Abstract
The dentate gyrus is a simple cortical region that is an integral portion of the larger functional brain system called the hippocampal formation. In this review, the fundamental neuroanatomical organization of the dentate gyrus is described, including principal cell types and their connectivity, and a summary of the major extrinsic inputs of the dentate gyrus is provided. Together, this information provides essential information that can serve as an introduction to the dentate gyrus — a “dentate gyrus for dummies.”
Keywords: neuroanatomy, circuits, connections, cell types
Introduction
The dentate gyrus is a simple cortical region that is an integral portion of the larger functional brain system called the hippocampal formation (Fig. 1) (Amaral and Lavenex, 2007). The regular organization of its principal cell layers coupled with the highly ordered laminar distribution of many of its inputs has encouraged its use as a model system for many facets of modern neurobiology. In this chapter, we present the fundamental principles of neuroanatomical organization of the dentate gyrus, its principal cell types and their connectivity, and a summary of the major extrinsic inputs of the dentate gyrus.
Fig. 1.
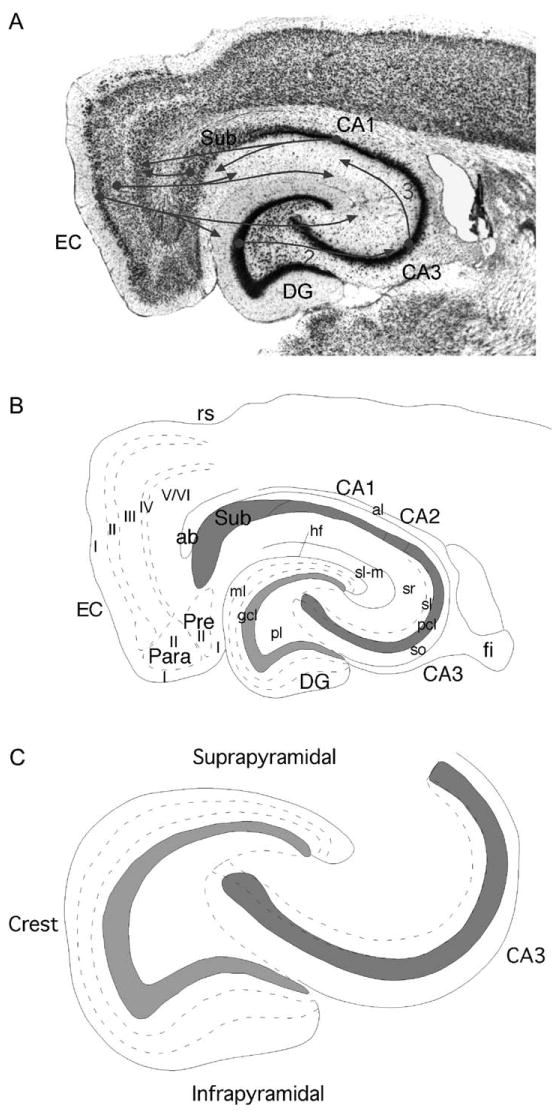
The rat hippocampal formation. (A) Nissl-stained horizontal section through the hippocampal formation of the rat. The major fields are indicated. Projections (1) originate from layer II of the entorhinal cortex (EC) and terminate in the molecular layer of the dentate gyrus (DG) and in the stratum lacunosum-moleculare of the CA3 field of the hippocampus. An additional component of the perforant path originates in layer III and terminates in the CA1 field of the hippocampus and the subiculum. Granule cells of the DG give rise to the mossy fibers (2) that terminate both within the polymorphic layer of the DG and within stratum lucidum of the CA3 field of the hippocampus. The CA3 field, in turn, gives rise to the Schaffer collaterals (3) that innervate the CA1 field of the hippocampus. Pyramidal cells in CA1 project to the subiculum and to the deep layers of the EC. The subiculum also gives rise to projections to the deep layers of the EC. (B) Line drawing to illustrate the major regional and laminar organization of the DG. The DG is divided into a molecular layer (ml) a granule cell layer (gcl) and a polymorphic layer (pl). The molecular layer is divided into three sublayers based on the laminar organization of inputs. The hippocampus is divided into CA3, CA2 and CA1 subfields. Within CA3, a number of layers are defined. The main cell layer is the pyramidal cell layer (pcl). Deep to the pyramidal cell layer is the stratum oriens (so); deep to this is the white matter of the alveus (al). Superficial to the pyramidal cell layer is stratum lucidum (sl), stratum radiatum (sr) and stratum lacunosum-moleculare (sl-m). Fields CA2 and CA1 have the same layers as CA3 except for stratum lucidum. The remainder of the hippocampal formation is made up of the subiculum (Sub), presubiculum (Pre), parasubiculum (Para) and EC. Layers of these latter structures are indicated with roman numerals. Additional abbreviations: ab, angular bundle; fi, fimbria; hf, hippocampal fissure. (C) Schematic illustration of DG and hippocampus to illustrate position of suprapyramidal blade, infrapyramidal blade and crest of the DG. This model is used in subsequent illustrations to demonstrate the major cell types and connections of the DG. (See Color Plate 1.1 in color plate section.)
One of the unique features of the hippocampal formation is that many of its connections are unidirectional (Fig. 1). Thus, the entorhinal cortex provides the major input to the dentate gyrus via fibers called the perforant path (see Chapter 3). However, the dentate gyrus does not return a projection to the entorhinal cortex. Since the entorhinal cortex is the source of much of the cortical sensory information that the hippocampal formation uses to carry out its functions, and since the dentate gyrus is the major termination of projections from the entorhinal cortex, it is reasonable to consider the dentate gyrus as the first step in the processing of information processing that ultimately leads to the production of episodic memories. Moreover, the unique neuroanatomy of the dentate gyrus predicts that it carries out a specific information-processing task with the information that it receives from the entorhinal cortex and ultimately conveys to the CA3 field of the hippocampus.
In this chapter, we will first give a general overview of the neuroanatomical organization of the dentate gyrus. We will then put the spotlight on three of its most important neurons: the dentate granule cell, the dentate pyramidal basket cell and the mossy cell. We will summarize the major structural features of these neurons as well as the connections they make within and beyond the dentate gyrus. We will then very briefly discuss some of the other neurons located in the dentate gyrus. Finally, we briefly summarize the origin and termination of the major extrinsic inputs to the dentate gyrus. Many of these topics will be discussed in greater detail in other chapters of this book. But, this chapter provides a synoptic view in order to serve as an introduction to the dentate gyrus — this is dentate gyrus for dummies.
Overall organization
The dentate gyrus has three layers (Figs. 1–2). There is a relatively cell-free layer called the molecular layer which, in the rat, is approximately 250 μm thick and is occupied by, among other things, the dendrites of the dentate granule cells. The other major occupants of the molecular layer are the fibers of the perforant path that originate in the entorhinal cortex. There are also a small number of interneurons that reside in the molecular layer and fibers from a variety of extrinsic inputs terminate there. The principal cell layer, the granule cell layer, is made up largely of densely packed granule cells. The thickness of the granule cell layer ranges from 4 to 8 neurons or ∼60 μm. While the granule cell layer is mainly made up of granule cells, there are some other neurons that are located at the boundary of the granule and polymorphic layers. The cell body of the dentate pyramidal basket cell, for example, is often located just within the granule cell layer at its border with the polymorphic layer. The granule cell layer encloses a cellular region, the polymorphic cell layer, which constitutes the third layer of the dentate gyrus (Fig. 1). A number of cell types are located in the polymorphic layer but the most prominent is the mossy cell that we will describe below.
Fig. 2.
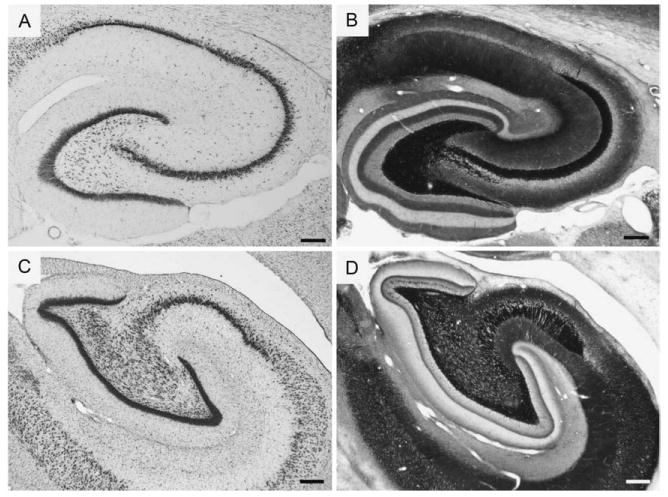
Comparative Nissl and Timm's staining of the rat and monkey dentate gyrus. Approximately the same portions of the rat (A & B) and monkey (C & D) hippocampal formation are illustrated. The magnification of the paired images are different. The increased complexity of the hilar region in the monkey compared to the rat is obvious in these photomicrographs. In the monkey, the CA3 field inserts more deeply into the dentate gyrus and accounts for a much greater area of the hilar region. While the darkly stained polymorphic layer is pronounced in the rat, it is much thinner in the monkey brain. The condensed layer of mossy fibers is also much more pronounced in the rat compared to the monkey. Also note that the strict lamination of the molecular layer into thirds in the rat is not as sharp as in the monkey. This corresponds to a more gradient-like termination of the medial and lateral perforant paths in the monkey compared to the rat. Calibration, 250 μm (A, B); 350 μm (C, D).
