Abstract
Extracorporeal photochemotherapy (ECP) has demonstrated immunological effects. The proposed cytotoxic lymphocyte antigen 4 (CTLA-4) involvement, together with forkhead box P3 (FoxP3) and transforming growth factor (TGF)-β are associated with regulatory T cell activity. The aim of the study was to evaluate the regulatory T cell-associated effect of ECP in recent onset type 1 diabetic (T1D) children. Children (n = 20) with T1D received photopheresis 8-methoxypsoralen + ECP or placebo + shampheresis. Peripheral blood mononuclear cells (PBMC) collected pretreatment (day 1) and post-treatment (day 90) were stimulated with phytohaemagglutinin (PHA) and T1D-associated glutamic acid decarboxylase 65 (GAD65) peptide a.a. 247–279. CTLA-4, sCTLA-4, FoxP3 and TGF-β mRNA transcription was quantified. Photopheresis-treated individuals' relative mRNA expression was generally maintained during the course of the study. Placebo individuals increased in spontaneous CTLA-4 mRNA (P< 0·05) but decreased in expression after stimulation with GAD65-peptide (P< 0·05) and PHA (P< 0·05). Spontaneous TGF-β (P< 0·05) increased whereas PHA- (P< 0·01) and GAD65-peptide (P< 0·01)-induced TGF-β expression decreased in the placebo group, whereas it was maintained in the treated group. Without intervention, expression of CTLA-4 and TGF-β, stimulated with PHA and GAD65 peptide, decreased with time, with a parallel reduction of GAD65-peptide and PHA-stimulated TGF-β expression. These parameters were counteracted by ECP. In conclusion, our results indicate that ECP maintains regulatory T cell-associated activity in recent-onset T1D.
Keywords: children, extracorporeal photochemotherapy, T1D, Treg
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by the infiltration of lymphocytes in the pancreas causing destruction of insulin-producing beta-cells [1]. Genetic factors are known to predispose individuals for the disease, while the role of environmental factors still remains elusive [2]. Molecular mimicry between Coxsackie enteroviruses and glutamic acid decarboxylase (GAD65), a.a. 247–279 has been suggested as one possible trigger mechanism [3]. GAD65 is considered to be one of the major autoantigens in T1D, and its administration is tested currently in T1D intervention trials [4,5]
Regulatory CD4+ CD25+ T cells (Treg) cells are characterized by expression of CD25 at high intensities, and pinpointed further by the expression of the transcription factor forkhead box P3 (FoxP3) [6,7]. Vital for tolerance and the Treg cells is the cytotoxic lymphocyte antigen 4 (CTLA-4) protein, expressed by the majority of Treg cells [8]. CTLA-4 is also expressed on activated T cells and acts as a general threshold for the immunological activation signal [9–11]. A splice variant of CTLA-4 that lacks the transmembrane domain and generates a soluble form of CTLA-4 (sCTLA-4) has been detected in humans as well as in mice and rats [12]. Initial reports indicate that increased concentrations are associated with autoimmune disease and T1D [13,14]. Native CTLA-4 binds to B7/CD80/82 in the immunological synapse, producing a suppressive immune response [9,15]. sCTLA-4 shares these characteristics, but the immunological effects mediated by endogenous sCTLA-4 have not yet been clarified.
The soluble and membrane-bound cytokine transforming growth factor (TGF)-β1 is also suggested to play a pivotal role in the function and development of Treg cells [16–19]. In addition to Treg there are other T cells with regulatory activities. The interleukin (IL)-10 generated T regulatory 1 (Tr1) cells secreting high levels of IL-10 and TGF-β[20] and high-secreting TGF-β T helper 3 (Th3) cells have both been shown to be essential in immunological tolerance development and function [18,21,22].
