Abstract
Objective
Sleep problems are a cardinal symptom of depression in children and adolescents and caffeine use is a prevalent and problematic issue in youth; yet little is known about caffeine use and its effects on sleep in youth with depression. We examined caffeine use and its relation to sleep and affect in youth’s natural environments.
Methods
Thirty youth with major depressive disorder (MDD) and 23 control youth reported on caffeine use, sleep, and affect in their natural environment using ecological momentary assessment at baseline and over 8 weeks, while MDD youth received treatment.
Results
Youth with MDD reported more caffeine use and sleep problems relative to healthy youth. Youth with MDD reported more anxiety on days they consumed caffeine. Caffeine use among youth with MDD decreased across treatment, but sleep complaints remained elevated.
Conclusions
Findings suggest that both sleep quality and caffeine use are altered in pediatric depression; that caffeine use, but not sleep problems, improves with treatment; and that caffeine may exacerbate daily anxiety among youth with depression.
Keywords: caffeine, depression, ecological momentary assessment, sleep
Caffeine is the most widely consumed stimulant in the US and perhaps the world (Barone & Roberts, 1996). In adults, caffeine can affect arousal (Barry et al., 2005; Lyvers, Brooks, & Matica, 2004), attention (Lorist & Tops, 2003; Yeomans, Ripley, Davies, Rusted, & Rogers, 2002), reaction time (Childs & deWit, 2006; Kenemans & Lorist, 1995), and sleep (for a review, see Boutrel & Koob, 2004; Drapeau et al., 2006). Those same effects on youth, however, have received little empirical study (for a review, see Hughes & Hale, 1998). Work in this area is a crucial undertaking, given that many youth use caffeine daily and caffeine use is associated with poor sleep and daytime fatigue (National Sleep Foundation, 2006; Rapoport, Berg, Ismond, Zahn, & Neims, 1984; Rapoport, Elkins, Neims, Zahn, & Berg, 1981). Understanding caffeine’s effects on sleep is particularly important in clinical disorders, such as depression, in which sleep difficulties are important features. The aim of the current study was to examine relationships between caffeine use and sleep in healthy and depressed youth using a natural approach to gather real-time information on how youth sleep and utilize caffeine in their daily lives.
Epidemiological work suggests that caffeine use in youth is worthy of empirical attention. 75–98% of youth consume at least one caffeinated beverage daily (Morgan, Stults, & Zabnick, 1982; National Sleep Foundation, 2006), with 31% reporting more than two per day (National Sleep Foundation, 2006). These rates approach the levels consumed by adults (Hughes & Oliveto, 1997). The subjective effects of high caffeine doses on youth are similar to those found in adults, such as nervousness and nausea (for a review, see Hughes & Hale, 1998). Behaviorally, caffeine use in youth has also been shown to improve performance on attention-related tasks. Children show improved performance and decreased self-reported “sluggishness” following moderate levels of caffeine consumption (Bernstein et al., 1994). On the other hand, when children who are regular caffeine users are asked to abstain, they report higher levels of negative affect (Goldstein & Wallace, 1997) and show decreased reaction times (Bernstein et al., 1998), suggesting those complex cycles of caffeine dependence can be set into motion even in childhood and adolescence.
Although less widely researched, caffeine may also play a cyclical role in affect regulation. Caffeine can contribute to arousal, anxiety, and irritability, thus exacerbating negative affect states (Brice & Smith, 2002; Childs & deWit, 2006; Smith, Sutherland, & Christopher, 2005). On the other hand, individuals may attempt to use caffeine as an affect regulator, much as they use other stimulants, such as cigarettes. Caffeine is a widely available, heavily marketed, and socially acceptable stimulant, even in child and adolescent populations. Caffeine may be particularly appealing to depressed youth seeking a “lift” due to fatigue or negative affect. In support of this speculation, self-reported anxious and depressive symptoms have been found to be elevated in adolescents with caffeine dependence (Bernstein et al., 1994; Bernstein, Carroll, Thuras, Cosgrove, & Roth, 2002). To address this question in our sample, we examined whether youth with depression used more caffeine than healthy controls and whether their caffeine use was associated with daily fluctuations in affect.
Caffeine use may have an important association with sleep quality. There is also evidence that, like adults (National Sleep Foundation, 2001), youth use caffeine to counteract daytime sleepiness. Caffeine use in youth tends to increase after Wednesday, peak on Saturday, and then decline (Pollack & Bright, 2003). In fact, adoles-cents who drank two or more caffeinated beverages a day were more likely to report an insufficient amount of sleep on school nights, a self-described sleep disturbance, and problems related to drowsiness, than those who drank one or less (National Sleep Foundation, 2006). In addition, children who were heavy caffeine users reported an increase in sleep disruption following a day of caffeine consumption (Pollack & Bright, 2003). This finding demonstrates the potential for caffeine consumption to contribute to cycles of sleep disruption in youth.