The dentate gyrus along with many other fields of the hippocampal formation create a banana-shaped structure that, in the rat, runs from the septal nuclei rostrally to the temporal cortex caudally. Thus, the long axis is typically called the septotemporal axis. The axis at right angles to the septotemporal axis is typically called the transverse axis. The dentate gyrus has a relatively similar structure at all septotemporal levels of the hippocampal formation and is not generally divided into subregions. However, it does tend to have more of a “V” shape septally and more of a “U” shape temporally (Fig. 3). In discussing features of the dentate gyrus, it is often useful to refer to a particular transverse portion of the “V”- or “U”- shaped structure. The portion of the granule cell layer that is located between the CA3 field and the CA1 field (separated by the hippocampal fissure) is called the suprapyramidal (above CA3) blade and the portion opposite to this, the infrapyramidal (below CA3) blade. The region bridging the two blades (at the apex of the “V” or “U”) is called the crest (Fig. 3).
Fig. 3.
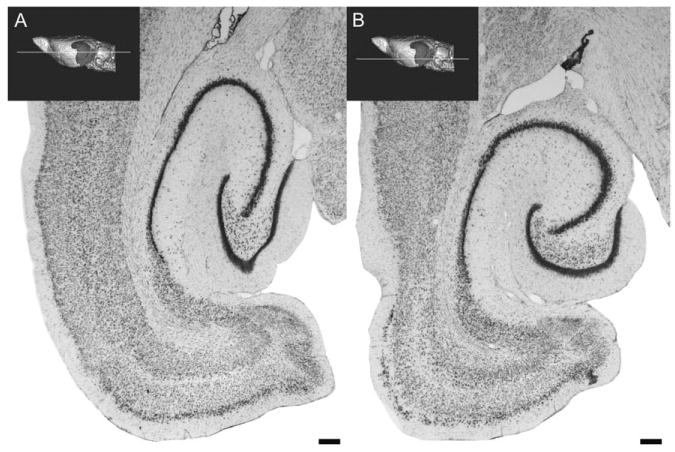
Horizontal sections through the rat hippocampal formation. This figure illustrates a more dorsally situated (A) and a more ventrally situated (B) horizontal section through the rat hippocampal formation. The approximate level of the section is illustrated on a 3D reconstruction of a magnetic resonance image series of the rat brain. Subtle differences in the cytoarchitectonic organization are seen throughout the hippocampal formation. The dentate gyrus, takes on a more “V” shape dorsally and a more “U” shape ventrally. Calibration bar = 250 μm. (See Color Plate 1.3 in color plate section.)
Comparative neuroanatomy of the dentate gyrus
The comparative neuroanatomy of the dentate gyrus is covered in detail in Chapter 2. However, we would like to raise a few issues for which there remains substantial confusion in the literature. First, the basic trilaminar structure of the dentate gyrus is common across all species studied. And, relative to other hippocampal structures such as the CA1 field of the hippocampus or the entorhinal cortex, there has not been substantial phylogenetic modification. For example, in the rat, there are approximately one million granule cells in the dentate gyrus. There are approximately ten times more granule cells in the monkey than in the rat, a ratio that parallels the overall volume differences. But there are only 15 times more dentate granule cells in humans when compared to rats, while the volume of the dentate gyrus plus the hippocampus is ∼100 times larger in humans than in rats (reviewed in more detail in Amaral and Lavenex, 2007).
Despite the similarities, there are also some obvious differences. In the rat, the CA3 field of the hippocampus inserts into the dentate gyrus and abuts the cells of the polymorphic layer. By using stains such as the Timm's stain that identifies depositions of metals such as zinc (Fig. 2), the heavily labeled polymorphic layer can be easily differentiated from the CA3 field. This differentiation is not so clear in the nonhuman primate or in the human dentate gyrus. This is because the CA3 field is much more prominent within the confines of the dentate gyrus and the polymorphic layer has thinned to a very narrow subgranular region (Fig. 2). In the early nomenclature espoused by Lorente de Nó, he used a term “CA4” that was vaguely defined but appears to have been intended for the part of CA3 that inserts within the dentate gyrus. However, sometimes Lorente de Nó appears to have used the CA4 term for the polymorphic layer. We have recommended that this term be abandoned and that even the part of CA3 that enters the limbs of the dentate gyrus be called CA3.
There are a variety of other differences in the rat, monkey and human dentate gyrus. The granule cells, which we will describe in much more detail shortly, only have apical dendrites in the rat. But in the monkey and human, many granule cells also have basal dendrites (Seress and Mrzljak, 1987). Similarly, the mossy cells of the rat have very rare dendrites that enter the molecular layer. Yet, in the macaque monkey, many of the mossy cells give rise to numerous dendrites that enter the molecular layer (Buckmaster and Amaral, 2001). The implication of this is that rat mossy cells get little if any perforant path input whereas monkey mossy cells could get a quite substantial perforant path input.
Unfortunately, many of the connections of the monkey and human dentate gyrus have yet to be investigated. One example of a projection that has been studied in both species is the commissural connection. While cells of the polymorphic layer (mainly the mossy cells) give rise to a very robust commissural projection to the molecular layer of the contralateral dentate gyrus in the rat, this projection does not exist in the nonhuman primate brain or in the human brain. As connections of the primate and human dentate gyrus are better studied, it is undoubtedly the case that there will be other fundamental differences that will affect not only normal function but also pathological processes. This raises the caveat that while the rodent dentate gyrus is an extremely valuable tool for evaluating function and potential mechanisms of pathology, caution must be exercised when these results are extrapolated to the human dentate gyrus. For the remainder of this chapter, we will focus on neuroanatomical findings from the rat brain.
Major cell types of the dentate gyrus — and their connections
The dentate granule cell
The principal cell type of the dentate gyrus is the granule cell (Figs. 4 and 5). The dentate granule cell has an elliptical cell body with a width of approximately 10 μm and a height of 18 μm (Claiborne et al., 1990). The granule cell bodies are tightly packed together and, in most cases, there is no glial sheath interposed between cells. The granule cell has a characteristic cone-shaped tree of spiny apical dendrites. The branches extend throughout the molecular layer and the distal tips of the dendritic tree end just at the hippocampal fissure or at the ventricular surface. The total length of the dendritic trees of granule cells located in the suprapyramidal blade are, on average, larger than those of cells in the infrapyramidal blade (3500 μm vs. 2800 μm, respectively). Dendrites of cells in the suprapyramidal blade have 1.6 spines/μm, whereas dendrites in the infrapyramidal blade have 1.3 spines/μm (Desmond and Levy, 1985). Thus, an estimate for the number of spines on the average suprapyramidal granule cell would be around 5600 and for an infrapyramidal cell 3640. Since virtually all of the excitatory inputs to granule cells are on these dendritic spines, these numbers indicate the approximate number of excitatory synapses that dentate granule cells receive from all sources. As noted earlier, the total number of granule cells in one dentate gyrus of the rat is ∼1.2 × 106 (West et al., 1991; Rapp and Gallagher, 1996). Although neurogenesis in the dentate gyrus persists into adulthood, and appears to be under environmental control, modern stereological studies have shown that the total number of granule cells does not vary in adult animals (Rapp and Gallagher, 1996). This implies that there is a steady state turnover of granule cells rather than a continuous accretion.
Fig. 4.
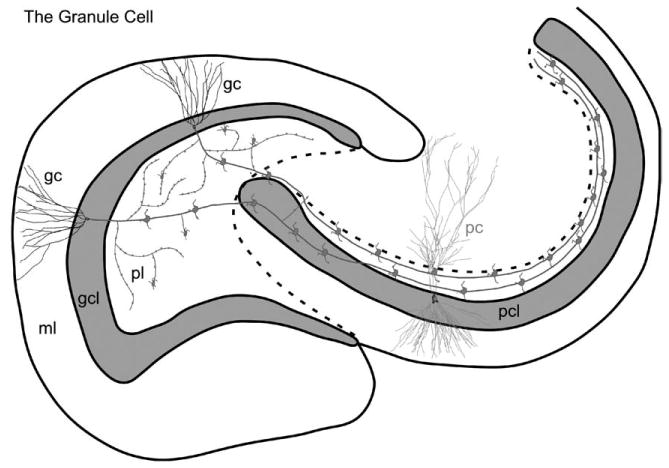
The dentate granule cell. The characteristic features of the dentate granule cell are illustrated, including its axonal arbor. A collateral plexus gives rise to numerous (∼200) typical synapses on cells located within the polymorphic layer. Most of these synapses are onto the dendrites of inhibitory interneurons. Some of the large mossy fiber expansions are also distributed in the polymorphic layer. Many of these terminate on the proximal dendrites of mossy cells. The mossy fiber axons ultimately enter the CA3 field where they travel through the full transverse extent of the field. On their course, they terminate with mossy fiber expansions on a small number (15–20) of CA3 pyramidal cells. Additional abbreviations: gc, granule cell; pc, pyramidal cell. (See Color Plate 1.4 in color plate section.)
Fig. 5.