A method for extracorporeal photochemotherapy (ECP) that could inhibit harmful immune system activity was developed in the late 1980s. Lymphocytes are removed from the patient, treated with ultraviolet-sensitizing 8-methoxypsoralen (8-MOP) and UVA, and are then returned to the patient [23]. Reinfusion of the treated ECP cells is thought to induce tolerance via dendritic cell or Treg activity [24]. It has also been suggested that the observed tolerance induction of ECP involves CTLA-4 activity [25].
A randomized, double-blind, placebo-controlled extra-corporeal photopheresis (ECP) intervention trail of recent onset T1D was performed previously at the Linköping University Hospital that showed immunological effects. Although clinical results were minor [26], the method is still of interest as it might be a complement to other types of immune intervention.
We hypothesize that ECP induces regulatory T cell-associated activity and suggest that this activity explains the effects recorded in the preceding ECP-trial. Our aim of the current study is to investigate how Treg-associated markers are affected by ECP treatment.
Materials and methods
Study population
Recent-onset T1D patients had participated previously in a randomized double-blind, placebo-controlled intervention trial (described in detail elsewhere [26]). Patients were assigned active treatment, 8-MOP and subsequent apheresis treatment (n = 19) or received placebo (placebo tablet and shampheresis, n = 21). The procedure was repeated on two consecutive days, the first performed approximately 5–6 days after T1D diagnosis. The double treatment was repeated at days 14, 28, 42 and 90. Coded blood and serum samples were collected before each double treatment session. Laboratory personnel were thus blinded for sample group and identity during analysis.
Peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated by Fiqoll Paque density gradient centrifugations (Pharmacia, Biotech, Sollentuna, Sweden) from sodium heparinized venous blood samples collected before (day 0) and after the total of five double treatments (day 90). The samples came from a random subsample of 10 photopheresis-treated patients and 10 shampheresis placebo controls (ages 10–17 years) matched by gender and age that had been frozen carefully directly after sample collection. Cryofrozen cells were now thawed in 37°C water and washed in RPMI-1640 supplemented with 10% fetal calf serum. PBMCs, 1 × 106 (viability > 90%), were resuspended in 500 μl AIM V serum-free medium (Gibco, Täby, Sweden) supplemented with 2 nM 1-glutamine, 50 μg/l streptomycin sulphate, 10 μg/l gentamycin and 2 × 10−5 M 2-mercaptoethanol (Sigma Chemical Co., St Louis, MO, USA), stimulated immediately with GAD65 peptide (a.a. 247–279) and phytohaemagglutinin (PHA), both at a concentration of 5 μg/ml for 48 h at 37°C in 5% CO2[27].
RNA isolation and cDNA synthesis
Total RNA was isolated from PBMC as described previously [28]. Total RNA were added in equal final concentrations (3·5 ng/μl) and transcribed using high capacity cDNA Archive Kit (no. 4322171; Applied Biosystems, Foster City, CA, USA), according to the manufacturer's recommendations. In addition to the manufacturer's protocol, RNAse inhibitor (no. N8080119; Roche, Basel, Switzerland) was added to a final concentration of 1 U/ml. Transcription reactions were run in a GeneAmp polymerase chain reaction (PCR) System 2700 (Applied Biosystems) at 25°C for 10 min followed by 37°C for 2 h.
For sCTLA-4 mRNA detection, trace genomic DNA was first removed by DNAse 1 treatment (no. 776785; Roche Diagnostics, Mannheim, Germany) at a concentration of 0·1 U/μl for 30 min at 37°C followed by 5 min at 95°C.
Multiplex real-time reverse transcription–PCR
cDNA was analysed with TaqMan gene expression assay (Applied Biosystems) with primers and 6-carboxyfluorescein-(FAM)-labelled probes for TGF-β (Hs00171257_m1), FoxP3 (Hs00203958_m1) and CTLA-4 (Hs00175480_m1), as well as primers and VIC®-labelled probes for rRNA (18S) as endogenous control (no. 4310893E; Applied Biosystems). Real-time (RT–PCR) was carried out in a final reaction volume of 25 μl containing TaqMan predeveloped assay reagents (PDAR) Universal MasterMix (no. 4304437; Applied Biosystems), 1 × PDAR primers/probes for target genes, and template cDNA in MicroAmp Optical 96-well plates covered with optical caps (Applied Biosystems). The reaction was amplified with an initial step of 95°C for 10 min followed by 50 cycles of 95°C for 15 s, 60°C for 60 s and 72°C for 60 s in a ABI Prism 7700 Sequence detector (Applied Biosystems).