The second focus of our study was on youth’s sleep behaviors in the natural context. Youth with major depressive disorder (MDD) frequently complain of sleep disturbances, regardless of caffeine use (Bertocci et al., 2005; Ryan et al., 1987). A large body of literature implicates sleep dysregulation in adult depression, with several studies suggesting that sleep difficulties precede the onset of depressive disorders (for a review, see Riemann & Voderholzer, 2003). Sleep complaints are extremely common in children and adolescents with MDD, with as many as 90% reporting significant sleep problems (Ryan et al., 1987). Reported sleep problems have included hypersomnia, nighttime awakenings, daytime sleepiness, and circadian reversal (Dahl et al., 1996). In a previous study, our group found that children and adolescents with depression, compared to controls, reported significantly worse subjective sleep in terms of sleep quality, number of awakenings, minutes awake, and ease of waking (Bertocci et al., 2005). The current study extends this work by examining group differences in subjective sleep behaviors in the natural environments of healthy and depressed youth over several months, as well as how these sleep behaviors are related to caffeine consumption.
A final, more exploratory goal was to examine whether subjective sleep and caffeine use change across the course of treatment for youth with depression. Although caffeine consumption is not specifically targeted in treatments for depression, it may change as participants stabilize and normalize their affect states and daily activities as a function of treatment via medication or psychosocial therapy. Alternatively, more specific treatments (or adjunctive treatment) addressing these behaviors may need to be developed. To the extent that sleep and caffeine behaviors are altered in pediatric depression, it will be important to understand whether standard treatments for these disorders impact these behaviors. This study represents a preliminary step toward addressing this question.
To address these questions, we utilized Ecological Momentary Assessment (EMA) to objectively measure affect, behavior, and caffeine use in the home environment. EMA is an ecologically valid method of gathering representative real-time data on affect and behavior in natural environments through the use of signaling devices (Axelson et al., 2003; Larson, Csikszentmihalyi, & Graef, 1980; Shiffman et al., 2006; Silk, Steinberg, & Morris, 2003). EMA can provide more accurate and objective data on day-to-day shifts in caffeine consumption and sleep, but has not been applied to examining these behaviors in youth with depression. In fact, most studies have relied on retrospective reports of caffeine intake and sleep habits—methods limited by memory biases.
In summary, this study builds on previous research to address four questions: (a) Do youth with depression use more caffeine in their daily lives than healthy youth?; (b) Do youth with depression report poorer sleep in their daily lives than healthy youth?; (c) How is daily caffeine use related to sleep and affect?; and (d) Do sleep and caffeine use change as youth with MDD go through treatment? We hypothesized that youth with MDD would report greater caffeine use and subjective sleep problems than healthy youth, that caffeine use would be associated with greater sleep problems that night and greater negative affect that day, especially for youth with MDD, and that both sleep and caffeine use would improve throughout treatment.
Method
Participants
This report includes data from 53 youth participating in a longitudinal clinical assessment study of neurobehavioral factors in pediatric affective disorder (Birmaher et al., 2000). Participants (34 females) ranged in age from 7–17 years (M=12.44, SD=2.88). Participants were divided into two groups based on current psychiatric diagnoses: MDD n=30; and healthy controls n=23. Sixty-three percent of participants with depression had a current comorbid anxiety disorder (Separation Anxiety Disorder, Generalized Anxiety Disorder, or Social Phobia) and 43% had a current comorbid behavioral disorder (Conduct Disorder, Oppositional Defiant Disorder, or Attention-deficit Hyperactivity Disorder). The retention rate was ~70% and there were no demographic or clinical differences between subjects retained and not retained in the study (Birmaher et al., 2004).
Inclusion Criteria
Youth with MDD met diagnostic criteria according to DSM-III-R (American Psychiatric Association, 1987) or DSM-IV (American Psychiatric Association, 1994) classification. All participants diagnosed with a psychiatric disorder received an 8-week treatment course consisting of Selective Serotonin Reuptake Inhibitors (SSRI’s; n=9) and/or Cognitive Behavioral Therapy (CBT; n=8), or both (n=13). The SSRI’s included citalopram 10–40 mg (n=8), escitalopram 5 mg (n=1), and fluoxetine 5–25 mg (n=10). Medication data were missing for three subjects.
Healthy control youth were required to be free of any lifetime psychopathology. In addition, they were required to have no first-degree relatives with a lifetime episode of any mood or psychotic disorder; no second-degree relatives with a lifetime history of childhood-onset, recurrent, psychotic, or bipolar depression or schizoaffective or schizophrenic disorder; and no >20% of second-degree relatives could have a lifetime episode of MDD.
Exclusion Criteria
Since the youth in this study were originally recruited to participate in a broad set of biological protocols including hormonal challenge probes and sleep electroencephalogram (Birmaher et al., 2000, 2004), the following exclusionary criteria applied at the time of the initial interview: (a) the use of any medication with central nervous system effects within the past 2 weeks or any lifetime use of fluoxetine (no subjects were taking serotonin reuptake inhibitors, stimulants, or other anti-depressant medications); (b) significant medical illness; (c) extreme obesity (weight >150% of ideal body weight) or growth failure (height or weight below the third percentile); (d) IQ of 70 or less; (e) inordinate fear of intravenous needles (because of the need to draw blood for biological assays); and (f) specific learning disabilities. Subjects with depression were also excluded if they had schizophrenic, schizoaffective, and bipolar disorders.