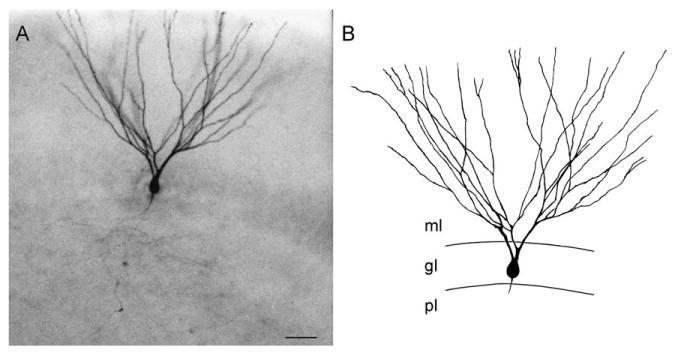
The dentate granule cell. A photomicrograph (A) and line drawing (B) of a prototypical granule cell that was filled with Lucifer yellow in a hippocampal slice. The dendrites arise primarily from the apical surface of the cell body, and the axon emerges from the basal surface. The spiny dendrites extend into the molecular layer until the hippocampal fissure, and the axon collateralizes profusely within the polymorphic layer. Calibration bar = 25 μm (A), 20 μm (B).
The packing number of granule cells and their ratio to CA3 pyramidal cells varies along the septotemporal axis (Gaarskjaer, 1978b); the packing density is higher septally than temporally. Since the packing density of CA3 pyramidal cells follows an inverse gradient, the net result is that at septal levels of the hippocampal formation, the ratio of granule cells to CA3 pyramidal cells is something on the order of 12:1, whereas at the temporal pole the ratio drops to 2:3. Since the CA3 pyramidal cells are the major recipients of granule cell innervation, and the number of mossy fiber synapses is roughly the same along the septotemporal axis, contact probability is much lower septally than temporally.
The mossy fibers — projections to the polymorphic layer
The granule cells give rise to distinctive unmyelinated axons which Ramón y Cajal called mossy fibers. The mossy fibers have unusually large boutons that form en passant synapses with the mossy cells of the polymorphic layer and with the CA3 pyramidal cells of the hippocampus. Less appreciated is the fact that the mossy fiber axons give rise to a distinctive set of collaterals that heavily innervate cells within the polymorphic layer of the dentate gyrus. Each principal mossy fiber (which is on the order of 0.2–0.5 μm in diameter) gives rise to about seven thinner collaterals within the polymorphic layer before entering the CA3 field of the hippocampus. As much as 2300 μm of collateral axonal plexus is generated by a single mossy fiber in the polymorphic layer (Claiborne et al., 1986). Within the polymorphic layer, the mossy fiber collaterals branch extensively and the daughter branches bear two types of synaptic varicosities. Numerous small (approximately 2 μm) spherical synaptic varicosities are distributed unevenly along these collaterals. There are ∼160–200 of these varicosities distributed throughout the axonal collateral plexus of a single granule cell, and these form contacts on dendrites located in the polymorphic layer. At the end of many of the collateral branches there are also larger (3–5 μm diameter), irregularly shaped varicosities that resemble, although are smaller than, the mossy fiber boutons found in CA3. The mossy fiber terminals in the polymorphic layer establish contacts with the proximal dendrites of the mossy cells (Ribak et al., 1985), the basal dendrites of the pyramidal basket cells as well as with other, unidentified cells. Acsady et al. (1998) reported that the vast majority of mossy fiber collaterals in the polymorphic cell layer terminate on GABAergic interneurons. Since there are 160–200 such varicosities (compared with ∼20 of the larger thorny excrescences), mossy fiber axons synapse on a larger number of interneurons than mossy cells or CA3 pyramidal cells. Mossy fiber collaterals occasionally enter the granule cell layer, but they rarely enter the molecular layer under normal conditions. The collaterals that enter the granule cell layer appear to terminate preferentially on the apical dendritic shafts of pyramidal basket cells. The lack of mossy fiber innervation of the molecular layer changes dramatically in pathological conditions and sprouting of mossy fibers is one of the major hallmarks of temporal lobe epilepsy (see Chapter 29).
The mossy fibers — projections to CA3
The rat dentate gyrus does not project to any brain region other than the CA3 field of the hippocampus (but see Chapter by Zimmer for exceptions in other species). The mossy fibers terminate in a relatively narrow zone mainly located just above the CA3 pyramidal cell layer (Blackstad et al., 1970; Swanson et al., 1978; Gaarskjaer, 1978a; Claiborne et al., 1986). In the proximal portion of CA3, mossy fibers are also located below and within the pyramidal cell layer. The layer of mossy fiber termination located just above the pyramidal cell layer is called stratum lucidum. There is no indication that dentate neurons other than the granule cells project to CA3. In particular, cells in the polymorphic layer do not project to the hippocampus, at least in the rodent. The dentate projection to CA3 stops near the border of CA3 with CA2, and the lack of granule cell input is one of the main features that distinguishes CA3 from CA2 pyramidal cells.
As far as can be determined, all dentate granule cells appear to project to CA3 and the axon trajectory is partially correlated with the position of the parent cell body. In the proximal portion of CA3 (closer to the dentate gyrus), mossy fibers are distributed below, within and above the pyramidal cell layer. The fibers located below the layer, i.e., those that are in the area occupied primarily by basal dendrites, are generally called the infrapyramidal bundle (Fig. 2). The fibers located within the pyramidal cell layer are called the intrapyramidal bundle and those located above the pyramidal cell layer (in the area occupied mainly by proximal apical dendrites) the suprapyramidal bundle. The suprapyramidal bundle occupies the stratum lucidum. At mid and distal portions of CA3, the intra- and infrapyramidal bundles are largely eliminated and those fibers that were in these regions cross the pyramidal cell layer and join the other mossy fibers within stratum lucidum.
Granule cells at all transverse positions within the granule cell layer generate mossy fibers that extend for the full transverse extent of CA3 (Gaarskjaer, 1981). Cells located in the infrapyramidal blade of the granule cell layer have axons that tend to enter CA3 in the infrapyramidal bundle, but ultimately cross the pyramidal cell layer to enter the deep portion of stratum lucidum. The axons of granule cells located in the crest of the dentate gyrus tend to enter CA3 in the intrapyramidal bundle and also ultimately ascend into stratum lucidum. Cells located in the suprapyramidal blade of the dentate gyrus give rise to axons that enter CA3 in the stratum lucidum and continue within the most superficial portion of stratum lucidum (Claiborne et al., 1986).
Early Golgi anatomists indicated that the mossy fiber axons were mainly oriented perpendicular to the long axis of the hippocampus. Blackstad and colleagues confirmed the largely transverse trajectory of the mossy fibers using degeneration track tracing methods. Mossy fibers originating from any particular septotemporal level travel through the full transverse extent of CA3 in a very narrow septotemporal zone of ∼1 mm, which has been described as a lamella. There is one peculiarity of the mossy fiber projection, however, that has eluded explanation. Lorente de Nó (1934) and McLardy (McLardy and Kilmer, 1970) indicated that the mossy fibers actually do change their course and take a longitudinal direction, but not until they reach the distal portion of CA3. The extent of this distal longitudinal projection was further clarified by Swanson and colleagues, using the autoradiographic method of neural tract tracing (Swanson et al., 1978). They showed that granule cells located at septal levels give rise to mossy fibers that travel throughout most of the transverse extent of CA3 at the same septotemporal level. But just at the CA3/CA2 border, they abruptly change course and travel toward the temporal pole for nearly 2 mm. The extent of this longitudinal component, however, appears to depend on the septotemporal location of the cells of origin. Granule cells in the mid to temporal portions of the dentate gyrus have mossy fibers that exhibit only a slight temporal inclination at their distal extremity. And mossy fibers that originate at the extreme temporal pole of the dentate gyrus barely extend to the CA3/CA2 border and have little or no longitudinal component. On the face of it, this indicates that some CA3 pyramidal cells that are located very close to the border with CA2 may be contacted by mossy fiber axons from granule cells spread out over a much broader septotemporal extent of the dentate gyrus. They might, therefore, form a special class of integrator CA3 cells. An elegant neuroanatomical verification of this has come from the work of Acsady et al. (1998) who stained dentate granule cells by using in vivo intracellular techniques. Not only do mossy fibers travel temporally for 2–3 mm, but they also demonstrate typical mossy fiber expansions along this portion of their trajectory.
There is substantial evidence indicating that the granule cells use glutamate as their primary transmitter, and the asymmetric contacts made between the mossy fiber expansions and the thorny excrescences would tend to confirm this notion. The mossy fibers are, nonetheless, also immunoreactive for several other neuroactive substances. At least some of the mossy fibers demonstrate immunoreactivity for the opioid peptide dynorphin and they are also immunoreactive for GABA (Walker et al., 2002). The chemical neuroanatomy of the dentate granule cell will be dealt with in more detail in Chapter 6.
The dentate pyramidal basket cell
As with most other brain regions, some neurons of the dentate gyrus, such as the granule cells, are excitatory whereas others are inhibitory. The most intensively studied type of inhibitory interneuron in the dentate gyrus is the pyramidal basket cell (Figs. 6 and 7; Ribak et al., 1978; Ribak and Seress, 1983). These cells are generally located along the interface between the granule cell layer and the polymorphic layer. They have pyramidal-shaped cell bodies that are substantially larger (25–35 μm in diameter) than the granule cells (10–18 μm). Ramón y Cajal first described the pyramidal basket cells as having a single, principal aspiny apical dendrite directed into the molecular layer that divides into several aspiny branches, and several basal dendrites that divide and extend into the polymorphic cell layer. The number of basket cells is not constant throughout either the transverse or septotemporal extents of the dentate gyrus (Seress and Pokorny, 1981). At septal levels, the ratio of basket cells to granule cells is 1:100 in the suprapyramidal blade and 1:180 in the infrapyramidal blade. At temporal levels, the number is 1:150 for the suprapyramidal blade and 1:300 for the infrapyramidal blade.