A custom-designed assay for sCTLA-4 mRNA was run separately at the same reaction conditions. Primers/probes were designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_http://www.cgi). sCTLA-4 forward primer 5′-CATCTGCAAGGTGGAGCTCAT, reverse primer 5′-GGCTTCTTTTCTTTAGCAATTACATAAATC and probe sequence 5′-6-FAM-ACCGCCATACTACCTGGGCATAGGCA-TAMRA (Applied Biosystems).
All RT–PCR plates were loaded with duplicate samples and with an internal calibrator duplicate sample from an adult donor with T1D as intra-assay control as well as a duplicate no template control. Cells from the internal calibrator were lysed and total RNA was isolated according to the manufacturer's protocol (Sigma GenElute Mammalian Total RNA kit (Sigma Chemical Co.).
The delta cycle threshold, ΔCT, was calculated for each sample by subtracting the 18S CT (VIC®) from the target gene CT (FAM). As samples were run in duplicates, the mean ΔCT of each duplicate was calculated. ΔΔCT was determined by calculating the ΔCT difference between each stimulated sample ΔCT and its corresponding spontaneous sample ΔCT. For example, GAD65-peptide stimulated FoxP3 expression was compared with spontaneous FoxP3 expression in each individual. Relative quantification was calculated as 2–ΔΔCT.
Results were generated from spontaneous as well as stimulated expression, and at disease onset (0) and after 90 days of treatment (3).
Immunoassay sCTLA-4
Cell supernatants from 20 patients in the same time interval as the cell samples were used for sCTLA-4 analysis. Gyrolab Bioaffy platform was used for a sandwich immunoassay detection of sCTLA-4 (Gyros AB, Uppsala, Sweden) (described in detail elsewhere [29]). Biotinylated anti-human CD152 (AS33-B; Antibody Solutions, Palo Alto, CA, USA) was used to capture and detection was made with a complementary anti-human CD152 antibody (BNI.3; BD PharMingen, San Diego, CA, USA) labelled previously with Alexa Flour 647 (Molecular Probes, Eugene, OR, USA), according to the manufacturer's instructions. The matching CD152 antibodies have been successfully implemented previously in sCTLA-4 sandwich immunoassay [12].
Statistics
As data are not expected to be normally distributed (even after logarithmic transformation), paired observations were computed by Wilcoxon's signed ranks test. Two groups were compared by Mann–Whitney U-test and three or more groups with the Kruskal–Wallis test for unpaired observations. Spearman's rank correlation test was used when comparing two variables non-parametrically. A probability level of < 0·05 was considered to be statistically significant, whereas a P-value of 0·05 < 0·1 was regarded as a tendency. Calculations were performed with spss for Windows version 11·5 and GraphPad Prism version 4·03.
Ethics
Informed consent was given by the participating children and their parents. The study was approved by the Research Ethics Committee of the Faculty of Health Sciences, Linköping University.
Results
Expression of Treg-associated markers pre-ECP treatment
We evaluated the effect of ECP on tolerance-associated markers in samples obtained from a clinical trial. The samples did not differ in mRNA levels of the Treg-associated markers FoxP3, CTLA-4, sCTLA-4 and TGF-β between the treated and placebo groups prior to treatment, except for PHA-stimulated FoxP3 mRNA expression, where the photopheresis group tended to be elevated compared with the placebo control group (P= 0·06) (data not shown).
Expression of Treg-associated markers post-ECP treatment
Cytotoxic lymphocyte antigen 4 mRNA increased during the course of the treatment in the ECP treated group (P= 0·04) (Fig. 1). Similarly, T1D placebo controls showed increased spontaneous CTLA-4 mRNA expression after 3 months of treatment (P= 0·02) (Fig. 2).