Procedures
The study was approved by the university’s Institutional Review Board. Participants were recruited from three sources: (a) community advertisements (primarily radio and newspaper ads), (b) inpatient and outpatient clinics at a major medical center in which the youth or their parents were being treated, and (c) referrals from other research studies or other participants in the present study. Youth and their parents were required to sign assents and informed consents, respectively. Structured diagnostic interviews were administered to establish lifetime and present youth psychiatric diagnoses and familial history of affective disorder. Qualifying participants were invited to participate in a multifaceted protocol that included: (a) for participants with MDD, an 8-week open treatment protocol using CBT and/or SSRI’s; (b) for all participants, a visit to the neurobehavioral laboratory during the baseline weekend of the study (Forbes et al., 2006; Ladouceur, et al., 2005); and (c) also for all participants, a home assessment protocol that included EMA and measures of sleep in the natural environment collected in biweekly intervals over the 8-week course of the study. The focus of this report is on data collected through the home assessment protocol.
Instruments
Structured Diagnostic Interviews
Each youth and his or her parent(s) were interviewed to determine the youth’s psychiatric history using the Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime version (K-SADS-PL, Kaufmann, Birmaher, Brent, & Rao, 1997). Parents and youth were interviewed separately, with clinical interviewers integrating data from both informants to arrive at a final diagnosis. To determine familial loading for mood disorders, parents were interviewed using the Structured Clinical Interview for the DSM-IV (Spitzer, Williams, Gibbon, & First, 1990). Other adult first-degree and second-degree relatives were assessed indirectly using a modified version of the Family History Interview (Weissman et al., 1986), with the youth’s parent(s) and other available relatives serving as informant(s). All interviews were carried out by trained BA- and MA-level research clinicians. Inter-rater reliabilities for diagnoses assessed during the course of this study were estimated to be k ≥0.70. The results of the interview were presented at a consensus case conference with a child psychiatrist, who reviewed the findings and preliminary diagnosis and provided a final diagnosis based on DSM-III-R or DSM-IV criteria.
Subjective Sleep Ratings
All youth completed one subjective sleep report each day collected in biweekly intervals for five extended weekends (Friday through Monday) beginning at baseline and across the 8-week treatment period (n=20 per participant). The subjective sleep reports included the participant’s estimates of (a) sleep quality (the level of restfulness the youth felt upon awakening), (b) ease of waking (the level of difficulty the youth had waking up), (c) the number of minutes to fall asleep, (d) the number of nighttime awakenings, (e) the number of minutes awake during the night, (f) bedtime, (g) total sleep time, and (h) morning wake time (Bertocci et al., 2005). For some analyses, weekend totals were created by averaging responses that occurred during each day of the assessment weekends.
Ecological Momentary Assessment
As part of a larger study, all participants completed an EMA protocol designed to provide real-time data on behavior, emotion, and social context in the child’s natural environment. Participants were given answer-only cellular phones on which they received calls from a trained staff member for five extended weekends beginning at baseline and across an the 8-week treatment period (Axelson et al., 2003). Participants were called 12 times between 4 p.m. Friday and 10 p.m. Monday each weekend, for a total of 60 calls in 8 weeks. Participants received two calls on Friday and Monday and four calls on Saturday and Sunday. Each call consisted of a brief structured interview to evaluate current behavior, affect, and social context. The present report focuses on affect ratings and caffeine consumption from the calls obtained during each extended weekend. At each call, participants were asked to rate their current affect on a subset of 5-point scales from the Positive and Negative Affect Schedule for Children (PANAS-C; Laurent et al., 1999). Ratings were obtained for four negative emotions (“sad,” “angry,” “nervous,” and “upset”). During the last call of each day, participants were asked, “Have you had any caffeine today?” followed by “How many servings of caffeine did you have?” For some analyses, weekend totals were created by averaging responses that occurred during each day of the assessment weekends.
Plan of Analyses
Data were analyzed using repeated measures linear mixed effects models to account for the nesting of assessments within subjects and across time. Because data on sleep and caffeine use were collected at one call per day, data were analyzed at the level of day rather than call. Data on affect were averaged across the 2–4 sampling points per day to create corresponding measures of daily affect. All mixed effects models included subject as a random effect and day as a repeated measure. Fixed effects were included for week (0, 2, 4, 6, or 8), diagnostic group, caffeine use, and/or subjective sleep, depending upon the specific hypothesis tested, as described subsequently. Preliminary analyses indicated that there were no significant differences in age (t[51]=−1.74; NS) or gender across diagnostic groups, therefore these variables were not included as covariates in the mixed models. Effect sizes for primary analyses were calculated using Effect Size Generator-Pro (Devilly, 2005).
Results
Caffeine Consumption
Independent samples t-tests revealed no gender differences in caffeine consumption (t[63]=0.66; NS; 95% confidence interval [CI]=−2.52–4.98); age was correlated with caffeine consumption (r=0.34, p<.01). Thus, age was included as a covariate in subsequent analyses.