Fig. 6.
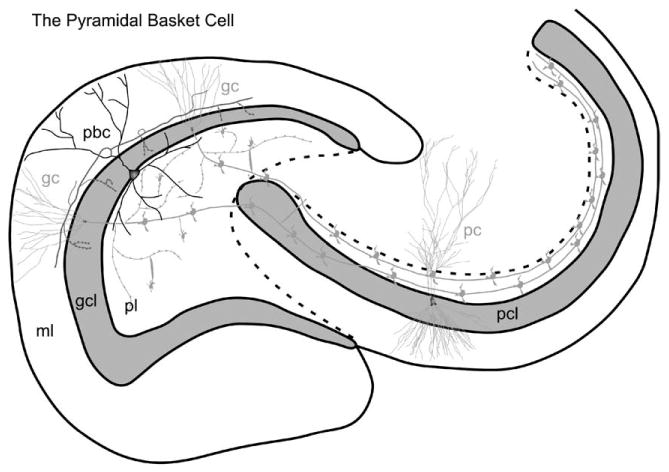
The pyramidal basket cell. The cell body of the pyramidal basket cell is located at the interface between the granule cell layer and the polymorphic cell layer. The axon (arrow) emerges from the apical dendrite. Collaterals of this axon form a curtain of terminals that synapse with the granule cell bodies. Additional abbreviations: pbc, pyramidal basket cell. (See Color Plate 1.6 in color plate section.)
Fig. 7.
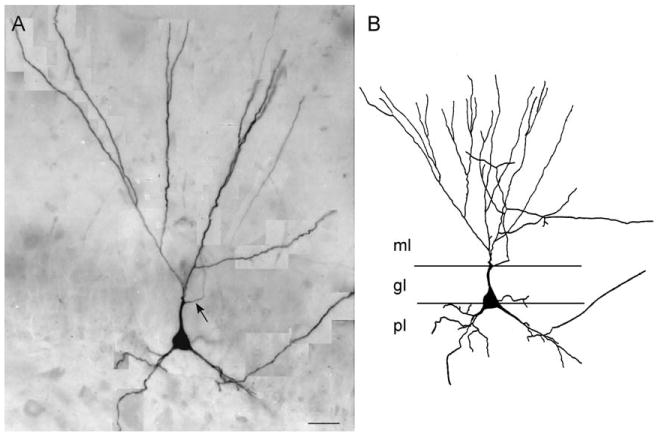
The pyramidal basket cell. A prototypical pyramidal basket cell is shown after intracellular injection of Neurobiotin. The montage that was created after visualization of the cell (A) and line drawing (B) illustrate the characteristics of this cell type. An arrow points to the axon. Calibration bar = 25 μm (A), 40 μm (B).
The basket portion of the name refers to the fact that the axon of these cells forms pericellular plexuses, like the covering of a chianti bottle, that form encompassing synapses with the cell bodies of granule cells. The dentate pyramidal basket cell along with other basket cells located just below the granule cell layer contribute to a very dense terminal plexus that is confined to the granule cell layer (Struble et al., 1978; Sik et al., 1997). The terminals in this basket plexus are GABAergic and form symmetric, inhibitory contacts, located primarily on the cell bodies and proximal dendritic shafts of the apical dendrites of the granule cells. Analysis of Golgi-stained axonal plexuses from single basket cells in the rat indicates that they extend for distances greater than 900 μm in the transverse axis and ∼1.5 mm in the septotemporal axis. This widely distributed axonal plexus would allow a single basket cell to influence as many as 10,000 (1%) of the granule cells.
The mossy cell
The polymorphic layer harbors a variety of neuronal cell types. The most common, and certainly the most impressive, is the mossy cell (Figs. 8 and 9) This cell type is probably what Ramón y Cajal referred to as the “stellate or triangular” cells located in his “subzone of fusiform cells”, and is undoubtedly what Lorente de Nó referred to as “modified pyramids”. The mossy cell received its current name from Amaral (1978) who studied this and other neurons of the polymorphic layer using the Golgi method. The cell bodies of the mossy cells are large (25–35 μm) and are often triangular or multipolar in shape. Three or more thick dendrites originate from the cell body and extend for long distances within the polymorphic layer. Each principal dendrite bifurcates once or twice and generally gives rise to a few side branches. While most of the dendritic branches remain within the polymorphic layer, an occasional dendrite pierces the granule cell layer and enters the molecular layer. The mossy cell dendrites virtually never enter the adjacent CA3 field in the rat.
Fig. 8.
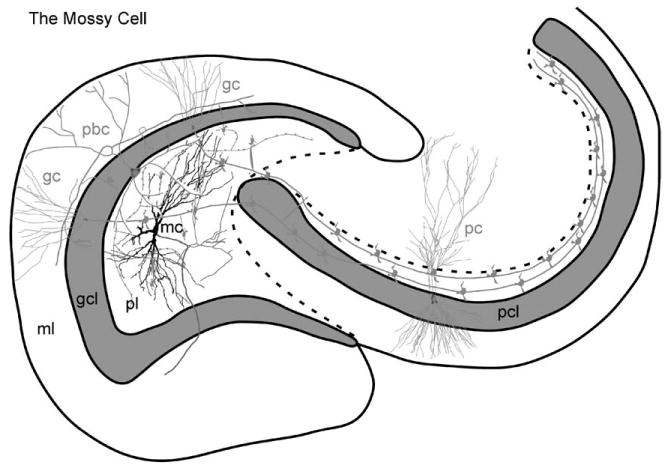
The mossy cell. A line drawing of a mossy cell (mc) in the polymorphic layer. The axon (arrow) develops a plexus within the polymorphic layer, and also an ipsilateral projection to the inner molecular layer, known as the associational pathway. The main axon also projects contralaterally to the inner molecular layer, forming the commissural pathway. The ipsilateral projection increases in density with distance from the cell body of origin. (See Color Plate 1.8 in color plate section.)
Fig. 9.
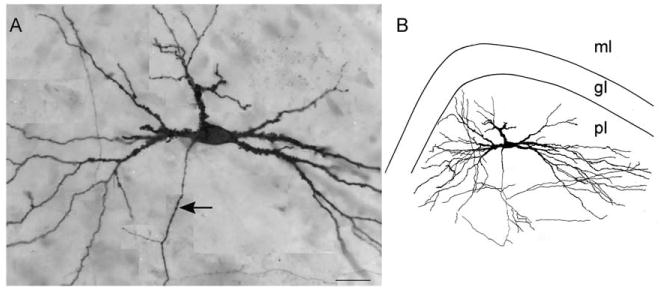
The mossy cell. A montage of several focal planes (A), and line drawing (B) of a classic mossy cell, illustrating the characteristic thorny excrescences and dendritic tree of this cell type. Thorny excrescences are present proximal to the soma. The dendrites of the cell extend throughout nearly the entire polymorphic region, but few enter either the granule cell or molecular layers. Calibration bar = 25 μm (A), 50 μm (B).
The most distinctive feature of the mossy cell is that all of its proximal dendrites are covered by very large and complex spines, the so-called thorny excrescences (Fig. 9). These are the distinctive sites of termination of the mossy fiber axons. While thorny excrescences are also observed on the proximal dendrites of CA3 pyramidal cells, they are never as dense or as complex as the ones on the mossy cells. The distal dendrites of the mossy cell have typical pedunculate spines that appear to be less dense than those on the distal dendrites of the hippocampal pyramidal cells.
Mossy cell projections
The inner third of the molecular layer (Fig. 2) receives a projection that originates exclusively from neurons in the polymorphic layer (Laurberg and Sorensen, 1981; Frotscher et al., 1991; Buckmaster et al., 1992, 1996). Since, in the rat, this projection originates both in the ipsilateral and contralateral sides of the dentate gyrus, it has been called the associational/commissural projection. As noted above, the commissural portion of this projection does not exist in the primate brain. There is substantial evidence from neural track tracing studies that this projection arises almost exclusively from cells in the polymorphic layer and mainly from the mossy cells. The fact that the mossy cells are immunoreactive for glutamate (Soriano and Frotscher, 1994) adds credence to the notion that the associational/commissural projection is excitatory. A direct electrophysiological demonstration of the excitatory nature of the mossy cell synapse on the granule cell has been provided by Scharfman (1994). Scharfman (1995) has also demonstrated that mossy cells innervate both granule cells and GABAergic interneurons. It should also be noted for the sake of completeness that a small component of the commissural connection in the rat does appear to arise from GABAergic neurons (Ribak et al., 1986).
There are a number of interesting features about the “feedback” projection from the mossy cells to the granule cells. First, the projection from mossy cells located at any particular level of the dentate gyrus is distributed widely along the longitudinal axis, both septally and temporally from the point of origin. Axons from any particular septotemporal point in the dentate gyrus may innervate as much as 75% of the long axis of the dentate gyrus (Amaral and Witter, 1989). Second, the projection to the molecular layer at the septotemporal level of origin is very weak, but gets increasingly stronger at levels that are progressively more distant from the cells of origin. Remembering that mossy cells are the recipients of massive innervation from the granule cells at their same level (via the mossy fiber collaterals into the polymorphic layer), it would appear that the mossy cells pass on the collective output of granule cells from one septotemporal level to granule cells located at distant levels of the dentate gyrus.
