Abstract
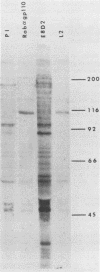
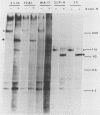
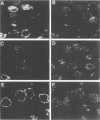
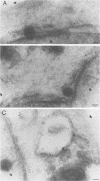
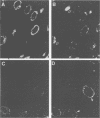
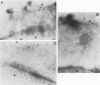
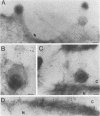
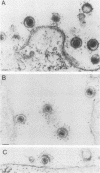
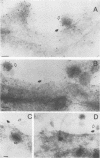
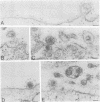
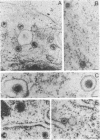
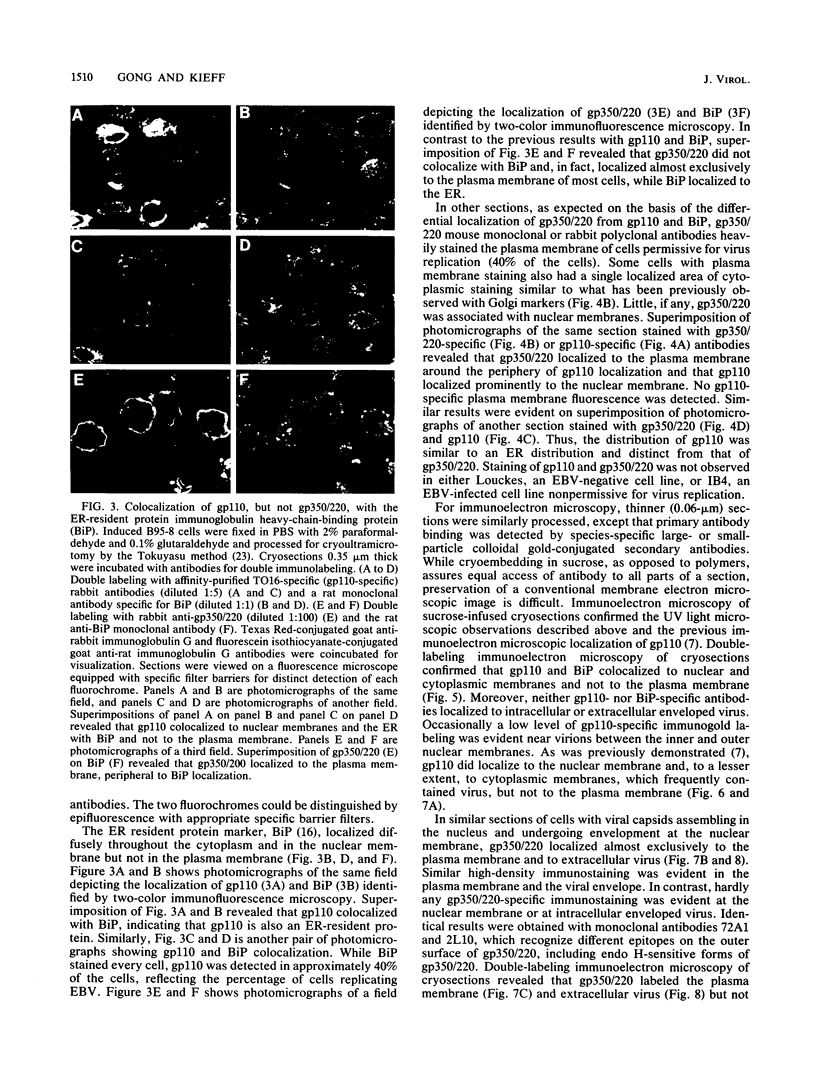
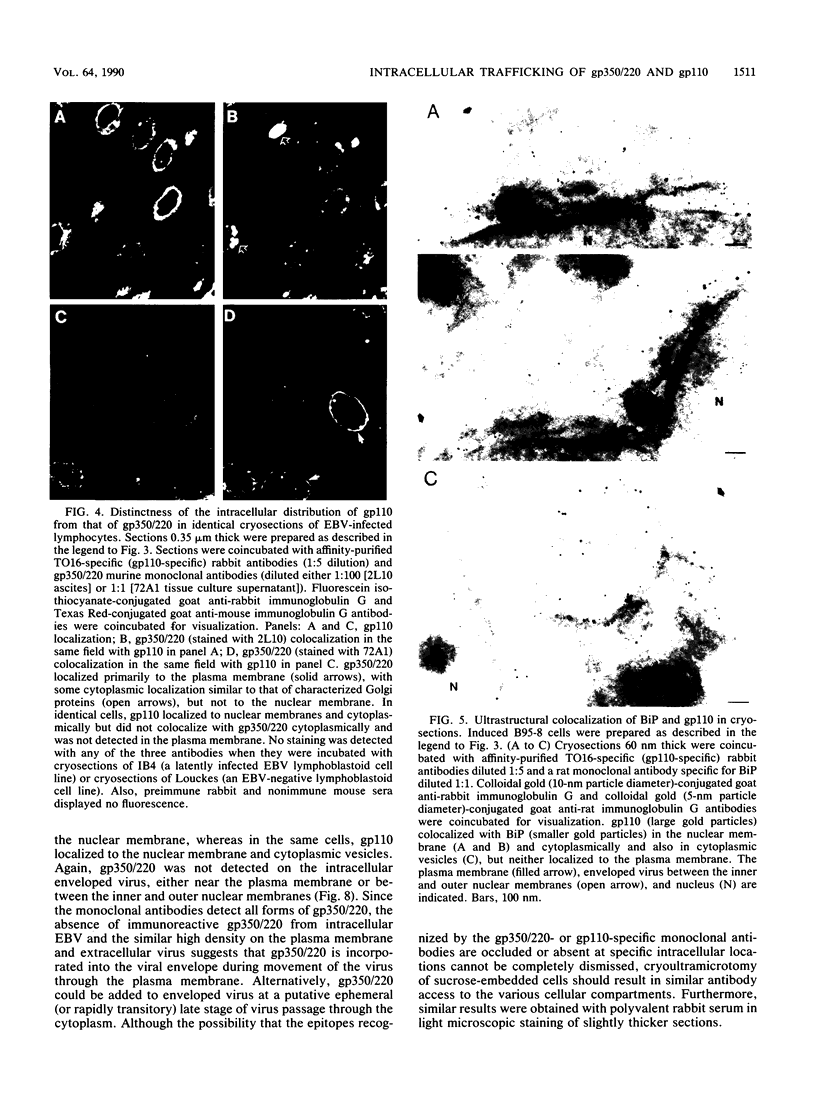
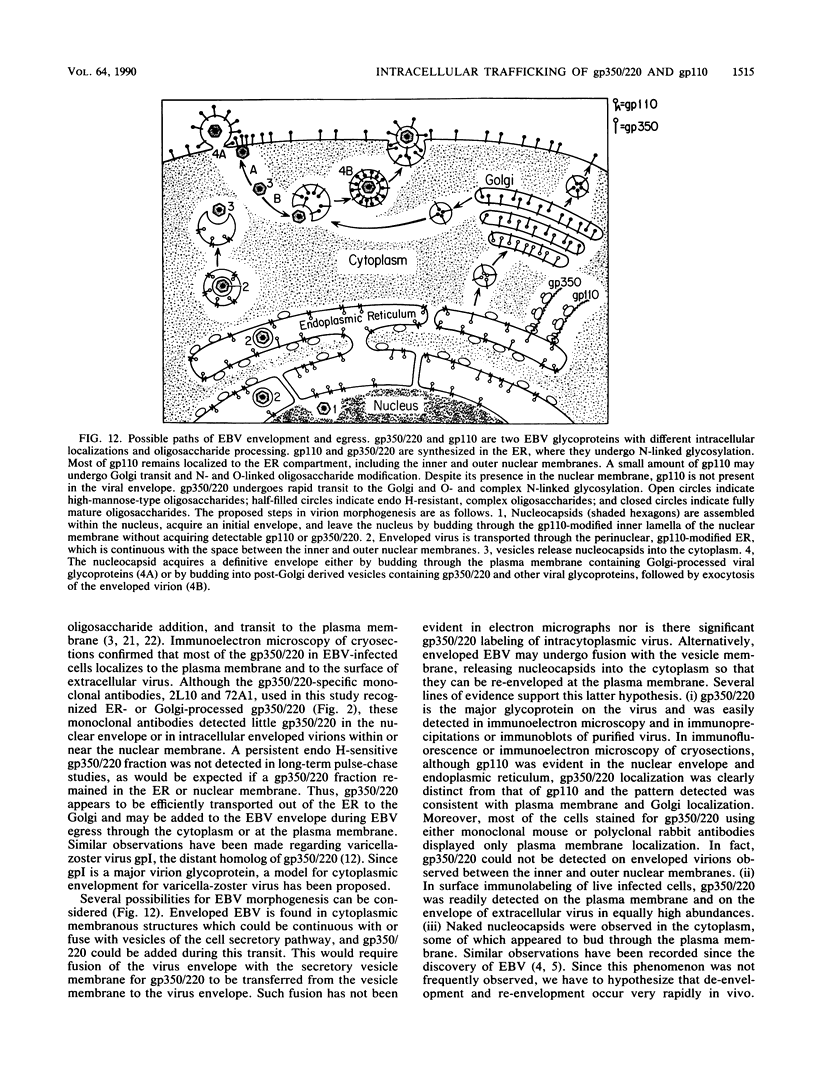
The processing and intracellular localization of the two predominant Epstein-Barr virus glycoproteins expressed in late lytic infection were investigated. Immune light or electron microscopy of frozen fixed sections revealed that gp110 colocalized to the endoplasmic reticulum and to the nuclear membrane with the endoplasmic reticulum-resident protein, heavy-chain-binding protein (BiP), while gp350/220 accumulated in low abundance in the endoplasmic reticulum and was present in higher abundance in cytoplasmic structures presumed to be Golgi and in plasma membranes. Consistent with endoplasmic reticulum and nuclear membrane localization, the bulk of gp110 was sensitive to endoglycosidase H, indicating high-mannose, pre-Golgi, N-linked glycosylation; while consistent with Golgi and plasma membrane localization, gp350/220 was mostly resistant to endoglycosidase H because of complex N- and O-linked glycosylation. gp350/220 was as abundant in extracellular enveloped virus as in the plasma membrane but was much less abundant or undetected in internal cytoplasmic or nuclear membranes. In contrast, gp110-specific antibodies did not label extracellular or intracellular virus. These data indicate that