A mixed effects model was computed examining the relationship between the numbers of caffeinated beverages consumed per day, diagnostic group, and week in the study. We also examined the interaction between diagnosis and week to test for treatment related changes in caffeine use, as only the group with MDD would be expected to show changes over time since the control group was not enrolled in any form of treatment throughout the study. This analysis revealed a main effect for group (F(1,504)=14.12; p<.001; 95% CI=−.45 to .01; d=1.06) indicating that youth with depression consumed greater amounts of caffeine per day across the study than healthy controls (Table I). To determine whether this main effect was driven by comorbid anxiety disorders in the sample, we computed a mixed effects model that added anxiety as a covariate. This analysis revealed a main effect for anxiety (F(1,208)=15.27; p<.001; 95% CI=.01 to .02; d=1.1) indicating that youth with depression and comorbid anxiety disorders consumed greater amounts of caffeine per day across the study than those without comorbid anxiety disorders.
Table I.
Mean Reports of Sleep and Caffeine Use in the Natural Environment by Diagnostic Group at Baseline and Week 8
| MDD |
Low-risk |
|||
|---|---|---|---|---|
| Baseline | Week 8 | Baseline | Week 8 | |
| Caffeine use | ||||
| M | 5.33 | 1.63 | 1.22 | 0.52 |
| SD | 10.47 | 3.77 | 1.86 | 0.99 |
| Sleep quality | ||||
| M | 60.06 | 67.24 | 77.00 | 80.94 |
| SD | 22.59 | 21.32 | 16.98 | 17.78 |
| Difficulty waking | ||||
| M | 51.55 | 61.43 | 70.73 | 70.63 |
| SD | 24.72 | 23.28 | 20.49 | 21.09 |
| Nighttime awakenings | ||||
| M | 1.51 | 4.10 | 0.38 | 2.11 |
| SD | 1.27 | 4.77 | 0.45 | 2.83 |
| Minutes to sleep | ||||
| M | 33.03 | 21.26 | 12.27 | 9.89 |
| SD | 46.31 | 21.21 | 8.80 | 9.31 |
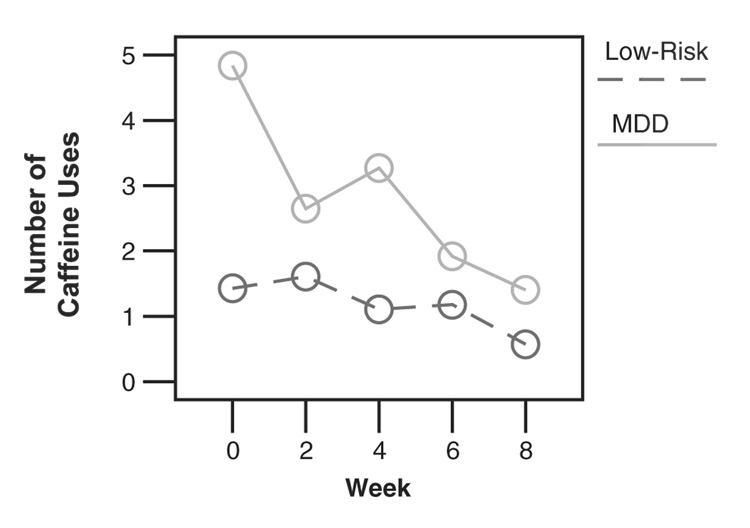
There was also an interaction between group and week (F(4, 236)=2.56; p<.05; 95% CI=Week 0 −.52 to .28, Week 1 −.20 to .46, Week 3 −.60 to .08, Week 5 −.08 to .52) in predicting caffeine use. To interpret this interaction, we conducted mixed effects models predicting caffeine use from week separately for each diagnostic group. This analysis indicated that week in the study was a predictor of caffeine use for youth with MDD (F(1, 157)=11.85; p<.001; 95% CI=Week 0 −.09 to .56, Week 1 −.23 to .29, Week 3.04 to .59, Week 5 −.35 to .10; d=.55) but not for controls (F(1, 43)=2.12; NS; 95% CI=Week 0.02 to .30, Week 1.06 to .36, Week 3 −.05 to .19, Week 5 −.02 to .26; d=.44). To examine the direction of this effect, we plotted mean caffeine use across each weekend in the study separately for each diagnostic group. As shown in Fig. 1, caffeine use decreased across the 8-week treatment protocol for youth with MDD, but not controls. There were no significant differences in posttreatment caffeine consumption among youth with MDD receiving CBT, SSRI, or CBT+SSRI treatment (F [2, 27]=0.27; NS; 95% CI=0.22 to 3.04).
Figure 1.
Changes in caffeine use across treatment.