Other neurons of the dentate gyrus
There has been an explosion in the number of interneurons identified in the rat hippocampal formation. A detailed overview of the characteristics of the various hippocampal interneurons has been published by Freund and Buzsaki (1996) and is also covered in Chapter 13 of this book. Many of the cell types can be distinguished on the basis of the distribution of their axonal plexus. Some have axons that terminate on cell bodies, whereas others have axons that terminate exclusively on the initial segments of other axons. Interneurons have also been distinguished on the basis of their inputs. Some are preferentially innervated, for example, by the serotonergic fibers originating from the raphe nuclei. Interneurons can also be differentiated from principal cells on the basis of their electrophysiological characteristics. At least some interneurons have high rates of spontaneous activity and fire in relation to the theta rhythm. For this reason, interneurons are often called theta cells (Sik et al., 1997).
Within the same subgranular region occupied by the cell bodies and dendrites of the pyramidal basket cells are several other cell types with distinctly different somal shapes, as well as different dendritic and axonal configurations. Some of these cells are multipolar with several aspiny dendrites entering the molecular and polymorphic layers, while others tend to be more fusiform-shaped with a similar dendritic distribution. As Ribak and colleagues have pointed out, many of these cells share fine structural characteristics such as infolded nuclei, extensive perikaryal cytoplasm with large Nissl bodies and intranuclear rods. Moreover, it appears that all of these cells give rise to axons that contribute to the basket plexus in the granule cell layer. Most of these neurons are immunoreactive for GABA, form symmetrical synaptic contacts with the cell bodies, proximal dendrites and occasionally with axon initial segments of granule cells, and therefore function as inhibitory interneurons. These cells are not neurochemically homogeneous, however, since subsets appear to colocalize distinct categories of other neuroactive substances (Ribak, 1992).
Neurons of the molecular layer
The molecular layer is occupied primarily by dendrites of the granule cells, pyramidal basket cells and polymorphic layer cells, as well as axons and terminal axonal arbors from the entorhinal cortex and other sources. At least two neuron types are also present in the molecular layer. The first is located deep in the molecular layer, has a multipolar or triangular cell body and gives rise to an axon that produces a substantial terminal plexus largely limited to the outer two thirds of the molecular layer. This neuron has aspiny dendrites that remain mainly within the molecular layer and has been called the MOPP cell (molecular layer perforant path-associated cell). This terminology was proposed by Han et al. (1993) to bring some order to naming interneurons in the hippocampal formation. The lettering system refers to the location of the cell body and to the region where the axon is distributed. Unfortunately, this terminology has not been embraced and more widely applied to neurons of the hippocampal formation.
Frotscher and colleagues have described a second type of neuron in the molecular layer that resembles the chandelier or axo-axonic cell originally found in the neocortex (Soriano and Frotscher, 1989). These cells are generally located immediately adjacent or even within the superficial portion of the granule cell layer. The axo-axonic cell is named for the fact that its axon descends from the molecular layer into the granule cell layer, collateralizes profusely and then terminates, with symmetric synaptic contacts, exclusively on the axon initial segments of granule cells. Thus, their shape resembles that of a chandelier. Each axo-axonic cell may innervate the axon initial segments of as many as 1000 granule cells. Since these cells are immunoreactive for markers of GABAergic neurons and make symmetrical synapses, it is likely that they provide an additional means of inhibitory control of granule cell output.
Neurons of the polymorphic cell layer
Besides the mossy cell, there are a number of fusiform cells in the polymorphic layer. The main difference between the fusiform cell types is whether they have spines or not and the characteristic shapes and sizes of the spines. One type, the long-spined multipolar cell first described by Amaral (1978) has recently been called the HIPP cell (hilar perforant path-associated cell) (Fig. 10; Han et al., 1993). The conspicuous feature of this cell is the distribution of copious, long and often branched spines over its cell body and dendrites. Intracellular staining techniques demonstrate that these cells have axons that ascend into the outer two-thirds of the molecular layer (i.e., the perforant path zone) and terminate with symmetrical and presumably inhibitory synapses on the dendrites of granule cells. An amazing feature of these neurons is that their axonal plexus can extend for as much as 3.5 mm along the septotemporal axis of the dentate gyrus (the entire length of the dentate gyrus in the rat is only ∼10 mm) and may generate as many as 100,000 synaptic terminals. Since inhibitory interneurons typically have aspiny dendrites and relatively local axonal plexuses, this long spined multipolar/HIPP cell is a very atypical interneuron! At least some of these HIPP cells appear to correspond to the somatostatin/GABA cells that give rise to the somatostatin innervation of the outer portion of the molecular layer.
Fig. 10.
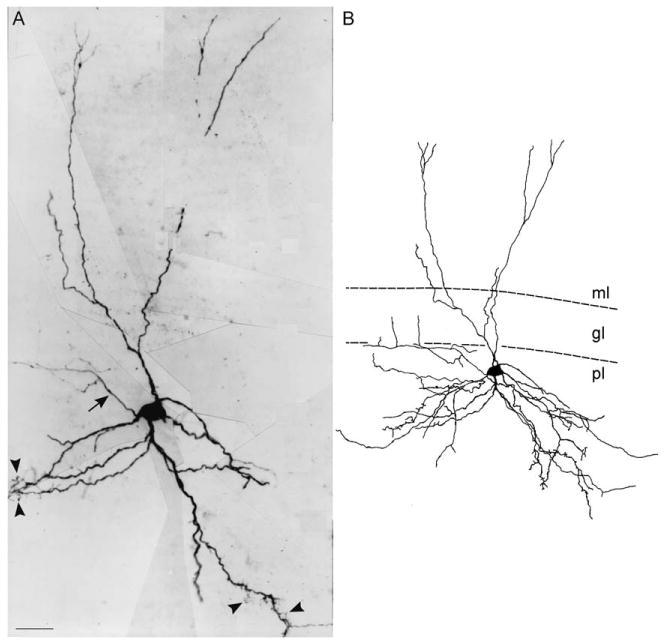
The long-spined cell. A montage (A) and line drawing (B) of a long-spined cell in the polymorphic layer. The extremely long spines that characterize this cell type are marked by arrowheads, and can be proximal as well as distal to the cell body, although in this example they were primarily located along distal dendrites. The axon of this cell collateralized in the molecular layer. Some of the long-spined cells correspond to the GABAergic interneurons that colocalize somatostatin, so-called HIPP cells. Calibration bar = 25 μm (A), 50 μm (B).
Antibodies directed against the peptide somatostatin have revealed that neurons such as the HIPP cells scattered throughout the polymorphic layer are immunoreactive for this peptide, and account for approximately 16% of the GABAergic cells in the dentate gyrus (Morrison et al., 1982; Bakst et al., 1986; Freund and Buzsaki, 1996; Sik et al., 1997; Boyett and Buckmaster, 2001). The somatostatin-positive cells all colocalize with GABA, and are the source of the somatostatin immunoreactive fibers and terminals in the outer two-thirds of the molecular layer. This system of fibers, which forms contacts on the distal dendrites of the granule cells, provides a third means for inhibitory control of granule cell activity, in addition to the GABAergic basket cell plexus and axo-axonic terminals of the chandelier cells. Since electron microscopic studies have demonstrated that the somatostatin cells are contacted by mossy fiber terminals, the projection to the outer molecular layer thus constitutes a local feedback inhibitory circuit.
Interestingly, unlike the mossy cell associational projection that terminates more heavily at distant levels of the dentate gyrus, the GABA/somatostatin projection terminates most heavily around the level of the cells of origin, and termination rapidly decreases within approximately 1.5 mm septally and temporally to the cells of origin. Thus, the mossy cell projection and the somatostatin/GABA cell projection have terminal fields that are spatially complementary in both radial and septotemporal axes. The distribution suggests that the two cell types mediate distal excitation and local inhibition, respectively.
There are also multipolar or triangular cells in the polymorphic layer with thin, aspiny dendrites that extend both within the hilus and within the molecular layer. The axons of these HICAP cells (hilar commissural-associational pathway related cells) extend through the granule cell layer and branch profusely in the inner third of the molecular layer. There is a variety of other neuron types in the polymorphic layer of the dentate gyrus whose axonal plexus have not yet been well described. A summary of the neurons in the polymorphic region, to some extent still incompletely understood, is shown in Fig. 11.
Fig. 11.