the major antigenic components of gp110 are not incorporated into or are occluded in virions and that gp350/220 is added to virus in cytoplasmic transit through a process of de-envelopment and re-envelopment at the plasma membrane or at post-Golgi vesicles. Consistent with cytoplasmic de-envelopment and re-envelopment at the plasma membrane was the finding of some free nucleocapsids in the cytoplasm of cells with intact nuclear membranes and nucleocapsids which appeared to bud through the plasma membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Pittari J., Hutt-Fletcher L. M. Detection by monoclonal antibodies of an early membrane protein induced by Epstein-Barr virus. J Virol. 1986 Nov;60(2):369–375. doi: 10.1128/jvi.60.2.369-375.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN M. A., HENLE G., ACHONG B. G., BARR Y. M. MORPHOLOGICAL AND BIOLOGICAL STUDIES ON A VIRUS IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. J Exp Med. 1965 May 1;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Thorley-Lawson D. A. Synthesis and processing of the three major envelope glycoproteins of Epstein-Barr virus. J Virol. 1983 May;46(2):547–556. doi: 10.1128/jvi.46.2.547-556.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G. The EB virus. Annu Rev Microbiol. 1973;27:413–436. doi: 10.1146/annurev.mi.27.100173.002213. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hoffman G. J., Lazarowitz S. G., Hayward S. D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A. 1980 May;77(5):2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Thorley-Lawson D., Kieff E. An Epstein-Barr virus DNA fragment encodes messages for the two major envelope glycoproteins (gp350/300 and gp220/200). J Virol. 1984 Feb;49(2):413–417. doi: 10.1128/jvi.49.2.413-417.