Abstract
Interferon (IFN)-γ is a major cytokine that regulates T helper 1-type immune reactions and serves as an important mediator in the pathogenesis of autoimmune diseases. Retinoic acid-inducible gene-I (RIG-I) is an IFN-γ-inducible gene and known to be involved in the inflammatory and immune reactions. In the present study, we found high levels of RIG-I expression in synovial tissues of rheumatoid arthritis (RA), while the expression in osteoarthritis tissues was low. Treatment of cultured fibroblast-like synoviocytes with IFN-γ markedly induced the expression of RIG-I. Knockdown of RIG-I in fibroblast-like synoviocytes, with specific siRNA, resulted in the inhibition of the IFN-γ-induced expression of chemokine (C-X-C motif) ligand 10 (CXCL10)/IFN-γ-inducible protein-10 (IP-10), a chemokine with chemotactic activity towards T cells. These findings suggest that RIG-I may play an important role in the pathogenesis of synovial inflammation in RA, at least in part, by regulating the IFN-γ-induced expression of CXCL10/IP-10.
Keywords: CXCL10/IP-10, fibroblast-like synoviocytes, IFN-γ, RIG-I
Introduction
The synovial lining seals the joint and contributes to the maintenance of the homeostasis of synovial microenvironment. Although the pathophysiological features of rheumatoid arthritis (RA) are complicated, inflammation in the synovium is one of the cardinal characteristics of RA [1]. Fibroblast-like synoviocytes constitute part of a complex cellular network in the synovial lining and may play an important role in both initiation and driving of inflammation in RA. Interferon (IFN)-γ plays the central role in T helper 1 (Th1)-type reactions, which are implicated in the basic mechanisms of RA. IFN-γ was detected in synovial fluids and T cells in synovial tissues from RA patients, while it was not detected in materials from osteoarthritis (OA) patients [2].
Retinoic acid-inducible gene-I (RIG-I) is a member of the DExH box family protein, and regarded to encode an RNA helicase [3], which may be involved in various reactions related to RNA metabolisms. The expression of RIG-I is induced by IFN-γ in several cell types including endothelial cells, smooth muscle cells, bronchial epithelial cells and macrophages [4–7], and RIG-I is considered to mediate reactions induced by IFN-γ[6]. RIG-I is associated with various chronic inflammatory diseases [8] including lupus nephritis, another autoimmune disease [9]. The present study was undertaken to address the role of RIG-I in RA by examining RIG-I expression in tissues from RA patients and in cultured fibroblast-like synoviocytes.
Materials and methods
Reagents
An anti-RIG-I antibody was raised as described previously [3]. Alexa Fluor 549-conjugated anti-rabbit immunoglobulin (Ig)G and Alexa Fluor 488-conjugated anti-mouse IgG were from Molecular Probes (Tokyo, Japan). An anti-vimentin mouse monoclonal antibody was from Dako (Kyoto, Japan). An RNeasy total RNA isolation kit, small interfering RNA (siRNA; SI03019646) against RIG-I and Taq DNA polymerase were from Qiagen (Hilden, Germany). Dulbecco's modified Eagle's medium, fetal bovine serum, a primer oligo(dT)12−18, dNTP mix, MMLV reverse transcriptase and Lipofectamine 2000 were purchased from Invitrogen (Frederick, MD, USA). Oligonucleotide primers for polymerase chain reaction (PCR) were custom synthesized by Greiner Japan (Atsugi, Japan). An iQTM SYBR® Green Supermix solution was from Bio-Rad (Hercules, CA, USA). Recombinant human [r(h)] IFN-γ was from Roche (Manheim, Germany). Anti-actin rabbit IgG was from Sigma (St Louis, MA, USA). A SuperSignal West Pico chemiluminescence substrate was from Pierce (Rockford, IL, USA). An enzyme-linked immunosorbent assay kit for chemokine (C-X-C motif) ligand 10 (CXCL10)/IFN-γ-inducible protein-10 (IP-10) was from R&D Systems (Minneapolis, MN, USA).