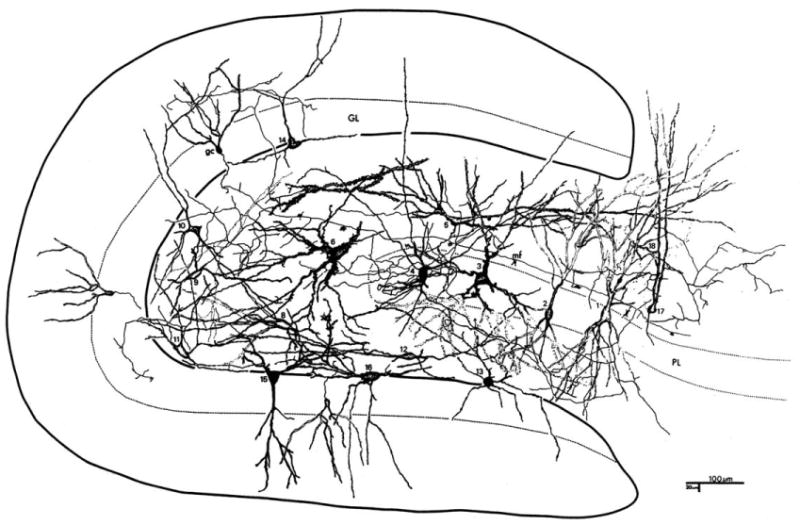
Neurons of the polymorphic region. The summary figure reprinted from Amaral (1978) illustrates the diversity of cells types within the dentate gyrus and proximal CA3 region of the rat. Based on Golgi staining and cameral lucida drawings of individual neurons from multiple preparations. See original paper for description of cell types.
Extrinsic afferents
Afferent projections
Although this topic is covered in more details in other chapters (see Chapters 3 and 4), we will provide a brief general synopsis of the afferent projections of the dentate gyrus.
Entorhinal cortex projection to the dentate gyrus
The dentate gyrus receives its major input from the entorhinal cortex, via the so-called perforant pathway (Ramón y Cajal, 1893). The projection to the dentate gyrus arises mainly from cells located in layer II of the entorhinal cortex (Fig. 1), although a minor component of the projection also comes from layers V and VI (Steward and Scoville, 1976; Deller et al., 1996). The entorhinal terminals are strictly confined to the outer two-thirds of the molecular layer where they form asymmetric synapses that account for nearly 85% of the total axospinous terminations (Nafstad, 1967; Hjorth-Simonsen and Jeune, 1972). These contacts occur primarily on the dendritic spines of granule cells, although a small number of perforant path fibers also form asymmetric synapses on the shafts of GABA-positive interneurons.
The perforant pathway can be divided into two parts based on the region of origin, pattern of termination and appearance in histochemical and immunohistochemical preparations. In the rat, the two divisions have been called the lateral and medial perforant paths because they originate from the lateral and medial entorhinal areas, respectively. Interestingly, while the cells of origin for these projections look very similar and the light microscopic appearance of their projections is indistinguishable, they do demonstrate substantial histochemical and chemical neuroanatomical differences which remain largely unexplored. Perforant path fibers originating in the lateral entorhinal area terminate in the most superficial third of the molecular layer, whereas the fibers originating from the medial entorhinal area terminate in the middle third of the molecular layer (see Chapter 3 for more detail on the perforant path projection). These terminal zones are readily distinguished by the classical Timm's stain method for visualization of heavy metals which demonstrates dense staining in the outer third of the molecular layer, a near absence of staining in the middle third and a dark staining in the inner third that is associated with the commissural/associational connection (Fig. 2). Projections from both areas of the entorhinal cortex innervate the entire transverse extent of the molecular layer. The thin axon branches (0.1 μm) in the molecular layer of the dentate gyrus show periodic varicosities with a thickness of 0.5–1.0 μm. Most of entorhinal cortex layer II spiny stellate cells project up to 2 mm in the septotemporal direction forming a sheet-like axon arbor in the molecular layer (Tamamaki and Nojyo, 1993).
While it is often assumed that the perforant path fibers from the entorhinal cortex are the only hippocampal input reaching the dentate gyrus, it is now clear that at least minor projections also arise in the presubiculum and parasubiculum (Kohler, 1985). These fibers enter the molecular layer of the dentate gyrus and ramify in a zone that is interspersed between the lateral and medial perforant path projections. The presubicular axons tend to be thicker than those from the entorhinal cortex and give rise to collaterals that take a radial course in the molecular layer. Virtually nothing is currently known about which cells these fibers innervate or what type of transmitter they use. Since the presubiculum receives the only direct input from the anterior thalamic nucleus, these fibers provide a potential link by which thalamic information could reach the dentate gyrus.
Basal forebrain inputs: projections from the septal nuclei
The dentate gyrus receives relatively few inputs from subcortical structures. The most robust is the projection from the septal nuclei (Mosko et al., 1973; Swanson, 1978; Amaral and Kurz, 1985). The septal projection arises from cells of the medial septal nucleus and the nucleus of the diagonal band of Broca. Septal fibers heavily innervate cells of the polymorphic layer, particularly in a narrow region just subjacent to the granule cell layer. Septal fibers are lightly distributed throughout the molecular layer.
A major portion of the fibers of the septal projection to the dentate gyrus are cholinergic. Thirty to fifty percent of the cells in the medial septal nucleus and 50–75% of the cells in the nucleus of the diagonal band that project to the hippocampal formation are cholinergic. Many of the other septal cells that project to the dentate gyrus are GABAergic. The most interesting facet of this heterogeneous septal projection is that the cholinergic and GABAergic components target different cell types. Fibers of the septal GABAergic projections terminate preferentially on other GABAergic nonpyramidal cells, such as the basket pyramidal cells of the dentate gyrus, and they form symmetrical, presumably inhibitory contacts. The heaviest GABAergic septal termination is on interneurons located in the polymorphic layer. The cholinergic septal projection to the dentate gyrus, in contrast, terminates mainly on granule cells, making asymmetric, presumably excitatory contacts on dendritic spines, chiefly in the inner third of the molecular layer. Only 5–10% of the septal cholinergic synapses are on dentate interneurons. The large mossy cells are innervated by cholinergic fibers (Lübke et al., 1997).
Supramammillary and other hypothalamic inputs
The major hypothalamic projection to the dentate gyrus arises from a population of large cells in the supramammillary area that caps and partially surrounds the medial mammillary nuclei (Wyss et al., 1979; Dent et al., 1983; Vertes, 1993; Magloczky et al., 1994). The supramammillary projection mainly terminates in a narrow zone located just superficial to the granule cell layer and lightly in the polymorphic layer or the remaining portion of the molecular layer. The vast majority of the supramammillary fibers terminate on the proximal dendrites of granule cells. This projection is excitatory and is likely using glutamate as a primary neurotransmitter (Kiss et al., 2000). Most, but not all, of the glutamatergic supramammillary neurons that project to the dentate gyrus also colocalize calretinin; some of these cells also colocalize substance P (Borhegyi and Leranth, 1997).
Brainstem inputs
The dentate gyrus receives a particularly prominent noradrenergic input from the nucleus locus coeruleus (Pickel et al., 1974; Swanson and Hartman, 1975; Loughlin et al., 1986). The noradrenergic fibers terminate mainly in the polymorphic layer of the dentate gyrus and extend into the stratum lucidum of CA3, as if preferentially terminating in the zones occupied by mossy fibers.
The dentate gyrus receives a minor and diffusely distributed dopaminergic projection that arises mainly from cells located in the ventral tegmental area. The dopaminergic fibers terminate mainly in the polymorphic layer.
The serotonergic projection that originates from median and dorsal divisions of the raphe nuclei also terminates most heavily in the polymorphic layer in an immediately subgranular portion of the layer (Conrad et al., 1974; Moore and Halaris, 1975; Köhler and Steinbusch, 1982; Vertes et al., 1999). A number of GABAergic interneurons appear to be preferentially innervated by the serotonergic fibers. The targets are often the pyramidal basket cells. Fusiform neurons in the region, particularly those that are stained for the calcium binding protein calbindin, are also very heavily innervated. As with the cholinergic projection from the septum, many of the cells in the raphe nuclei that project to the hippocampal formation appear to be nonserotonergic, but their transmitter is not known.
Conclusions
In the remainder of this book, the reader will receive far greater detail on many of the neuroanatomical features of the dentate gyrus that were only briefly touched on in this chapter. Every effort will also be made to correlate the neuroanatomy of the dentate gyrus with both its electrophysiological and functional attributes. We will close with a short reflection on the position of the dentate gyrus both within the hippocampal formation and within the brain at large.
It has been fashionable at times to highlight the simple neuroanatomy of the dentate gyrus and to claim that it provides a heuristic for studying and understanding the much more complicated neocortex. This is probably a misguided strategy. There are numerous features of the dentate gyrus that make it absolutely unique from a neuroanatomical point of view and thus presumably from a functional point of view as well. The largely unidirectional nature of its inputs and outputs is one distinctive feature. The distinctive mossy fibers that give rise to massive synaptic endings that have as many as 40 active sites onto postsynaptic neurons is another. Our view is that the distinctive neuroanatomy of the dentate gyrus has been sculpted by evolution to play a precise and unique role in the hippocampal information processing that ultimately leads to the production of declarative memories. Whatever computations the dentate gyrus executes will undoubtedly be different from, and will work in concert with, the computations carried out by field CA3 and other portions of the hippocampal formation. The challenge for the future will be to understand enough about the input/output patterns of the dentate gyrus to identify the specific contribution it makes to memory formation.
There are other unique features of the dentate gyrus. Why, for example, is there the conspicuous production during adult life of new granule cells and how do these support the unique function of the dentate gyrus? Does the formation of these new neurons have significance for the broader questions of stem cell recovery of function or does it reflect again the unique attributes of one particular brain region? Despite the “simplicity” of the dentate gyrus, it will undoubtedly remain the topic of extensive research for decades to come. And, the chapters that constitute the remainder of this book will provide the reader both with a valuable summary of the state of dentate gyrus science and guidance for research into the future.
