Abstract
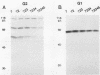
The middle (M) RNA segment of Rift Valley fever virus (RVFV) encodes four proteins: the major viral glycoproteins G2 and G1, a 14-kilodalton (kDa) protein, and a 78-kDa protein. These proteins are derived from a single large open reading frame (ORF) present in the virus-complementary M-segment mRNA. We used recombinant vaccinia viruses in which sequences representing the M-segment ORF were engineered as a surrogate system to study phlebovirus protein expression. To investigate the translational initiation codon requirements for synthesis of these proteins, we constructed a series of vaccinia virus recombinants containing specific sequence changes which eliminated select ATG codons found in the region of the ORF preceding the mature glycoprotein-coding sequences (the preglycoprotein region). Examination of phleboviral proteins synthesized in cells infected with these vaccinia virus recombinants clearly showed that the first ATG of the ORF was required for the production of the 78-kDa protein, while synthesis of the 14-kDa protein was absolutely dependent on the second in-phase ATG codon. Efficient biosynthesis of glycoprotein G2 was shown to depend on one or more ATG codons within the preglycoprotein region, but not the first one of the ORF. Synthesis of about one-half of the total glycoprotein G1 was affected by the amino acid changes that eliminated ATG codons, while production of the remainder appeared to be independent of all ATG codons in the preglycoprotein region. These data indicated that the means for glycoprotein G1 biosynthesis was distinct from those of the other three M-segment gene products. The results presented herein suggest that a surprisingly complex expression strategy is employed by the RVFV M segment. Although the full nature of the mechanisms involved in the biogenesis of the four RVFV M-segment proteins remains unclear, it does involve the use of at least two (ATG codons 1 and 2), and likely more, distinct translation start sites within the same ORF to produce its complete complement of gene products.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A., Rubin C. M., Westbrook C. A., Paskind M., Baltimore D. The first intron in the human c-abl gene is at least 200 kilobases long and is a target for translocations in chronic myelogenous leukemia. Mol Cell Biol. 1987 Sep;7(9):3231–3236. doi: 10.1128/mcb.7.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Calisher C. H., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Lvov D. K., Marshall I. D., Oker-Blom N., Pettersson R. F. Bunyaviridae. Intervirology. 1980;14(3-4):125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Pryciak P., Ganem D., Varmus H. E. Biosynthesis of the reverse transcriptase of hepatitis B viruses involves de novo translational initiation not ribosomal frameshifting. Nature. 1989 Jan 26;337(6205):364–368. doi: 10.1038/337364a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K. Forms of pp60v-src isolated from Rous sarcoma virus-transformed cells. J Virol. 1987 May;61(5):1593–1601. doi: 10.1128/jvi.61.5.1593-1601.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S. Messenger RNA of the M segment RNA of Rift Valley fever virus. Virology. 1986 May;151(1):151–156. doi: 10.1016/0042-6822(86)90114-5. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Keegan K., Frazier S., Hays W., Anderson D. K., Parker M. D., Schmaljohn C., Schmidt J., Dalrymple J. M. Complete nucleotide sequence of the M RNA segment of Rift Valley fever virus. Virology. 1985 Jul 15;144(1):228–245. doi: 10.1016/0042-6822(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M. J., Jacoby D. R., Janssen R. S., Gonzalez-Scarano F., Nathanson N. The large viral RNA segment of California serogroup bunyaviruses encodes the large viral protein. J Gen Virol. 1989 Jan;70(Pt 1):223–228. doi: 10.1099/0022-1317-70-1-223. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Patwardhan S. ACG, the initiator codon for a Sendai virus protein. J Biol Chem. 1988 Jun 25;263(18):8553–8556. [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Internal initiation of translation on the vesicular stomatitis virus phosphoprotein mRNA yields a second protein. J Virol. 1986 Jun;58(3):797–804. doi: 10.1128/jvi.58.3.797-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara T., Akashi H., Bishop D. H. Novel coding strategy (ambisense genomic RNA) revealed by sequence analyses of Punta Toro Phlebovirus S RNA. Virology. 1984 Jul 30;136(2):293–306. doi: 10.1016/0042-6822(84)90166-1. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakach L. T., Suzich J. A., Collett M. S. Rift Valley fever virus M segment: phlebovirus expression strategy and protein glycosylation. Virology. 1989 Jun;170(2):505–510. doi: 10.1016/0042-6822(89)90442-x. [DOI] [PubMed] [Google Scholar]
- Kakach L. T., Wasmoen T. L., Collett M. S. Rift Valley fever virus M segment: use of recombinant vaccinia viruses to study Phlebovirus gene expression. J Virol. 1988 Mar;62(3):826–833. doi: 10.1128/jvi.62.3.826-833.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan K., Collett M. S. Use of bacterial expression cloning to define the amino acid sequences of antigenic determinants on the G2 glycoprotein of Rift Valley fever virus. J Virol. 1986 May;58(2):263–270. doi: 10.1128/jvi.58.2.263-270.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott A. C., Ward V. K., Nuttall P. A. The S RNA segment of Sandfly Fever Sicilian virus: evidence for an ambisense genome. Virology. 1989 Apr;169(2):341–345. doi: 10.1016/0042-6822(89)90159-1. [DOI] [PubMed] [Google Scholar]
- McGinnes L., McQuain C., Morrison T. The P protein and the nonstructural 38K and 29K proteins of Newcastle disease virus are derived from the same open reading frame. Virology. 1988 May;164(1):256–264. doi: 10.1016/0042-6822(88)90643-5. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeher V. L., Jorgensen E. M., Garber R. L. Multiple transcripts from the Antennapedia gene of Drosophila melanogaster. Mol Cell Biol. 1986 Dec;6(12):4667–4675. doi: 10.1128/mcb.6.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzich J. A., Collett M. S. Rift Valley fever virus M segment: cell-free transcription and translation of virus-complementary RNA. Virology. 1988 Jun;164(2):478–486. doi: 10.1016/0042-6822(88)90562-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wasmoen T. L., Kakach L. T., Collett M. S. Rift Valley fever virus M segment: cellular localization of M segment-encoded proteins. Virology. 1988 Sep;166(1):275–280. doi: 10.1016/0042-6822(88)90174-2. [DOI] [PubMed] [Google Scholar]