Immunohistochemistry
Synovial tissues were obtained from patients with RA or OA during joint replacement surgery, and all patients provided written consent. The study was approved by the ethics committees in Hirosaki University, St Marianna University and Kumamoto University. Expression of RIG-I protein in the tissue was examined by immunohistochemistry as described previously [7,10,11]. Briefly, deparaffinized tissue sections were incubated with an anti-RIG-I antibody (1:1000) or non-immune rabbit IgG, and the samples were incubated with a biotinylated secondary antibody and horseradish peroxidase (HRP)–streptavidin. The immunoreactivity was detected using the DAB/H2O2 system, and the nuclei counterstained with haematoxylin. Random five high-power fields were photographed, and the RIG-I-positive cell count in total of 100 cells in each field was obtained.
In an attempt to identify the RIG-I-positive cells, the tissue samples were also subjected to double staining for RIG-I and vimentin [11]. Briefly, the sections were incubated with the anti-RIG-I antibody, followed by incubation with an Alexa Fluor 549-conjugated anti-rabbit antibody. After washing, the sections were incubated with an anti-vimentin monoclonal antibody and then with Alexa Fluor 488-conjugated anti-mouse IgG. The fluorescent signals were evaluated using a confocal microscope (Bio-Rad Radiance 2100; Bio-Rad, Osaka, Japan).
Cells
Fibroblast-like synoviocytes were isolated from the tissues by enzymatic dispersion and cultured as described previously [10], and the cells of passages 3–8 were used for the experiments. The cells were identified as fibroblast-like synoviocytes by detecting the expression of fibroblast-specific protein-1 (FSP-1) (Fig. 3c). The cells were subjected to RNA interference against RIG-I using a Lipofectamine 2000 reagent as described [12].
Fig. 3.
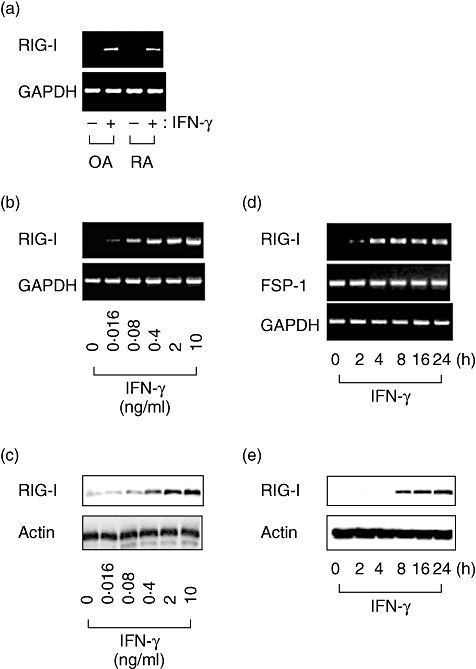
Expression of retinoic acid-inducible gene-I (RIG-I) and fibroblast-specific protein-1 in cultured human fibroblast-like synoviocytes. Fibroblast-like synoviocytes obtained from patients with osteoarthritis or rheumatoid arthritis were cultured and treated with 2 ng/ml interferon (IFN)-γ for 4 h, and reverse transcription–polymerase chain reaction (RT–PCR) was performed (a). Cultured fibroblast-like synoviocytes were treated with various concentrations of IFN-γ for 24 h, and the expression of RIG-I mRNA or protein was examined by RT–PCR (b) or Western blot analysis (c). The cells were treated with 2 ng/ml IFN-γ for up to 24 h, and RT–PCR (d) and Western blot analyses (e) were performed.
RNA extraction and reverse transcription–PCR analysis
Total RNA was extracted from the tissues or cultured cells using an RNeasy kit. Single-strand cDNA was synthesized from 1 μg of total RNA using oligo(dT)12−18 primer and MMLV reverse transcriptase. The expression of mRNAsfor RIG-I and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the synovial tissues was quantified by real-time PCR using a Chromo4TM real-time PCR System (Bio-Rad). The cDNA for RIG-I, FSP-1, CXCL10/IP-10, CXCL9/monokine induced by IFN-γ (MIG) or GAPDH was amplified by PCR using Taq DNA polymerase. The primers are shown in Table 1.
Table 1.
