown that multiple mailings of tailored print materials outperformed a single, one-time print intervention to promote CRC screening (Marcus, et.al., 2005).
Noar and colleagues found significant, but small, effect sizes in favor of tailored interventions (r=.074, 95% CI=0.66–0.82) in their metanalysis of 57 studies (Noar, Benac & Harris, 2007). These investigators identified several moderators of the effectiveness of tailored interventions including the type of health behavior change being promoted, type of comparison condition, type of print material, number of intervention contacts, length of follow-up, as well as number and type of theoretical constructs and other variables used for tailoring. Larger effect sizes were observed for interventions that: 1) focused on screening and preventive behaviors; 2) had more than one contact; 3) used ipsative feedback; 4) used a control, rather than a comparison, group; 5) had shorter follow-up; and 6) and tailored on four to five constructs. Significant interactions observed in this study indicated that the intervention effect differed by race, objective CRC risk, and baseline stage of adoption for colonoscopy. The interaction between income and intervention effect was marginally significant.
Numerous studies have documented the receipt of a recommendation for CRC screening from one’s healthcare provider as the most important predictor of participation. Rimer and colleagues (1999), who compared several interventions to promote breast and cervical cancer screening in community health centers, reported those who received a combination of tailored print materials along with a provider prompt did no better than those who received a provider prompt alone. The interventions in this study did not include any recommendation from the participants’ providers. Community-based interventions that do not incorporate a provider recommendation may have limited effectiveness, especially when outcomes that require a provider’s referral, such as colonoscopy, are of interest.
In this study, participation in screening would have required contact with a health care provider to obtain a FOBT or referral for endoscopy. Participants were residing in the community and may not have seen a health care provider in the 3 month timeframe we used for follow-up. When asked to report the main reason they had not had a CRC screening test, the most frequently cited reasons included the lack of insurance coverage (n=37), and lack of physician recommendation (n=17). We did not mail FOBT cards with the print interventions.
We conducted what Glasgow referred to as a “practical clinical trial” [32] in that we compared a tailored print intervention with a clinically relevant alternative that we considered a “usual care” or control condition for this at-risk population. The nontailored American Cancer Society brochure was readily available, inexpensive, required no baseline assessment or computerized tailoring algorithms to generate. Increasing FDRs’ awareness of their CRC risk and the importance of screening should constitute “usual care”. The lack of a true control group in this study, however, could be viewed as a limitation. It is possible that the modest increases observed in CRC screening could be the result of secular trends rather than the print interventions, but such trends should have affected outcomes for both groups equally.
One strength of this study was that we included only participants who were non-adherent for screening at baseline. Numerous studies on CRC screening have included people who were up-to-date with screening recommendations, thereby directing interventions at individuals who did not need them. These studies may waste scarce resources; they also make it difficult to determine intervention effectiveness. At the same time, our decision to enroll only those FDRs who, despite having had a family member recently affected by CRC, were not adherent to screening recommendations may result in a sample of particularly “hard-to-reach” individuals. Because 30% of the FDRs we contacted were ineligible for the study due to their adherence to screening guidelines, there may be some support for this hypothesis.
Several limitations of this study should be noted including the limited power related to the small sample size. The effect of this limited power is to increase the width of the 95% confidence intervals (i.e., reduced precision) which impacts the degree of confidence one can place in the odds ratio point estimates. Nevertheless, the results suggest that tailored print intervention was efficacious to some degree for particular subgroups in this sample. The fact that screening outcomes were based on self-report is another potential limitation. However, evidence suggests that self-reports of CRC screening, especially colonoscopy, are highly accurate and that medical record documentation of FOBT can be limited (Madlensky, McLaughlin & Goal, 2003; Baier, et. al., 2000). Any systematic under- or over-reporting of screening by participants in this study should be comparable between intervention groups.
The absence of significant differences might be explained by the fact that 100% of the tailored intervention group received colonoscopy-specific messages and little to no information about FOBT or sigmoidoscopy (as opposed to the nontailored group). The tailored intervention was designed to deliver messages about the recommended screening tests based on CRC risk assessment. In order to deliver tailored messages to overcome barriers to a specific test, messages were also tailored based on participants’ stage of adoption for a particular test. Because all FDRs in this study who were at average risk were in precontemplation or contemplation for colonoscopy, they received messages about that test.
In addition, because this was an intervention development and feasibility study, only three months were allowed for follow-up. This 3-month timeframe was likely inadequate to fully capture screening participation, especially for colonoscopy, in this community-based sample. For this reason, we considered forward stage movement to be a valid outcome in addition to screening behavior.