Fig. 1.
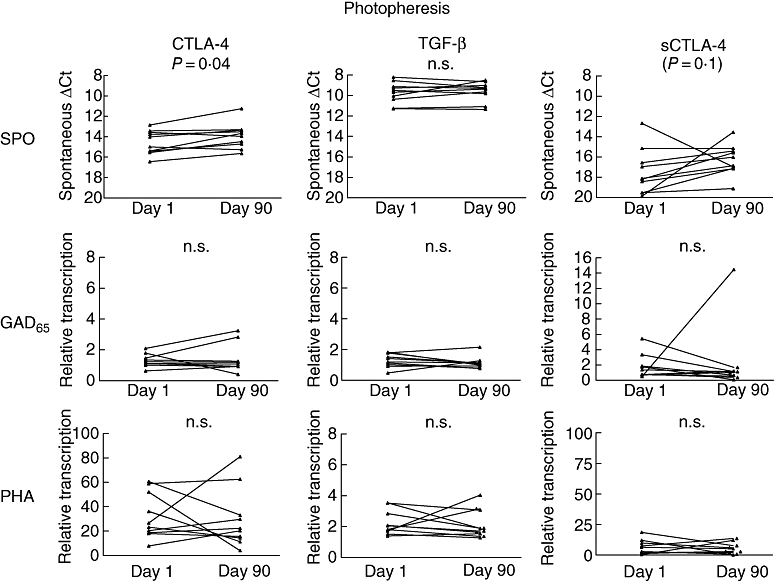
Extracorporeal photochemotherapy (ECP)-treated children (n = 10) showed few changes in target gene transcription during the course of the study. mRNA transcription displayed at days 0–90. Spontaneous (unstimulated), glutamic acid decarboxylase (GAD65)-peptide or phytohaemagglutinin (PHA) stimulated for the same target gene are organized horizontally. Target genes are displayed vertically. Note that spontaneous transcription is displayed at an inverted axis because of raw delta cycle threshold-values. Spontaneous expression of cytotoxic lymphocyte antigen 4 increased during treatment (P= 0·04). n = 10.
Fig. 2.
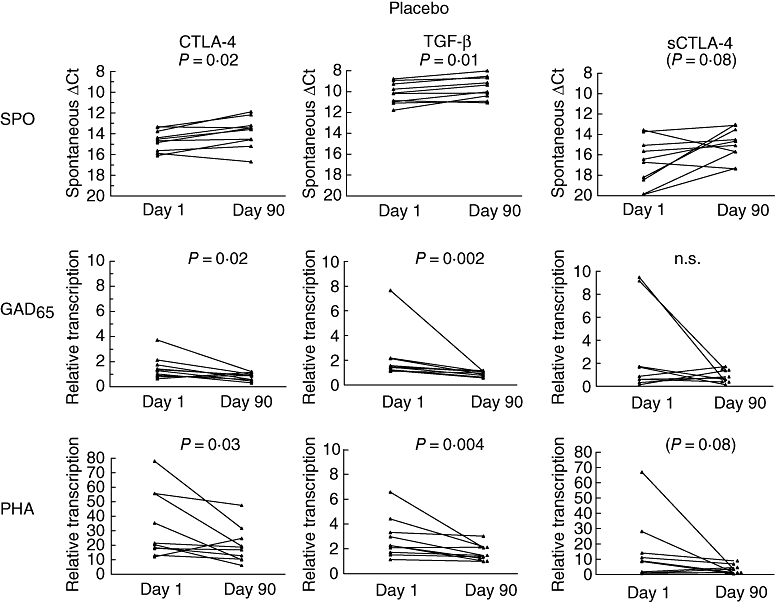
Placebo controls (n = 10) showed several changes in target gene transcription. Transcription shown at days 0–90. Spontaneous (unstimulated), glutamic acid decarboxylase (GAD65)-peptide or phytohaemagglutinin (PHA) stimulated for the same target gene are organized horizontally. Target genes are displayed vertically. Note that spontaneous transcription is displayed at an inverted axis because of raw delta cycle threshold values. Spontaneous cytotoxic lymphocyte antigen 4 (CTLA-4) expression increased during the study (P= 0·02), whereas GAD65-peptide and PHA-stimulated responses decreased (P= 0·02 and P = 0·03 respectively). Similarly, spontaneous transforming growth factor (TGF)-β increased (P= 0·01) and GAD65-peptide and PHA stimulated responses decreased (P= 0·002 and P = 0·004 respectively). n = 10 except for in GAD65-peptide stimulated sCTLA-4 (n = 9).