(a) Oligonucleotide primers for real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR); (b) oligonucleotide primers for RT–PCR.
| cDNA | Primers | Annealing (°C) | Cycles | Product (bp) |
|---|---|---|---|---|
| (a) | ||||
| RIG-I | Forward: 5′-GTGCAAAGCCTTGGCATGT-3′ | 55 | 40 | 115 |
| Reverse: 5′-TGGCTTGGGATGTGGTCTACTC-3′ | ||||
| GAPDH | Forward: 5′-GCACCGTCAAGGCTGAGAAC-3′ | 55 | 40 | 142 |
| Reverse: 5′-ATGGTGGTGAAGACGCCAGT-3′ | ||||
| (b) | ||||
| RIG-I | Forward: 5′-GCA TATTGACTGGACGTGGCA-3′ | 60 | 30 | 644 |
| Reverse: 5′-CAGTCATGGCTGCAGTTCTGTC-3′ | ||||
| FSP-1 | Forward: 5′-AGCTTCTTGGGGAAAAGGAC-3′ | 60 | 30 | 200 |
| Reverse: 5′-CCCCAACCACATCAGAGG-3′ | ||||
| IP-10 | Forward: 5′-ACCTCCAGTCTCAGCACCATG-3′ | 60 | 30 | 761 |
| Reverse: 5′- TGGGAGGATGGCAGTGGAAG-3′ | ||||
| MIG | Forward: 5′-TTCCTCTTGGGCATCATCTTGCTG-3′ | 58 | 30 | 336 |
| Reverse: 5′-GGTCTTTCAAGGATTGTAGGTGGA-3′ | ||||
| GAPDH | Forward: 5′-CCACCCATGGCAAATTCCATGGCA-3′ | 60 | 30 | 598 |
| Reverse: 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ | ||||
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IP-10, interferon-γ-inducible protein-10; FSP-1, fibroblast-specific protein-1; MIG, monokine induced by interferon-γ (MIG); RIG-I, retinoic acid-inducible gene-I; bp, base pairs.
Western blot analysis
Cells were lysed using Laemmli's reducing sample buffer. The lysate was subjected to electrophoresis on a 7·5% polyacrylamide gel, and the proteins were transferred to a polyvinylidene difluoride membrane. RIG-I protein was detected using an anti-RIG-I antibody (1:10 000 dilution), as described previously [3]. Actin was detected using anti-actin rabbit IgG (1:250 dilution). Immunodetection was performed using an HRP-labelled anti-rabbit IgG antibody and a SuperSignal West Pico chemiluminescence substrate.
Results
Enhanced expression of RIG-I in RA synovial tissue
Intense RIG-I immunoreactivity was observed in synovial tissues from patients with RA (Fig. 1a), while the expression in OA synovial tissues was faint. All the three RA specimens examined were positive for RIG-I, and the percentage of RIG-I positive cells in RA tissue was 79 ± 6, which was significantly higher than that of OA tissue: 9 ± 3 (n = 3, P < 0·01 by Student's t-test). The RIG-I-positive cells in synovial tissue were also stained for vimentin, a marker for fibroblasts (Fig. 1b).
Fig. 1.
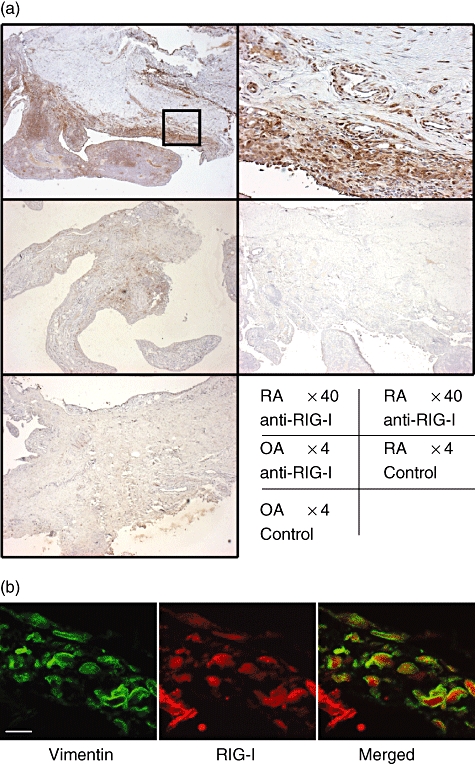
Immunohistochemical identification of retinoic acid-inducible gene-I (RIG-I) in human synovial tissues. (a) Tissues obtained from patients with rheumatoid arthritis (RA) or osteoarthritis (OA) were subjected to immunostaining using an anti-RIG-I antibody or non-immune rabbit immunoglobulin G (control). Intense RIG-I immunoreactivity was detected in synovial tissues from RA patients but not in tissues from OA patients. Upper right panel shows high power view of the square in upper left panel. The data shown are representative of three experiments. (b) Confocal microscopic images of double immunofluorescence staining for vimentin (left panel; green) and RIG-I (middle panel; red). The overlapping image was shown in right panel. Scale bar shows 10 μm.