Subjective Sleep Reports
A series of mixed effects models were computed predicting subjective ratings of sleep quality from diagnostic group, week in the study, and the interaction between diagnosis and week in the study. These analyses revealed main effects for group indicating that youth with MDD reported taking longer to fall asleep (F(1, 548)=56.42; p<.001; 95% CI=−17.22 to −4.94; d=.64), more nighttime awakenings (F(1, 569)=64.13; p<.001; 95% CI=−.75 to −.20; d=.75, more difficulty waking up (F(1, 765)=29.97; p<.001; 95% CI=1.22 to 19.71; d=.51) and a lower rating of overall subjective sleep quality (F(1, 682)=51.12; p<.001; 95% CI=.69 to 17.88; d=.67) than healthy controls. These analyses also revealed a general trend for subjective ratings of sleep to improve across the course of this study, with main effects of week for time to fall asleep (F(4, 326)=3.49; p<.01; 95% CI=Week 0 −0.43 to 16.66, Week 1 6.24 to 22.48, Week 3 −6.12 to 4.98, Week 5 −8.19 to 2.89; d=.53), nighttime awakenings (F(4, 255)=4.92; p<.001; 95% CI=Week 0.37 to .99, Week 1.13 to .72, Week 3 −.22 to .34, Week 5 −.36 to .31; d=.63), and overall subjective sleep quality (F(4, 263)=3.71; p<.01; 95% CI=Week 0 −11.96 to 3.69, Week 1 −15.53 to −.15, Week 3 −3.54 to 10.82, Week 5 −6.33 to 8.99; d=.54) (Table I). To determine whether these main effects were driven by comorbid anxiety disorders in the sample, we computed mixed effects models that added anxiety as a covariate. These analyses revealed a main effect for anxiety indicating that depressed youth with comorbid anxiety disorders reported more nighttime awakenings (F(1,307)=6.64; p<.05; 95% CI=.00 to .02; d=.73), more difficulty waking up (F(1, 385)=8.23; p<.01; 95% CI=.08 to .45; d=.81), and a lower rating of overall subjective sleep quality (F(1, 373)=10.95; p<.001; 95% CI=.11 to .42; d=.93) across the study than those without comorbid anxiety disorders.
However, there were no weeks by diagnosis interactions predicting any of the sleep variables, suggesting that sleep did not improve as a function of treatment for depression. Furthermore, t-tests conducted on sleep variables aggregated across the posttreatment weekend indicated that at the end of treatment, youth with MDD reported greater minutes to sleep (t[29]=−2.29; p<.05; 95% CI=−21.51 to −1.23; d=.65) and lower sleep ratings (t[44]=2.34; p<.05; 95% CI=1.89 to 25.51; d=.66) than healthy youth. There were no significant differences among youth with MDD receiving CBT, SSRI, or CBT+SSRI treatment in posttreatment subjective sleep rating (F[2, 22]=0.32; NS; 95% CI=58.44 to 76.04), minutes to sleep (F[2, 19]=3.55; p=.05; 95% CI=11.86 to 30.66), times awake (F[2, 17]=0.57; NS; 95% CI=1.87 to 6.33), and difficulty waking (F[2, 20]=0.62; NS; 95% CI=51.37 to 71.50).
Relationships Between Caffeine Use and Sleep
Next, we examined the relationship between youth’s caffeine use and subjective ratings of their sleep in the natural environment. Because of the potential bidirectional relationships between sleep and caffeine use, we tested two sets of lagged linear mixed effects models: (a) subjective sleep predicting caffeine use the next day, and (b) caffeine use during the day predicting that night’s sleep ratings. Mixed models included fixed effects for diagnostic group, sleep or caffeine use, and the interaction between the two. There were no significant main effects or interactions in any of the models testing whether subjective sleep predicted caffeine use the next day (all p’s >.05). There were also no main effects or interactions in the models testing whether caffeine use during the day predicted that night’s sleep ratings (all p’s>.05), with the exception of a trend for greater caffeine use during the day to predict more nighttime awakenings that night (F(1, 350)=3.23; p=.07; 95% CI=−.12 to .00; d=.19).
Relationships Between Caffeine Use and Negative Affect
Finally, we examined whether caffeine use was associated with youth’s negative affect. Separate models were computed for each of the four negative affect scales: “sadness,” “anger,” “nervous,” and “upset.” Mixed effects models included main effects for number of caffeinated beverages and diagnostic group as well as the interaction between caffeine consumption and diagnostic group predicting mean levels of negative affect across the day. Caffeine use was not related to sadness, anger or feeling upset (all p’s >.05), however, there was an interaction between diagnostic group and caffeine use in predicting youth’s feelings of nervousness (F(1, 638)=6.02; p<.05; 95% CI=−.12 to −.01; d=.69). To interpret this interaction, we conducted mixed effects models predicting nervousness from caffeine use separately for each diagnostic group. This analysis indicated that daily caffeine use was positively associated with daily nervousness for youth with MDD (coefficient=.03, t[160]=2.99, p<.01; 95% CI=.01 to .04) but not for controls (coefficient=−.02, t[46]=−1.56, p=.13; 95% CI=−.04 to .01).
Discussion
This is the first study of which we are aware to assess both caffeine use and sleep in the natural environments of youth with depression. We found significant differences between healthy and depressed youth in caffeine use and sleep during the baseline weekend before the youth with depression received therapy and/or medication. These differences in caffeine use diminished during the course of treatment. Even though the youth were not explicitly told to abstain from caffeine during treatment, those with MDD experienced a 4-fold decrease in caffeine consumption across treatment. However, daily sleep did not improve as a function of treatment for depression.