Plate 1.1.
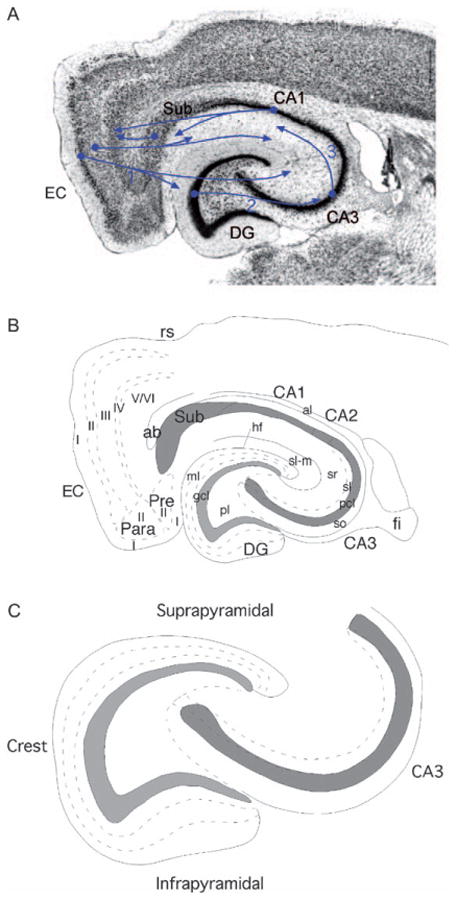
The rat hippocampal formation. (A) Nissl-stained horizontal section through the hippocampal formation of the rat. The major fields are indicated. Projections (1) originate from layer II of the entorhinal cortex (EC) and terminate in the molecular layer of the dentate gyrus (DG) and in the stratum lacunosum-moleculare of the CA3 field of the hippocampus. An additional component of the perforant path originates in layer III and terminates in the CA1 field of the hippocampus and the subiculum. Granule cells of the DG give rise to the mossy fibers (2) that terminate both within the polymorphic layer of the DG and within stratum lucidum of the CA3 field of the hippocampus. The CA3 field, in turn, gives rise to the Schaffer collaterals (3) that innervate the CA1 field of the hippocampus. Pyramidal cells in CA1 project to the subiculum and to the deep layers of the EC. The subiculum also gives rise to projections to the deep layers of the EC. (B) Line drawing to illustrate the major regional and laminar organization of the DG. The DG is divided into a molecular layer (ml) a granule cell layer (gcl) and a polymorphic layer (pl). The molecular layer is divided into three sublayers based on the laminar organization of inputs. The hippocampus is divided into CA3, CA2 and CA1 subfields. Within CA3, a number of layers are defined. The main cell layer is the pyramidal cell layer (pcl). Deep to the pyramidal cell layer is the stratum oriens (so); deep to this is the white matter of the alveus (al). Superficial to the pyramidal cell layer is stratum lucidum (sl), stratum radiatum (sr) and stratum lacunosum-moleculare (sl-m). Fields CA2 and CA1 have the same layers as CA3 except for stratum lucidum. The remainder of the hippocampal formation is made up of the subiculum (Sub), presubiculum (Pre), parasubiculum (Para) and EC. Layers of these latter structures are indicated with roman numerals. Additional abbreviations: ab, angular bundle; fi, fimbria; hf, hippocampal fissure. (C) Schematic illustration of DG and hippocampus to illustrate position of suprapyramidal blade, infrapyramidal blade and crest of the DG. This model is used in subsequent illustrations to demonstrate the major cell types and connections of the DG. (For B/W version, see page 4 in the volume.)
Plate 1.3.
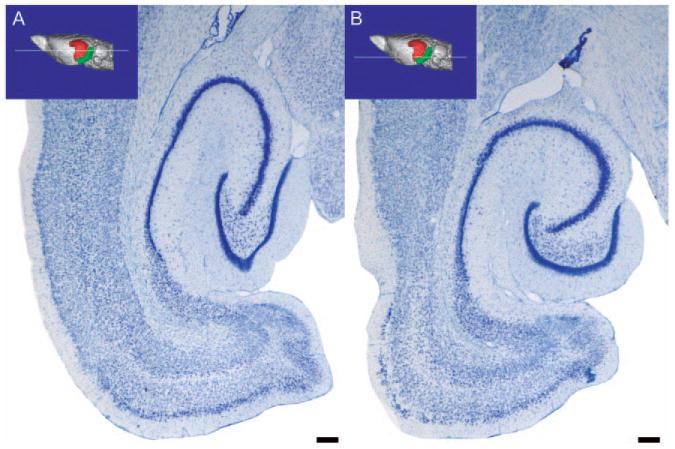
Horizontal sections through the rat hippocampal formation. This figure illustrates a more dorsally situated (A) and a more ventrally situated (B) horizontal section through the rat hippocampal formation. The approximate level of the section is illustrated on a 3D reconstruction of a magnetic resonance image series of the rat brain. Subtle differences in the cytoarchitectonic organization are seen throughout the hippocampal formation. The dentate gyrus, takes on a more “V” shape dorsally and a more “U” shape ventrally. Calibration bar = 250 μm. (For B/W version, see page 7 in the volume.)
Plate 1.4.
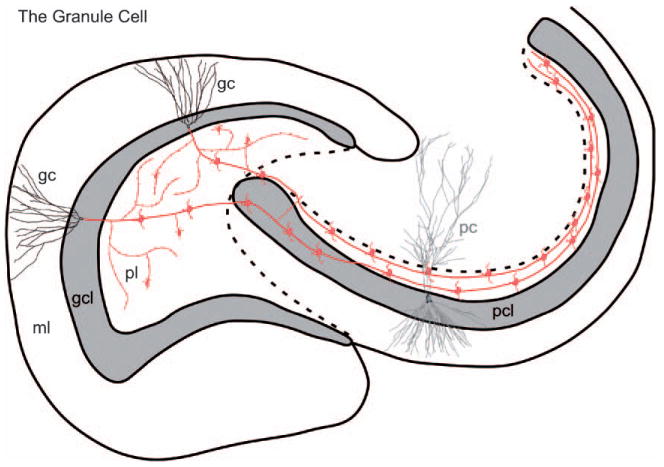
The dentate granule cell. The characteristic features of the dentate granule cell are illustrated, including its axonal arbor. A collateral plexus gives rise to numerous (∼200) typical synapses on cells located within the polymorphic layer. Most of these synapses are onto the dendrites of inhibitory interneurons. Some of the large mossy fiber expansions are also distributed in the polymorphic layer. Many of these terminate on the proximal dendrites of mossy cells. The mossy fiber axons ultimately enter the CA3 field where they travel through the full transverse extent of the field. On their course, they terminate with mossy fiber expansions on a small number (15–20) of CA3 pyramidal cells. Additional abbreviations: gc, granule cell; pc, pyramidal cell. (For B/W version, see page 8 in the volume.)
Plate 1.6.
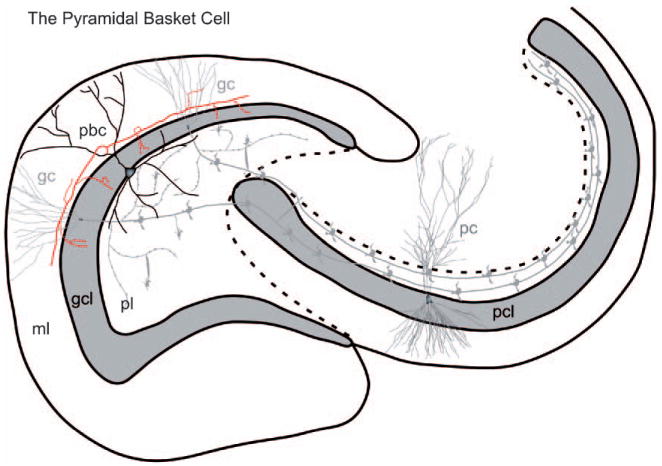
The pyramidal basket cell. The cell body of the pyramidal basket cell is located at the interface between the granule cell layer and the polymorphic cell layer. The axon (arrow) emerges from the apical dendrite. Collaterals of this axon form a curtain of terminals that synapse with the granule cell bodies. Additional abbreviations: pbc, pyramidal basket cell. (For B/W version, see page 12 in the volume.)
Plate 1.8.
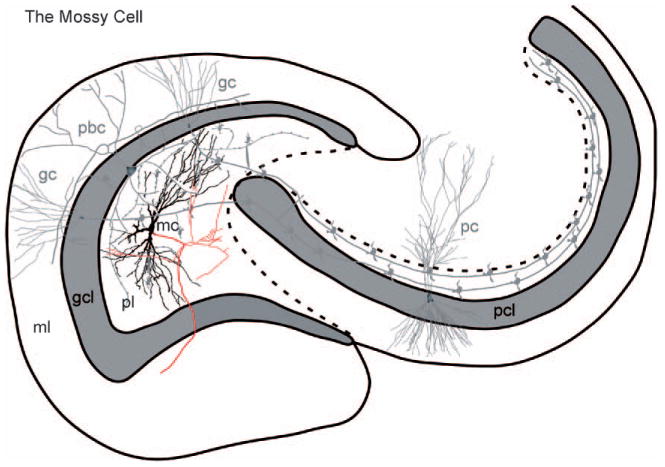
The mossy cell. A line drawing of a mossy cell (mc) in the polymorphic layer. The axon (arrow) develops a plexus within the polymorphic layer, and also an ipsilateral projection to the inner molecular layer, known as the associational pathway. The main axon also projects contralaterally to the inner molecular layer, forming the commissural pathway. The ipsilateral projection increases in density with distance from the cell body of origin. (For B/W version, see page 13 in the volume.)