The level of RIG-I mRNA was significantly higher in RA synovial tissues compared with OA tissues (Fig. 2).
Fig. 2.
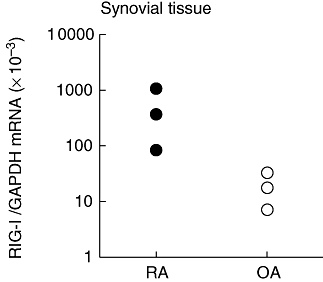
Expression of retinoic acid-inducible gene-I (RIG-I) mRNA in human synovial tissues. RNA was extracted from tissues obtained from patients with rheumatoid arthritis (RA) (n = 3) or osteoarthritis (OA) (n = 3), and was subjected to real-time quantitative reverse transcription–polymerase chain reaction analysis for RIG-I mRNA expression. Note that the level of RIG-I mRNA was shown in log scale.
Interferon-γ induces the expression of RIG-I in cultured fibroblast-like synoviocytes
Figure 3 shows the expression of RIG-I in fibroblast-like synoviocytes in culture. The basal expression of RIG-I mRNA was negligible in cultures derived from both RA and OA tissues. IFN-γ induced the expression of RIG-I mRNA, and the magnitude of the induction was virtually similar in both type of cells (Fig. 3a). The levels of RIG-I expression was enhanced by IFN-γ in a concentration- and time-dependent manner (Fig. 3b–e).
Retinoic acid-inducible gene-I siRNA suppresses IFN-γ-induced CXCL10/IP-10 expression in fibroblast-like synoviocytes
Interferon-γ enhanced the expression of both CXCL10/IP-10 and RIG-I. The IFN-γ-induced CXCL10/IP-10 up-regulation was inhibited significantly with siRNA against RIG-I (Fig. 4). The cells produced 14 ± 1·8 ng/105 cells of CXCL10/IP-10 in response to IFN-γ, and RIG-I siRNA reduced the production to 5·5 ± 0·7 ng/105 cells (mean ± standard deviation, n = 3; P < 0·01, based on Student's t-test). The IFN-γ-induced expression of CXCL9/MIG was not affected by RIG-I siRNA.
Fig. 4.
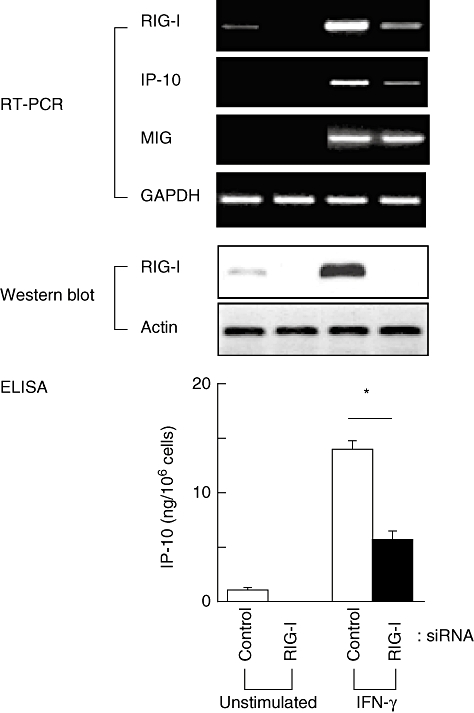
Effect of retinoic acid-inducible gene-I (RIG-I) knockdown on the expression of interferon (IFN)-γ-inducible protein-10 (IP-10) in cultured fibroblast-like synoviocytes. The cells were transfected with siRNA against RIG-I, and after 24 h the cells were treated with 2 ng/ml IFN-γ. The cells were incubated for additional 48 h, and culture medium and cell lysates were collected. The expression of mRNAs for RIG-I, IP-10 and monokine induced by IFN-γ were examined by reverse transcription–polymerase chain reaction (upper panel). The result of Western blot analysis for RIG-I was shown in the middle panel. The concentration of IP-10 in the culture medium was examined by an enzyme-linked immunosorbent assay (lower panel). Means ± standard deviation (n = 3) are shown. *P < 0·01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, ELISA, enzyme-linked immunosorbent assay.