The finding that youth with depression used more caffeine than healthy controls at baseline suggests that youth with MDD may use caffeine to help treat symptoms of depression. This is especially interesting given that these differences were found before the youth with MDD began therapy and/or medication. Youth with depression often lack energy and complain of chronic tiredness. These youth may self-medicate with caffeine to increase alertness (Goldstein, Kaizer, & Whitby, 1969; Rapoport et al., 1984). The stimulating effect of caffeine is necessarily followed by a period of withdrawal and return to the original state of low energy, and many youth counter these effects by consuming more caffeine (Goldstein, 1987). This cycle may contribute to increased negative affect and depressive symptoms, particularly during the withdrawal period.
However, contrary to our hypotheses, caffeine use and sleep were not directly related to each other. We found that youth who used more caffeine did not report more trouble sleeping that night, with the exception of a trend for youth who used more caffeine to report more awakenings that night. Surprisingly, youth who had more trouble sleeping did not report using more caffeine the next day. This finding suggests that the youth in our sample were not using caffeine to combat sleepiness specifically associated with poor sleep the previous night, although it is still possible that they were experiencing generalized fatigue and low energy associated with depression. It is also possible that the timing of caffeine use may impact sleep differently. For example, caffeine use in the evening may be more related to sleep difficulties than caffeine use in the morning. This should be assessed in future studies.
Another possibility is that youth with depression were attempting to utilize caffeine as an affect regulator. The finding that, among youth with MDD only, caffeine use was associated with greater nervousness on the same day supports the suggestion that caffeine plays a role in the regulation of anxiety for youth with MDD. Unfortunately, because we only assessed caffeine use once a day, we are not able to disentangle whether nervous affect led to greater use of caffeine, or whether greater use of caffeine led to greater nervousness among youth with depression. In fact, it is likely that bidirectional relationships exist between caffeine use and nervousness in youth with depression that can lead to a spiraling of irritability and anxious arousal. It seems that comorbid anxiety disorders in the youth with MDD were driving the overall effect of diagnosis. This further supports our speculation that caffeine may be used as an affect regulator. In addition, healthy youth with higher levels of anxiety may consume higher levels of caffeine. It will be important for future studies to address whether youth are using caffeine because they are anxious, or whether they are anxious because they are using caffeine.
Youth may also use caffeine for other reasons unrelated to sleep, such as fitting in with peers or attempting to increase positive emotion or arousal. To tap into youth’s motivation for using caffeine, future research should focus on the type and amount of caffeine youth use in different environments. For example, adolescents may be more likely to drink soda or coffee when socializing with friends. It would also be interesting to ask youth their reasons for choosing to use or not use caffeine at a given time, as well as to examine parents’ role in influencing their youth’s caffeine consumption.
It is important to note that we found group differences in caffeine use even though we did not select youth for the study based on a history of high caffeine consumption. Many studies examining caffeine in youth have selected samples with moderate to high levels of caffeine consumption (Bernstein et al., 1998, 2002; Orbeta, Overpeck, Ramcharren, Kogan, & Ledsky, 2006). In our study, at baseline, our healthy controls were consuming an average of one caffeinated beverage per weekend and our youth with depression were consuming an average of five per weekend. In comparison, most research on caffeine use in youth has only studied those reporting more than one drink per day or after the laboratory administration of high caffeine doses. Thus, it is possible that we would have found stronger relations between sleep and caffeine use in a sample that was selected specifically for higher rates of caffeine consumption.
It is particularly intriguing that caffeine use in youth with depression improved over the course of treatment, despite the fact that these youth were involved in heterogeneous treatments, including cognitive behavioral therapy and medication management with selective serotonin reuptake inhibitors. This decrease in caffeine use occurred naturally and was not recommended as part of either treatment course. As both type of treatments are presumed to decrease reactivity to and increase ability to cope with negative emotion, this may be one mechanism through which they also contribute to decreased caffeine consumption. Another possible mechanism is improvements in energy and motivation. Future research using larger samples and more homogeneous treatment modes are, however, needed to replicate and explore this finding.
Consistent with previous reports (Bertocci et al., 2005), the results of this study also show that youth with depression rate their sleep as more disrupted than control youth when asked to subjectively assess their sleep. This finding expands upon previous studies by showing that subjective sleep disturbances are present in the home environment across a 2-month window of time. Furthermore, we found that although all the youth in the study showed a tendency to rate their sleep as somewhat improved across the 2-month window, there was no improvement specific to being in treatment, and youth with depression still showed several elevated sleep complaints relative to healthy controls following treatment. There are several potential reasons that subjective sleep difficulties persist after treatment for depression. First, after only 8 weeks of treatment, youth in the MDD group may still be in the process of recovery and their sleep patterns may not have returned to normal. Just as it takes a while for sleep patterns to become dysregulated, it may also take a while for them to become regulated. Second, sleep problems could be trait markers that precede the development of MDD and persist after recovery (Ford & Patrick, 2001). Finally, it may be necessary to develop adjunctive sleep treatments to enhance the effectiveness of depression treatment programs in eliminating sleep problems in youth with depression.