References
- Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Lavenex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; New York: 2007. p. 872. [Google Scholar]
- Amaral DG, Witter MP. The three dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Bakst I, Avendaño C, Morrison JH, Amaral DG. An experimental analysis of the origins of the somatostatin immunoreactive fibers in the dentate gyrus of the rat. J Neurosci. 1986;6:1452–1462. doi: 10.1523/JNEUROSCI.06-05-01452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstad TW, Brink K, Hem J, Jeune B. Distribution of hippocampal mossy fibers in the rat. An experimental study with silver impregnation methods. J Comp Neurol. 1970;138:433–450. doi: 10.1002/cne.901380404. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Leranth C. Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats — a species difference between rats and monkeys. Exp Brain Res. 1997;115:369–374. doi: 10.1007/pl00005706. [DOI] [PubMed] [Google Scholar]
- Boyett JM, Buckmaster PS. Somatostatin-immunoreactive interneurons contribute to lateral inhibitory circuits in the dentate gyrus of control and epileptic rats. Hippocampus. 2001;11:418–422. doi: 10.1002/hipo.1056. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Amaral DG. Intracellular recording and labeling of mossy cells and proximal CA3 pyramidal cells in macaque monkeys. J Comp Neurol. 2001;430:264–281. doi: 10.1002/1096-9861(20010205)430:2<264::aid-cne1030>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Strowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992;2:349–362. doi: 10.1002/hipo.450020403. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Wenzel HJ, Kunkel DD, Schwartzkroin PA. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J Comp Neurol. 1996;366:271–292. doi: 10.1002/(sici)1096-9861(19960304)366:2<270::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A quantitative three-dimensional analysis of granule cell dendrites in the rat dentate gyrus. J Comp Neurol. 1990;302:206–219. doi: 10.1002/cne.903020203. [DOI] [PubMed] [Google Scholar]
- Conrad LCA, Leonard CM, Pfaff DW. Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1974;156:179–206. doi: 10.1002/cne.901560205. [DOI] [PubMed] [Google Scholar]
- Deller T, Martinez A, Nitsch R, Frotscher M. A novel entorhinal projection to the rat dentate gyrus — direct innervation of proximal dendrites and cell bodies of granule cells and gabaergic neurons. J Neurosci. 1996;16:3322–3333. doi: 10.1523/JNEUROSCI.16-10-03322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JA, Galvin NJ, Stanfield BB, Cowan WM. The mode of termination of the hypothalamic projection to the dentate gyrus: An EM autoradiographic study. Brain Res. 1983;258:1–10. doi: 10.1016/0006-8993(83)91220-9. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Granule cell dendritic spine density in the rat hippocampus varies with spine shape and location. Neurosci Lett. 1985;54:219–224. doi: 10.1016/s0304-3940(85)80082-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. Review. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi. II. Experimental analysis of fiber distribution with silver impregnation methods. J Comp Neurol. 1978a;178:73–88. doi: 10.1002/cne.901780105. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi. I. Terminal area related to number of granule and pyramidal cells. J Comp Neurol. 1978b;178:49–72. doi: 10.1002/cne.901780104. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. The hippocampal mossy fiber system of the rat studied with retrograde tracing techniques. Correlation between topographic organization and neurogenetic gradients. J Comp Neurol. 1981;203:717–735. doi: 10.1002/cne.902030409. [DOI] [PubMed] [Google Scholar]
- Han ZS, Buhl EH, Lorinczi Z, Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A, Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972;144:215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csaki A, Bokor H, Shanabrough M, Leranth C. The supramammillo-hippocampal and supramammillo-septal glutamatergic/aspartatergic projections in the rat: a combined. Neuroscience. 2000;97:657–669. doi: 10.1016/s0306-4522(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol. 1985;236:504–522. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- Köhler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Laurberg S, Sorensen KE. Associational and commissural collaterals of neurons in the hippocampal formation (hilus fasciae dentate and subfield CA3) Brain Res. 1981;212:287–300. doi: 10.1016/0006-8993(81)90463-7. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Lübke J, Deller T, Frotscher M. Septal innervation of mossy cells in the hilus of the rat dentate gyrus — an anterograde tracing and intracellular labeling study. Exp Brain Res. 1997;114:423–432. doi: 10.1007/pl00005651. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Acsady L, Freund TF. Principal cells are the postsynaptic targets of supramammillary afferents in the hippocampus of the rat. Hippocampus. 1994;4:322–334. doi: 10.1002/hipo.450040316. [DOI] [PubMed] [Google Scholar]
- McLardy T, Kilmer WL. Hippocampal circuitry. Am Psychol. 1970;25:563–566. doi: 10.1037//0003-066x.25.6.563. [DOI] [PubMed] [Google Scholar]
- Moore RY, Halaris AE. Hippocampal innervation by serotonin neurons of the midbrain raphe in the rat. J Comp Neurol. 1975;164:171–184. doi: 10.1002/cne.901640203. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Benoit R, Magistretti PJ, Ling N, Bloom FE. Immunohistochemical distribution of prosomatostatin-related peptides in hippocampus. Neurosci Lett. 1982;34:137–142. doi: 10.1016/0304-3940(82)90165-3. [DOI] [PubMed] [Google Scholar]
- Mosko S, Lynch G, Cotman CW. The distribution of septal projections to the hippocampus of the rat. J Comp Neurol. 1973;152:163–174. doi: 10.1002/cne.901520204. [DOI] [PubMed] [Google Scholar]
- Nafstad PHJ. An electron microscope study on the termination of the perforant path fibres in the hippocampus and the fascia dentata. Zeitsch Zellforsch Mikrosk Anat. 1967;76:532–542. doi: 10.1007/BF00339754. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Segal M, Bloom FE. A radioautographic study of the efferent pathways of the nucleus locus coeruleus. J Comp Neurol. 1974;155:15–42. doi: 10.1002/cne.901550103. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Estructura del asta de Ammon y fascia dentata. Ann Soc Esp Hist Nat. 1893;22 [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE. Local circuitry of GABAergic basket cells in the dentate gyrus. Epilepsy Res Suppl. 1992;7:29–47. [PubMed] [Google Scholar]
- Ribak CE, Seress L. Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J Neurocytol. 1983;12:577–597. doi: 10.1007/BF01181525. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Peterson GM, Seroogy KB, Fallon JH, Schmued LC. A GABAergic inhibitory component within the hippocampal commissural pathway. J Neurosci. 1986;6:3492–3498. doi: 10.1523/JNEUROSCI.06-12-03492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Vaughn JE, Saito K. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978;140:315–332. doi: 10.1016/0006-8993(78)90463-8. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Evidence from simultaneous intracellular recordings in rat hippocampal slices that area CA3 pyramidal cells innervate dentate hilar mossy cells. J Neurophysiol. 1994;72:2167–2180. doi: 10.1152/jn.1994.72.5.2167. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J Neurophysiol. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. [DOI] [PubMed] [Google Scholar]
- Seress L, Mrzljak L. Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations. Brain Res. 1987;405:169–174. doi: 10.1016/0006-8993(87)91003-1. [DOI] [PubMed] [Google Scholar]
- Seress L, Pokorny J. Structure of the granular layer of the rat dentate gyrus. A light microscopic and Golgi study. J Anat. 1981;133:181–195. [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Buzsaki G. Interneurons in the hippocampal dentate gyrus — an in vivo intracellular study. Eur J Neurosci. 1997;9:573–588. doi: 10.1111/j.1460-9568.1997.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Soriano E, Frotscher M. A GABAergic axo-axonic cell in the fascia dentata controls the main excitatory hippocampal pathway. Brain Res. 1989;503:170–174. doi: 10.1016/0006-8993(89)91722-8. [DOI] [PubMed] [Google Scholar]
- Soriano E, Frotscher M. Mossy cells of the rat fascia dentata are glutamate-immunoreactive. Hippocampus. 1994;4:65–69. doi: 10.1002/hipo.450040108. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Struble RG, Desmond NL, Levy WB. Anatomical evidence for interlamellar inhibition in the fascia dentata. Brain Res. 1978;152:580–585. doi: 10.1016/0006-8993(78)91113-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The anatomical organization of septo-hippocampal projections. In: Gray A, editor. Functions of the Septo-Hippocampal System. Elsevier North Holland; Amsterdam: 1978. pp. 25–48. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–716. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Projection of the entorhinal layer-II neurons in the rat as revealed by intracellular pressure-injection of neurobiotin. Hippocampus. 1993;3:471–480. doi: 10.1002/hipo.450030408. [DOI] [PubMed] [Google Scholar]
- Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1993;326:595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. Review. [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Do mossy fibers release GABA? Epilepsia. 2002;43:196–202. doi: 10.1046/j.1528-1157.43.s.5.6.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Swanson LW, Cowan WM. Evidence for an input to the molecular layer and the stratum granulosum of the dentate gyrus from the supramammillary region of the hypothalamus. Anat Embryol. 1979;156:165–176. doi: 10.1007/BF00300012. [DOI] [PubMed] [Google Scholar]