Discussion
In addition to its role in innate immunity [13], RIG-I is implicated in various inflammatory disorders [8]. In the present study, we found high levels of RIG-I expression in the synovial tissues from RA patients; expression in the tissues from OA patients was almost negligible. The cells expressing RIG-I were also positive for vimentin, and this supports the identification of the cells as fibroblast-like synoviocytes. Proliferation of synoviocytes, with transformed phenotypes, is one of the characteristics of RA [14], and the increased RIG-I expression may be related to these changes in RA fibroblast-like synoviocytes.
Expression of RIG-I in fibroblast-like synoviocytes was studied further using cell cultures. We compared the expression of RIG-I between fibroblast-like synoviocytes from patients with OA and RA, but the RIG-I level was similar between cultures from the two groups. Thus the role of RIG-I, if any, in fibroblast-like synoviocytes proliferation and phenotypic transformation was not confirmed in ex vivo cultures. However, we found that treatment of fibroblast-like synoviocytes with IFN-γ induced expression of RIG-I, which may be involved in the Th1-type reactions in the synovium. Close communication between tissue-infiltrating inflammatory cells and fibroblast-like synoviocytes is a consequence of chronic inflammation. In the initial step of inflammatory reactions, T cells pass through the vascular endothelium and localize into synovial tissue. CXCL10/IP-10 has an activity to stimulate the directional migration of T cells, particularly Th1 cells. IFN-γ is secreted by Th1-type cells and activates fibroblast-like synoviocytes to produce CXCL10/IP-10. It has been reported that CXCL10/IP-10 levels are elevated in synovial fluids from patients with RA [15], and neutralization of CXCL10/IP-10 suppresses experimentally induced arthritis in rats [16]. Therefore, IFN-γ and CXCL10/IP-10 may constitute a positive feedback loop for synovial inflammation. We have shown previously that overexpression of RIG-I in BEAS-2B cells, a human bronchial epithelial cell line, resulted in enhanced expression of CXCL10/IP-10 [6]. In the present study, RIG-I knockdown with siRNA resulted in suppression of the IFN-γ-induced CXCL10/IP-10 expression in fibroblast-like synoviocytes. RIG-I may mediate the function of IFN-γ by amplifying the retention and proinflammatory function of T cells, at least in part, through the enhanced production of CXCL10/IP-10. The expression of CXCL9/MIG was not altered with RIG-I siRNA, and RIG-I may regulate selectively the expression of CXCL10/IP-10. It is reported that RIG-I serves as an intracellular receptor of double-stranded RNA (dsRNA) and activates a transcriptional factor IFN-regulatory factor 3 upon the infection with RNA virus [13]. The signalling pathway for IFN-γ may differ from that for dsRNA, and further studies are required to clarify the molecular mechanisms by which RIG-I mediates the IFN-γ-induced CXCL10/IP-10 expression.
A recent study showed that, in addition to Th1 cells, Th17 cells are involved in the pathogenesis of RA [17]. Th17 cells are activated by a CC chemokine CCL20/macrophage inflammatory protein-3α (MIP-3α). In fibroblast-like synoviocytes, neither IFN-γ treatment nor RIG-I siRNA affected the expression of CCL20/MIP-3α in synoviocytes (data not shown), and RIG-I may not be involved in Th17 type reactions.
We conclude that RIG-I is expressed at a high level in synovial tissues of RA and IFN-γ induces the expression of RIG-I in cultured fibroblast-like synoviocytes. RIG-I may play a role in the initiation and amplification of the inflammatory reactions in the synovium, at least in part, by enhancing the expression of CXCL10/IP-10.