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jones F., Grose C. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J Virol. 1988 Aug;62(8):2701–2711. doi: 10.1128/jvi.62.8.2701-2711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kishishita M., Luka J., Vroman B., Poduslo J. F., Pearson G. R. Production of monoclonal antibody to a late intracellular Epstein-Barr virus-induced antigen. Virology. 1984 Mar;133(2):363–375. doi: 10.1016/0042-6822(84)90402-1. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nemerow G. R., Wolfert R., McNaughton M. E., Cooper N. R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J Virol. 1985 Aug;55(2):347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Malagolini N., Nanni M., Dall'Olio F., Campadelli-Fiume G., Tanner J., Kieff E. Characterization of N- and O-linked oligosaccharides of glycoprotein 350 from Epstein-Barr virus. Virology. 1989 May;170(1):1–10. doi: 10.1016/0042-6822(89)90345-0. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Adams M. R., Rabin H. Glycosylation pathways of two major Epstein-Barr virus membrane antigens. Virology. 1983 May;127(1):168–176. doi: 10.1016/0042-6822(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Tanner J., Whang Y., Sample J., Sears A., Kieff E. Soluble gp350/220 and deletion mutant glycoproteins block Epstein-Barr virus adsorption to lymphocytes. J Virol. 1988 Dec;62(12):4452–4464. doi: 10.1128/jvi.62.12.4452-4464.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Torrisi M. R., Cirone M., Pavan A., Zompetta C., Barile G., Frati L., Faggioni A. Localization of Epstein-Barr virus envelope glycoproteins on the inner nuclear membrane of virus-producing cells. J Virol. 1989 Feb;63(2):828–832. doi: 10.1128/jvi.63.2.828-832.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]