Extracorporeal photochemotherapy treated children showed few significant changes in the observed markers during the course of the treatment; no detectable changes could be observed in relative GAD65-peptide or PHA-induced CTLA-4 transcription. The same was true for spontaneous, GAD65-peptide and PHA-induced sCTLA-4 transcription. The results were consistent in TGF-β expressions, where neither spontaneous, GAD65-peptide nor PHA stimulation resulted in differences between the two studied time-points in ECP-treated patients.
Children receiving exclusively placebo
Relative transcription of GAD65-peptide- and PHA-induced CTLA-4 was decreased in the placebo group after 3 months (P= 0·02 and 0·03 respectively) (Fig. 2). Additionally, spontaneous sCTLA-4 mRNA expression tended to increase in the placebo group (P= 0·08) (Fig. 2). sCTLA-4 mRNA response to the GAD65-peptide did not yield differences in the placebo group, whereas PHA-stimulated sCTLA-4 mRNA tended to decrease in the placebo controls (P= 0·08) (Fig. 2).
Spontaneous TGF-β mRNA expression increased in the group of children receiving placebo (P= 0·01) (Fig. 2). In contrast, GAD65-peptide and PHA-stimulated TGF-β expressions were observed to decrease during the trial in the placebo group (P= 0·002 and P = 0·004 respectively) (Fig. 2).
In addition, relative changes in transcription was significantly greater in the placebo controls than actively photopheresis treated in both GAD65-peptide-stimulated CTLA-4 and TGF-β expressions (P= 0·04 for both) (Fig. 3a and b). This relationship also tended to persist in spontaneous TGF-β mRNA expression (P= 0·09) (Fig. 3c). Relative change in transcription of remaining marker antigen responses did not reach statistical significance.
Fig. 3.
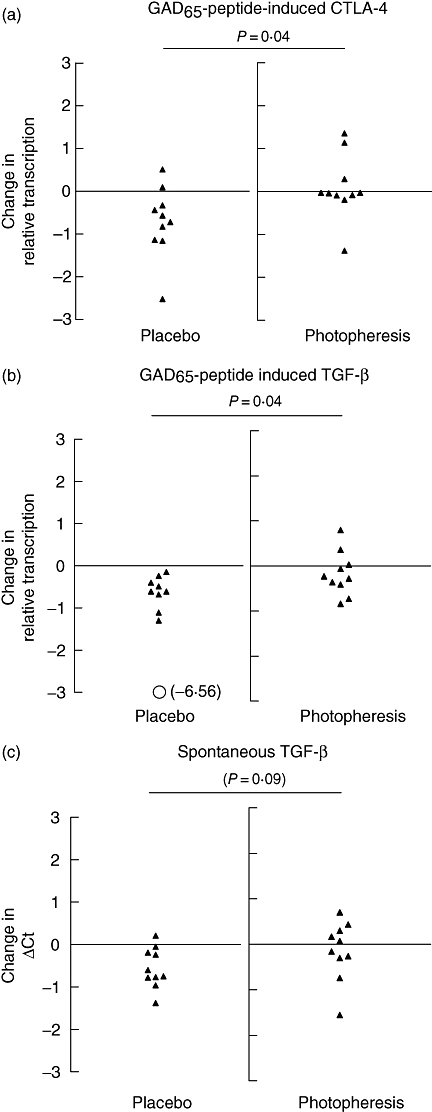
Relative change in transcription from days 0–90. P-values indicating significant difference between relative change in target transcription in photopheresis versus placebo controls. Glutamic acid decarboxylase (GAD65)-induced response of both cytotoxic lymphocyte antigen 4 (a) and transforming growth factor (TGF)-β (b) declined significantly more in placebo compared with photopheresis-treated type 1 diabetes patients (P= 0·04 for both). Spontaneous TGF-β (c) transcription tended to increase (note raw delta cycle threshold) more in the placebo group than the photopheresis-treated patients. n = 10 in each group.