Several limitations of the present study should be addressed. First, because the MDD sample was relatively small, we were unable to conduct comparison analyses based on the type of treatment received or type of SSRI. The majority of youth with depression in our sample were prescribed SSRI’s and we lacked statistical power to determine whether the types of SSRI’s impact sleep and caffeine use differently. Since anxiety was only assessed in youth with MDD, we were unable to determine whether higher levels of anxiety in healthy youth may impact their caffeine consumption. We included a relatively broad age range and were not able to test interactions between diagnostic group, gender, and development due to sample size limitations. Also, because this project utilized subjective reporting of sleep and caffeine use, there was no objective confirmation of caffeine intake. In addition, youth were required to make their own interpretations on what products contain caffeine, since a list of items containing caffeine was not provided. Finally, we did not collect information on the specific type of caffeine consumed (e.g., coffee vs. soda) or the exact timing of caffeine consumption, which could have differential effects on affect and sleep, and should be explored in future research.
The study also has several strengths. It utilized an innovative, intensive EMA protocol providing daily use data on caffeine consumption and its links to sleep patterns and daily affect. The study advances previous work in this area by focusing on a rigorously diagnosed clinical sample of youth with depression and utilizing an approach that provides data collected in natural home environments over an extended period of time, and throughout a course of clinical treatment. These findings have potential clinical and methodological implications, suggesting that EMA is a useful approach for understanding sleep and caffeine related-behaviors. Findings suggest that caffeine consumption may have a role in the clinical presentation of depression, and perhaps anxiety, and that it is sensitive to treatment, but that more work is needed to understand the role of treatment in improving sleep in youth with depression.
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) Grant P01 MH41712 (N.D.R., PI, R.E.D., Co-PI). We are grateful to Laura Trubnick, Michelle Bertocci, and the staff of the Child and Adolescent Neurobehavioral Laboratory for their invaluable role in assessing the participants in this study.
Footnotes
A portion of this data was presented at the Society for Research in Child Development Biennial Meeting, March–April 2007, Boston, MA, USA.
Conflict of interest: None declared.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Third edition revised (DSM-III-R) Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Axelson DA, Bertocci MA, Lewin DS, Trubnik LS, Birmaher B, Williamson DE, et al. Measuring mood and complex behavior in natural environments: Use of ecological momentary assessment in pediatric affective disorders. Journal of Child and Adolescent Psychopharmacology. 2003;13:253–266. doi: 10.1089/104454603322572589. [DOI] [PubMed] [Google Scholar]
- Barone J, Roberts H. Caffeine consumption. Food and Chemical Toxicology. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Rushby JA, Wallace MJ, Clarke AR, Johnstone SJ, Zlojutro I. Caffeine effects on resting-state arousal. Clinical Neurophysiology. 2005;116(11):2693–2700. doi: 10.1016/j.clinph.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Carroll ME, Crosby RD, Perwien AR, Go FS, Benowitz NL. Caffeine effects on learning, performance, and anxiety in normal school-age children. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:407–415. doi: 10.1097/00004583-199403000-00016. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Carroll ME, Dean NW, Crosby RD, Perwien AR, Benowitz NL. Caffeine withdrawal in normal school-age children. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:858–865. doi: 10.1097/00004583-199808000-00016. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Carroll ME, Thuras PD, Cosgrove KP, Roth ME. Caffeine dependence in teenagers. Drug and Alcohol Dependence. 2002;66:1–6. doi: 10.1016/s0376-8716(01)00181-8. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Dahl RE, Williamson DE, Iosif A, Birmaher B, Axelson D, et al. Subjective sleep complaints in pediatric depression: A controlled study and comparison with EEG measures of sleep and waking. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(11):1158–1166. doi: 10.1097/01.chi.0000179057.54419.17. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Dahl RE, Williamson DE, Perel JM, Brent DA, Axelson DA, et al. Growth hormone secretion in children and adolescents at high risk for major depressive disorder. Archives of General Psychiatry. 2000;57(9):867–872. doi: 10.1001/archpsyc.57.9.867. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Williamson DE, Dahl RE, Axelson DA, Kaufman J, Dorn LD, et al. Clinical presentation and course of depression in youth: Does onset in childhood differ from onset in adolescence? Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:63–70. doi: 10.1097/00004583-200401000-00015. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Koob GF. What keeps us awake: The neuropharmacology of stimulants and wakefulness-promoting medications. Sleep: Journal of Sleep and Sleep Disorders Research. 2004;27(6):1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- Brice CF, Smith AP. Effects of caffeine on mood and performance: A study of realistic consumption. Psychopharmacology. 2002;164(2):188–192. doi: 10.1007/s00213-002-1175-2. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Subjective, behavioral, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology. 2006;185(4):514–523. doi: 10.1007/s00213-006-0341-3. [DOI] [PubMed] [Google Scholar]
- Dahl R, Ryan N, Matty M, Birmaher B, Al-Shabbout M, Williamson D, et al. Sleep onset abnormalities in depressed adolescents. Biological Psychiatry. 1996;39:400–410. doi: 10.1016/0006-3223(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Devilly GJ. Clintools software for windows. Swinburne University, Australia: Brain Sciences Institute; 2005. [Google Scholar]