Acknowledgments
The authors thank Dr Hiroshi Kijima, Kumiko Munakata and Miho Ono for their assistance.
References
- 1.Smith JB, Haynes MK. Rheumatoid arthritis − a molecular understanding. Ann Intern Med. 2002;136:908–22. doi: 10.7326/0003-4819-136-12-200206180-00012. [DOI] [PubMed] [Google Scholar]
- 2.Steiner G, Tohidast-Akrad M, Witzmann G, et al. Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology (Oxf) 1999;38:202–13. doi: 10.1093/rheumatology/38.3.202. [DOI] [PubMed] [Google Scholar]
- 3.Imaizumi T, Aratani S, Nakajima T, et al. Retinoic acid-inducible gene-I (RIG-I) is induced in endothelial cells by LPS and regulates expression of COX-2. Biochem Biophys Res Commun. 2002;292:274–9. doi: 10.1006/bbrc.2002.6650. [DOI] [PubMed] [Google Scholar]
- 4.Imaizumi T, Yagihashi N, Hatakeyama M, et al. Expression of retinoic acid-inducible gene-I in vascular smooth muscle cells stimulated with interferon-γ. Life Sci. 2004;75:1171–80. doi: 10.1016/j.lfs.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Imaizumi T, Hatakeyama M, Yamashita K, et al. Interferon-γ induces retinoic acid-inducible gene-I in endothelial cells. Endothelium. 2004;11:169–73. doi: 10.1080/10623320490512156. [DOI] [PubMed] [Google Scholar]
- 6.Imaizumi T, Kumagai M, Taima K, et al. Involvement of RIG-I in IFN-γ/STAT1 signaling pathway in BEAS-2B cells. Eur Resp J. 2005;25:1077–83. doi: 10.1183/09031936.05.00102104. [DOI] [PubMed] [Google Scholar]
- 7.Imaizumi T, Yagihashi N, Kubota K, et al. Expression of retinoic acid-inducible gene-I (RIG-I) in macrophages: possible involvement of RIG-I in atherosclerosis. J Atheroscler Thromb. 2007;14:51–5. doi: 10.5551/jat.14.51. [DOI] [PubMed] [Google Scholar]
- 8.Imaizumi T, Mori F, Yagihashi N, et al. Retinoic acid-inducible gene-I (RIG-I) and diseases. Hirosaki Med J. 2007;59:S137–42. [Google Scholar]
- 9.Suzuki K, Imaizumi T, Tsugawa K, et al. Expression of retinoic acid-inducible gene-I in lupus nephritis. Nephrol Dial Transplant. 2007;22:2407–9. doi: 10.1093/ndt/gfm175. [DOI] [PubMed] [Google Scholar]
- 10.Amano T, Yamasaki S, Yagishita N, et al. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 2003;17:2436–49. doi: 10.1101/gad.1096603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki M, Sakata KM, Oomizu S, et al. Beneficial effect of galectin 9 on rheumatoid arthritis by induction of apoptosis of synovial fibroblasts. Arthritis Rheum. 2007;56:3968–76. doi: 10.1002/art.23076. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida H, Imaizumi T, Lee SJ, et al. Retinoic acid-inducible gene-I mediates RANTES/CCL5 expression in U373MG human astrocytoma cells stimulated with double-stranded RNA. Neurosci Res. 2007;58:199–206. doi: 10.1016/j.neures.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 14.Lafyatis R, Remmers EF, Roberts AB, et al. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-β and retinoids. J Clin Invest. 1989;83:1267–76. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel DD, Zachariah JP, Whichard LP. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin Immunol. 2001;98:39–45. doi: 10.1006/clim.2000.4957. [DOI] [PubMed] [Google Scholar]
- 16.Salmon I, Netzer N, Wildbaum G, et al. Targeting the function of IFN-γ-inducible protein 10 supresses ongoing adjuvant arthritis. J Immunol. 2002;169:2685–93. doi: 10.4049/jimmunol.169.5.2685. [DOI] [PubMed] [Google Scholar]
- 17.Hirota K, Yoshitomi H, Hashimoto M, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–12. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]