Expression of FoxP3 mRNA
Although no significant changes could be observed in FoxP3 expression in either group of treatment (data not shown), spontaneous TGF-β expression in the total group was correlated significantly with spontaneous FoxP3 both prior to (R= 0·66, P = 0·002) (Fig. 4a) and post-ECP treatment (R= 0·7, P = 0·001) (Fig. 4b).
Fig. 4.
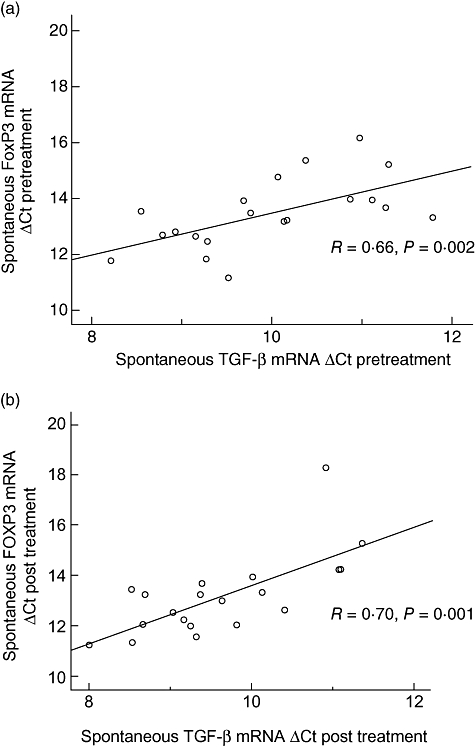
Spontaneous mRNA transcription (delta cycle threshold) of transforming growth factor-β and forkhead box P3 correlated both prior to (R= 0·66, P = 0·002) (day 0) (a) and after (R= 0·7, P = 0·001) the study (day 90) (b) n = 20.
Secretion of sCTLA-4
Low secretion of sCTLA-4 could be detected in unstimulated as well as PHA- and GAD65-peptide 48 h-stimulated cell supernatants (data not shown). No significant differences were observed in sCTLA-4 levels between photopheresis-treated and placebo controls, neither pre- nor post-treatment. Secreted sCTLA-4 did not correlate with either spontaneous sCTLA-4 mRNA or GAD65-peptide, or PHA-stimulated expression.
Discussion
Much research effort has been made to find an intervention for the autoimmune process in T1D. Although T1D can be intervened by several therapeutic strategies in animal models, successful clinical trials to preserve residual beta cell function have so far been achieved only partially in humans. Current trials of anti-CD3 [30–32] and GAD65-‘vaccination’[4,5] have shown promising results, but it may well be so that they need to be supported by other measures to regulate the immune system successfully. In this context photopheresis still is of interest.
Clinical outcome of T1D photopheresis treatment certainly showed very limited results. However, we find that a maintained immune regulation is induced by ECP treatment, and that it is active on GAD65-peptide-specific stimulation. Thus, even if ECP alone is not successful as a T1D intervention therapy, it might be a way to boost effect of anti-CD3- or GAD65-induced immune-modulated tolerance in recent-onset T1D individuals.
Recently it was discovered that CTLA-4 is active in the ECP-induced tolerance [25]. Our data support the theory that ECP-induced tolerance acts via regulatory T cells. It is a plausible mechanism, as only a small fraction of the total lymphocyte population needs to be treated in order for the ECP-treatment to have effect.