Although recruitment challenges are confronted in every study, recruitment was particularly difficult in this study because we began during the implementation of HIPAA. Hypervigilence about privacy issues among providers grew out of concerns about litigation and liability, especially among smaller practices with less experience with behavioral studies. Responding to recruitment challenges is critically important to the success of a study. Therefore, we searched for alternative approaches, including direct advertising of the study to FDRs via websites, newspapers, and radio. Though these alternative approaches seemed reasonable at the time, their impact on study outcomes cannot be discounted. In behavioral studies, participant motivation upon entry into a study can directly affect behavioral outcomes such as screening. In light of the fact that some of these participants were motivated to join the study, the large number of FDRs who remained unscreened at three months was disappointing. Similarly, Marcus and colleagues reported that despite receipt of well developed tailored print materials, approximately 75% of their participants remained non-adherent at 6 months.
4.2. Conclusion
Results of this study and others indicate that tailored print interventions may be more effective with certain subgroups of the population. Nontailored print interventions may be a cost-effective approach to motivating FDRs of colon cancer survivors to be screened. Examining moderators of intervention efficacy is important if we are to fully understand the impact of our interventions. Not every intervention will work for everyone. The diversity of the sample with regard to race and income allowed us to examine interactions and results demonstrated important differences in intervention effect. This tailored print intervention resulted in greater forward stage movement for Caucasians, those with high income, and those in the early stages of behavioral adoption at baseline.
Further research is needed to understand how we can most effectively motivate populations who are at risk and at average risk for developing CRC. A reasonable next step would be to test a tailored print intervention in the context of a managed care setting where access, cost, and insurance coverage can be removed as barriers. Embedding provider recommendations into tailored interventions may also be necessary to increase effects. Finally, multiple contacts and conversations may be needed, especially since awareness of the need for CRC screening is still low, even among those at increased risk for this often preventable disease.
4.3. Practice Implications
Interventions to promote screening among individuals who are at increased risk for CRC can save lives. Studies are needed to examine whether the efficacy of mailed print interventions could be increased if accompanied by a recommendation from the individual’s primary care provider or delivered in conjunction with a scheduled visit. Identifying those with a family history of CRC is often a challenge, so being proactive when a relative is diagnosed with CRC may increase intervention efficacy. Patients who have CRC are often ambassadors for screening who are eager for their relatives to avoid this disease, so family-based interventions during active treatment may hold promise as another approach to decreasing the CRC burden in families at risk.
Supplementary Material
Acknowledgements
This study was funded by the National Cancer Institute, Grant No. R21 CA093454-01. The authors would like to thank Dr. Phyllis Dexter for her careful review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burn J, Chapman PD, Bishop DT, Mathers J. Diet and cancer prevention: the concerted action polyp prevention (CAPP) studies. Proc Nutr Soc. 1998;57:183–186. doi: 10.1079/pns19980030. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: Clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 4.Burt RW. Screening of patients with a postive family history of colorectal cancer. Gastrointest Endosc Clin N Am. 1997;7:65–79. [PubMed] [Google Scholar]
- 5.Lovett E. Family studies in cancer of the colon and rectum. Br J Surg. 1976;63:13–18. doi: 10.1002/bjs.1800630103. [DOI] [PubMed] [Google Scholar]
- 6.Houlston RS, Murday V, Harocopos C, Williams CB, Slack J. Screening and genetic counselling for relatives of patients with colorectal cancer in a family cancer clinic. BRIT MED J. 1990;301:366–368. doi: 10.1136/bmj.301.6748.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St. John JDB, McDermott FT, Hopper JL, Debney EA, Johnson WR, Hughes ESR. Cancer risk in relatives of patients with common colorectal cancer. American College of Physicians. 1993;118:785–790. doi: 10.7326/0003-4819-118-10-199305150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Slattery ML, Kerber RA. Family history of cancer and colon cancer risk: The Utah Population Database. J Natl Cancer Inst. 1994;86:1618–1626. doi: 10.1093/jnci/86.21.1618. [DOI] [PubMed] [Google Scholar]