- Drapeau C, Hamel-Hebert I, Robillard R, Selmaoui B, Filipini D, Carrier J. Challenging sleep in aging: The effects of 200 mg of caffeine during the evening in young and middle-aged moderate caffeine consumers. Journal of Sleep Research. 2006;15(2):133–141. doi: 10.1111/j.1365-2869.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: An fmri study. Journal of Child Psychology and Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Patrick LC. Sleep disturbances and mood disorders: An epidemiological perspective. Depression and Anxiety. 2001;14:3–6. doi: 10.1002/da.1041. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Criteria of a pharmacologic withdrawal syndrome. Archives of General Psychiatry. 1987;44:392. doi: 10.1001/archpsyc.1987.01800160108016. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Kaizer S, Whitby O. Psychotropic effects of caffeine in man. IV. Quantitative and qualitative differences associated with habituation to coffee. Clinical Pharmacology and Therapeutics. 1969;10:489–497. doi: 10.1002/cpt1969104489. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Wallace ME. Caffeine dependence in school children? Experimental and Clinical Psychopharmacology. 1997;5:388–392. doi: 10.1037//1064-1297.5.4.388. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hale KL. Behavioral effects of caffeine and other methylxanthines on children. Experimental and Clinical Psychopharmacology. 1998;6:87–95. doi: 10.1037//1064-1297.6.1.87. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Oliveto AH. A systematic survey of caffeine intake in Vermont. Experimental and Clinical Psychopharmacology. 1997;5:393–398. doi: 10.1037//1064-1297.5.4.393. [DOI] [PubMed] [Google Scholar]
- Kaufmann J, Birmaher B, Brent D, Rao U. Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Lorist MM. Caffeine and selective visual processing. Pharmacology, Biochemistry, and Behavior. 1995;52:461–471. doi: 10.1016/0091-3057(95)00159-t. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey B. Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. Journal of Abnormal Child Psychology. 2005;33:165–177. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- Larson R, Csikszentmihalyi M, Graef R. Mood variability and the psychosocial adjustment of adolescents. Journal of Youth and Adolescence. 1980;9:469–490. doi: 10.1007/BF02089885. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE, Jr, Rudolph KD, Potter KI, Lambert S, et al. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11:326–338. [Google Scholar]
- Lorist MM, Tops M. Caffeine, fatigue, and cognition. Brain and Cognition. 2003;53:82–94. doi: 10.1016/s0278-2626(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Brooks J, Matica D. Effects of caffeine on cognitive and autonomic measures in heavy and light caffeine consumers. Australian Journal of Psychology. 2004;56:33–41. [Google Scholar]
- Morgan KJ, Stults VJ, Zabnik ME. Amount and dietary sources of caffeine and saccharin intake by individuals ages 5 to 18 years. Regulatory Toxicology and Pharmacology. 1982;2:296–307. doi: 10.1016/0273-2300(82)90003-4. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. 2001 Sleep in America Poll: Summary of findings. Washington, DC: National Sleep Foundation; 2001. [Google Scholar]
- National Sleep Foundation. 2006 Sleep in America Poll: Summary of findings. Washington, DC: National Sleep Foundation; 2006. [Google Scholar]
- Orbeta RL, Overpeck MD, Ramcharren D, Kogan MD, Ledsky R. High caffeine intake in adolescents: Associations with difficulty sleeping and feeling tired in the morning. Journal of Adolescent Health. 2006;38:451–453. doi: 10.1016/j.jadohealth.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Pollack CP, Bright D. Caffeine consumption and weekly sleep patterns in US seventh, eighth, and ninth graders. Pediatrics. 2003;111(1):42–46. doi: 10.1542/peds.111.1.42. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Berg CJ, Ismond DR, Zahn TP, Neims A. Behavioral effects of caffeine in children. Relationship between dietary choice and effects of caffeine challenge. Archives of General Psychiatry. 1984;41:1073–1079. doi: 10.1001/archpsyc.1983.01790220063010. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Elkins R, Neims A, Zahn TE, Berg CJ. Behavioral and autonomic effects of caffeine in normal boys. Developmental Pharmacology Therapy. 1981;3:74–82. doi: 10.1159/000457425. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U. Primary insomnia: A risk factor to develop depression? Journal of Affective Disorders. 2003;76:255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Ryan ND, Puig-Antich J, Ambrosini P, Rabinovich H, Robinson D, Nelson B, et al. The clinical picture of major depression in children and adolescents. Archives of General Psychiatry. 1987;44:854–861. doi: 10.1001/archpsyc.1987.01800220016003. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, et al. Natural history of nicotine withdrawal. Addiction. 2006;101(12):1822–1832. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74(6):1869–1180. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Smith A, Sutherland D, Christopher G. Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. Journal of Psychopharmacology. 2005;19(6):620–626. doi: 10.1177/0269881105056534. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. User’s guide for the structured clinical interview for DSM-III-R: SCID. Washington, DC: American Psychiatric Association; 1990. [Google Scholar]
- Weissman MM, Merikangas KR, John K, Wickramaratne P, Prusoff BA, Kidd KK. Family-genetic studies of psychiatric disorders: Developing technologies. Archives of General Psychiatry. 1986;43:1104–1116. doi: 10.1001/archpsyc.1986.01800110090012. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Ripley T, Davies LH, Rusted JM, Rogers PJ. Effects of caffeine on performance and mood depend on the level of caffeine abstinence. Psychopharmacology. 2002;164(3):241–249. doi: 10.1007/s00213-002-1204-1. [DOI] [PubMed] [Google Scholar]