Our current results show a pre- to post-ECP treatment increase in spontaneous CTLA-4 mRNA expression in both treated and placebo control individuals. After stimulation of lymphocytes we detect disease progression-associated decreased specific CTLA-4 mRNA expression in response to GAD65-peptide as well as PHA stimulation in placebo children. This was followed by a parallel reduction of TGF-β in response to the same antigen stimulations. Interestingly, this process of decreased antigen responses could not be observed in actively treated individuals. The GAD65-peptide used in the study is not human leucocyte antigen (HLA)-restricted. There is, however, a minor overlap of five amino acids in a GAD65-sequence that is HLA class II DRB1*0401-restricted [33]. This small overlap is not expected to influence the results.
The disease-associated reduction in the studied markers was significantly greater in GAD65-peptide responses of both CTLA-4 and TGF-β, suggesting that ECP has a greater effect on a T1D antigen-specific response rather than mitogen responses such as PHA. Another possibility is that the GAD65-peptide response drops more during the initial development compared with PHA, which results in a more clear ECP-induced effect.
The collected results are consistent with the earlier finding regarding lymphocyte activation [34]. Maintained levels of TGF-β mRNA expression taken together with increased IL-4 and IL-10 expression makes it possible to assume that it could be Tr1 cells that are active in this tolerance maintenance.
Previous results from our ECP trial also showed that treated individuals had increased levels of IL-10 compared with placebo controls [27]. Additionally, there was an indication that activated CD4+ and CD8+ cells increased in the placebo controls during the course of the study, an increase that was not observed in the actively treated group [34].
The interaction of both TGF-β and FoxP3-expression is well documented in the field of Treg cell research [17,25,35,36], and we now find further support of this relationship. The reason we fail to dissect the possible role of FoxP3 in ECP-induced tolerance could be because FoxP3 is part of many and complex immunological tolerance-associated interactions.
The role and function of Treg cells in the pathogenesis of T1D is still subject to much debate. It has been shown that Treg in T1D individuals are unable to suppress cell proliferation associated with T1D, although the cell population numbers are the same as in healthy individuals [37]. These results contradict earlier results suggesting a reduced Treg number in T1D development [38]. Even though the mechanism is unclear, increasing evidence suggest a role for Treg cells in maintaining tolerance against T1D development [17,22,39,40]. What complicates the discussion is that we have limited understanding of how to separate the pathogenic mechanisms from the immune system's defence mounted to resist T1D pathogenesis.
We speculate that the observed antigen-specific parallel decrease in the immunoregulatory markers CTLA-4 and TGF-β mRNA expression represents the natural course of the T1D progression. No such changes were recorded in patients who received active treatment; rather, ECP-treated patients maintain their regulatory T cell-associated activity towards T1D-specific autoantigen.
In conclusion, our results indicate that photopheresis acts to maintain immune regulation and thereby inhibit spontaneous islet autoimmunity.
Acknowledgments
We are grateful to the Pediatric department and to Professor Gösta Berlin and Professor Jan Ernerud, who were collaborators in the initial photopheresis study. We acknowledge Professor Åke Lernmark for help in interpreting the initial results and statistical design. We also thank Birgit Olsson, Liza Alkhori and Elizabeth A. Rutledge for help with the sCTLA-4 mRNA assay. This study was supported by the Children's Diabetes fund Sweden (Barndiabetesfonden) and Landstinget i Östergötland.