- 9.Lynch HT, Lynch J, Conway T, Severin M. Psychological aspects of monitoring high risk women for breast cancer. CA. 1994;74:1184–1192. doi: 10.1002/1097-0142(19940801)74:3+<1184::aid-cncr2820741530>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Burt RW. Familial risk and colon cancer. International Journal of Cancer. 1996;69:44–46. doi: 10.1002/(SICI)1097-0215(19960220)69:1<44::AID-IJC10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman D. Colonoscopy as a mass screening tool. Eur J Gastroenterol Hepatol. 1998;10:225–229. doi: 10.1097/00042737-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman D. How to screen for colon cancer. Annual Review of Internal Medicine. 1998;46:163–172. doi: 10.1146/annurev.med.49.1.163. [DOI] [PubMed] [Google Scholar]
- 13.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: Update of early detection guidelines for prostate, colorectal, and endometrial cancers. Ca: A Cancer Journal of Clinicians. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 14.Kreuter MW. Dealing with competing and conflicting risks in cancer communication. J Natl Cancer Inst Monogr. 1999;25:27–35. doi: 10.1093/oxfordjournals.jncimonographs.a024203. [DOI] [PubMed] [Google Scholar]
- 15.Skinner CS, Campbell MK, Rimer BK, Curry S, Prochaska JO. How effective is tailored print communication? Annals of Behavioral Medicine. 1999;21:290–298. doi: 10.1007/BF02895960. [DOI] [PubMed] [Google Scholar]
- 16.Marcus AC, Mason M, Wolfe P, et al. The efficacy of tailored print materials in promoting colorectal cancer screening: results from a randomized trial involving callers to the National Cancer Institute's Cancer Information Service. J Health Commun. 2005;10 Suppl 1:83–104. doi: 10.1080/10810730500257754. [DOI] [PubMed] [Google Scholar]
- 17.Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133:673–693. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- 18.Macrae FA, Hill DJ, St John DJ, Ambikapathy A, Garner JF. Predicting colon cancer screening behavior from health beliefs. Prev Med. 1984;13:115–126. doi: 10.1016/0091-7435(84)90044-6. [DOI] [PubMed] [Google Scholar]
- 19.Morrow GR, Way J, Hoagland AC, Cooper R. Patient compliance with self-directed Hemoccult testing. Prev Med. 1982;11:512–520. doi: 10.1016/0091-7435(82)90065-2. [DOI] [PubMed] [Google Scholar]
- 20.Manne S, Markowitz A, Winawer S, et al. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psy. 2002 Feb;21:3–15. [PubMed] [Google Scholar]
- 21.Rawl SM, Champion VL, Menon U, Loehrer P, Skinner CS. Society of Behavioral Medicine's 21st Annual Meeting; 2000. Nashville, TN: 2000. First-degree relatives of colorectal cancer patients: knowledge, beliefs, and cancer screening behavior; p. S119. [Google Scholar]
- 22.Richardson JL, Danley K, Mondrus GT, Deapen D, Mack T. Adherence to screening examinations for colorectal cancer after diagnosis in a first-degree relative. Prev Med. 1995;24:166–170. doi: 10.1006/pmed.1995.1030. [DOI] [PubMed] [Google Scholar]
- 23.Sandler RS, DeVellis BM, Blalock SJ, Holland KL. Participation of high-risk subjects in colon cancer screening. CA. 1989;63:2211–2215. doi: 10.1002/1097-0142(19890601)63:11<2211::aid-cncr2820631125>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Harris MA, Byles JE. A survey of screening compliance among first degree relatives of people with colon cancer in New South Wales. Jouranl of Medical Screening. 1997;4:29–34. doi: 10.1177/096914139700400110. [DOI] [PubMed] [Google Scholar]
- 25.Strecher VJ, Rosenstock IM. The Health Belief Model. In: Glanz K, Lewis FM, Rimer B, editors. Health Behavior and Health Education. 2nd Edition ed. San Francisco, CA: Jossey-Bass Publishers; 1997. pp. 41–59. [Google Scholar]
- 26.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 27.Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 28.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, Lewis FM, Rimer BK, editors. Health Behavior and Health Education. San Francisco, CA: Jossey-Bass Publishers; 1997. pp. 60–84. [Google Scholar]
- 29.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 30.Skinner CS, Strecher VJ, Hospers H. Physicians' recommendations for mammography: do tailored messages make a difference? American Journal of Public Health. 1994;84:43–49. doi: 10.2105/ajph.84.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Champion VL, Skinner CS, Foster JL. The effects of standard care counseling or telephone/in-person counseling on beliefs, knowledge, and behavior related to mammography screening. Oncol Nurs Forum. 2000;27:1565–1571. [PubMed] [Google Scholar]
- 32.Glasgow RE, Magid DJ, Beck A, Ritzwoller D, Estabrooks PA. Practical clinical trials for translating research to practice: design and measurement recommendations. Med Care. 2005;43:551–557. doi: 10.1097/01.mlr.0000163645.41407.09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.