References
- 1.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–36. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 2.Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl. 2):S125–36. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94:2125–9. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005;19:238–46. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Ludvigsson JCR, Vaarala O, Forsander O, et al. The Swedish GAD-vaccination Trial: outcomes of a phase II safety and efficacy trial with Diamyd (TM) for preservation of beta cell function in children with T1D. Diabetologia. 2006;49:149. [Google Scholar]
- 6.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Birebent B, Lorho R, Lechartier H, et al. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34:3485–96. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 9.Linsley PS, Greene JL, Tan P, et al. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsten T, Lee KP, Harris ES, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489. [PubMed] [Google Scholar]
- 11.Wang XB, Zheng CY, Giscombe R, Lefvert AK. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand J Immunol. 2001;54:453–8. doi: 10.1046/j.1365-3083.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 12.Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA-4. Cell Immunol. 2000;201:144–53. doi: 10.1006/cimm.2000.1649. [DOI] [PubMed] [Google Scholar]
- 13.Pawlak E, Kochanowska IE, Frydecka I, Kielbinski M, Potoczek S, Bilinska M. The soluble CTLA-4 receptor: a new marker in autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2005;53:336–41. [PubMed] [Google Scholar]
- 14.Purohit S, Podolsky R, Collins C, et al. Lack of correlation between the levels of soluble cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and the CT-60 genotypes. J Autoimmune Dis. 2005;2:8. doi: 10.1186/1740-2557-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper K, Balzano C, Rouvier E, Mattei MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147:1037–44. [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–7. doi: 10.1073/pnas.0400810101. Epub 2004 March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi R, Anderson DE, Weiner HL. Cutting edge: immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-beta-dependent manner. J Immunol. 2007;178:4017–21. doi: 10.4049/jimmunol.178.7.4017. [DOI] [PubMed] [Google Scholar]
- 20.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 21.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 22.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta–TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–83. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliven A, Shechter Y. Extracorporeal photopheresis: a review. Blood Rev. 2001;15:103–8. doi: 10.1054/blre.2001.0155. [DOI] [PubMed] [Google Scholar]
- 24.Heshmati F. Mechanisms of action of extracorporeal photochemotherapy. Transfus Apheresis Sci. 2003;29:61–70. doi: 10.1016/S1473-0502(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz A, Beissert S, Grosse-Heitmeyer K, et al. Evidence for functional relevance of CTLA-4 in ultraviolet-radiation-induced tolerance. J Immunol. 2000;165:1824–31. doi: 10.4049/jimmunol.165.4.1824. [DOI] [PubMed] [Google Scholar]
- 26.Ludvigsson J, Samuelsson U, Ernerudh J, Johansson C, Stenhammar L, Berlin G. Photopheresis at onset of type 1 diabetes: a randomised, double blind, placebo controlled trial. Arch Dis Child. 2001;85:149–54. doi: 10.1136/adc.85.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faresjo MK, Ernerudh J, Berlin G, Garcia J, Ludvigsson J. The immunological effect of photopheresis in children with newly diagnosed type 1 diabetes. Pediatr Res. 2005;58:459–66. doi: 10.1203/01.pdr.0000176906.42001.c3. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson MG, Garcia J, Ludvigsson J. Cows' milk proteins cause similar Th1- and Th2-like immune response in diabetic and healthy children. Diabetologia. 2001;44:1140–7. doi: 10.1007/s001250100611. [DOI] [PubMed] [Google Scholar]
- 29.Mayans S, Lackovic K, Nyholm C, et al. CT60 genotype does not affect CTLA-4 isoform expression despite association to T1D and AITD in northern Sweden. BMC Med Genet. 2007;8:3. doi: 10.1186/1471-2350-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–7. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herold KC. Achieving antigen-specific immune regulation. J Clin Invest. 2004;113:346–9. doi: 10.1172/JCI20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold KC, Bluestone JA, Montag AG, et al. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992;41:385–91. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- 33.Wicker LS, Chen SL, Nepom GT, et al. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J Clin Invest. 1996;98:2597–603. doi: 10.1172/JCI119079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ernerudh J, Ludvigsson J, Berlin G, Samuelsson U. Effect of photopheresis on lymphocyte population in children with newly diagnosed type 1 diabetes. Clin Diagn Lab Immunol. 2004;11:856–61. doi: 10.1128/CDLI.11.5.856-861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+) CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+) CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 38.Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–40. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 40.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–10. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]


