Abstract
Natural killer (NK) cells contribute to immunity as the first line of defence in numerous infections by early cytokine secretion and cytotoxicity. In Leishmania infection, NK cells contribute with interferon-γ and may assist in directing the immune response towards T helper type 1, which is essential for successful control of the parasites. Thus, NK cells may play an important role in both resistance and control of the infection. However, during Leishmania infection NK cells show signs of suppression. To explore the reason for this suppression, we exposed naive and interleukin (IL)-2 activated NK cells directly to promastigotes of Leishmania major in vitro. As a rapid consequence of contact between naive NK cells and promastigotes, expression of NK cell receptors show significant changes. We identify one of the major surface molecules of promastigotes, glycoprotein (gp) 63, as an important agent for these suppressive effects by using promastigotes of a gp63ko strain of L. major. Furthermore, proliferation of IL-2-activated purified NK cells is suppressed after exposure to the wild-type but not to gp63ko promastigotes. However, gp63ko L. major induced no NK cell proliferation when NK cells were co-cultured with peripheral blood mononuclear cells populations such as CD14+ monocytes or T cells.
Keywords: cutaneous leishmaniasis, gp63, human NK cells, Leishmania, proliferation
Introduction
Leishmania species are members of the trypanosomatidae family which cause a spectrum of diseases, ranging from life-threatening visceral disease to self-healing, localized cutaneous lesions of short duration. The various species of Leishmania have distinct properties that lead to different disease manifestations, and within the cluster causing cutaneous leishmaniasis (CL) there are many species differences which are yet to be elucidated fully. In mammalian hosts the extracellular promastigote form injected by the sandfly transforms into obligate intracellular amastigotes, with macrophages as the main host cells within which the amastigotes evade immune mechanisms [1].
Leishmania major, the agent of local CL, like most Leishmania spp., express predominantly three classes of molecules to form a surface glycocalyx: protein-free lipophosphoglycan (LPG), the glycosylphosphatidylinositol-anchored highly glycosylated proteophosphoglycan and glycoproteins (gp) of which gp63, a 63 kDa surface proteinase, is the most prominent. gp63 is expressed with > 500 000 copies (0.5–1% of total cell protein) and distributed over the entire promastigote body, including flagellum and flagellar pocket, and can be capped with antibodies which denotes that it is free to move in the plane of the membrane [2].
Glycoprotein 63, also described as leishmanolysin or major surface protease, is a zinc metalloprotease and has been reported to mediate entry into macrophages, enhancing phagocytosis and survival within the macrophage [3,4]. Furthermore, the proteinase activity of leishmanolysin has been demonstrated to exert control over complement activation, resulting in enhanced resistance to complement-mediated lysis [3,5]. In general, in mammals metalloproteases catalyse matrix remodelling but also facilitate recruitment of lymphocytes to sites of infection and cytokine and chemokine processing [6].
Because of the abundance of gp63 and its ability to mediate resistance against infectious promastigotes, gp63 has been suggested as a candidate for vaccination against Leishmania infection [7,8]; furthermore, murine dendritic cells (DC), when loaded with gp63 as antigen, enhanced the capability to control the parasite burden [9]. However, L. major knock-out (gp63ko) promastigotes exhibited decreased infectivity but caused a well-established infection in mice [10]. It is clear that a better understanding is necessary of how this abundant molecule of Leishmania interacts with the host immune system.
Natural killer (NK) cells are defined classically as CD3- and CD16/56+ and participate in innate immunity. Following infection by a broad range of pathogens such as viruses, bacteria and parasites, NK cells contribute to the immune response through cytotoxic activity and early cytokine production before the adaptive immunity is established (reviewed by [11]). In general NK cells are activated by cytokines mainly by interleukin (IL)-2, which induces strong proliferation of NK cells, but also by IL-12, tumour necrosis factor (TNF)-α or type I interferons (IFN) produced by infected cells [12]. NK cells also express a distinct repertoire of receptors that induce NK cell activity, including NKG2D, NKp46, NKp30 that are constitutively expressed on NK cells, and NKp44 that has been found only on activated NK cells [13]. On the other hand, NK cell activity is regulated to recognize host cells and prevent destruction or inflammatory activity by the binding of inhibitory NK receptors such as killer immunoglobin-like receptors and CD94/NKG2A to major histocompatibility complex (MHC) class I molecules [14]. The lack of MHC class I molecules make target cells susceptible to NK cell cytotoxicity, which is articulated in the missing-self theory [15].
During Leishmania infection, NK cells direct the immune response of T cells towards T helper type 1 (Th1) by releasing IFN-γ a few hours after infection [16]. Murine infection models show NK deficiency as a susceptibility factor leading to significantly higher replication and distribution of parasites comparable to vulnerability of mice after depletion of IFN-γ[17,18]. We have shown previously that live promastigotes of certain Leishmania species induce IFN-γ in human NK cells [19]. Because Leishmania enters the host cell in a ‘silent’ manner by inhibition of IL-12 and TNF-α induction [20], this activation is due most probably to receptor–ligand interactions, although LPG seems to have no influence because LPG-deficient strains of Leishmania still induce IFN-γ[19]. However, in ongoing CL NK cells seem to be suppressed after an initial activation, as patients reveal a significantly reduced number of NK cells [21].
In this study we investigated further the interaction of L. major with NK cells and demonstrate suppression of IL-2-stimulated NK cell proliferation and cytokine response after exposure to live L. major promastigotes. We identified gp63 as an important inhibitory molecule that acts through direct binding to a subset of NK cells.
Materials and methods
Donors
Patients with active CL [n = 21, all male, aged 19–45 (median: 21) years], infected when stationed in an L. major endemic area (Mehran, Iran), with lesion duration of 1–7 months volunteered for the study. Most patients had multiple lesions. Diagnosis was made based on clinical examination of lesions, isolation of parasites and their characterization using monoclonal antibodies and polymerase chain reaction, which confirmed L. major as the causative agent. Cells were analysed freshly or after cryopreservation. As control subjects for these donors, healthy Iranian volunteers [manual labourers, n = 10, all male, aged 29–50 (median: 33) years] with no history of leishmaniasis who were willing to participate and sign informed consent were recruited. Approximately 20–25 ml of blood was collected from each donor.
To assess the in vitro effect of Leishmania on NK cells and their function, blood-derived cells from healthy Swedish laboratory workers and blood donors were used.
Ethical approval for these studies was given by the Ministry of Health and Medical Education, Islamic Republic of Iran, and by the regional ethical committee at Karolinska Institutet, Stockholm, Sweden.
Preparation and isolation of NK cells, monocytes and T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from defibrinated or heparinized blood on a Ficoll gradient, as described previously [19,22]. NK cells and T cells were isolated by negative selection using the magnetic affinity cell sorting (MACS) NK cell isolation kit II and MACS Pan T cell isolation kit II (Miltenyi Biotech, Bergisch-Gladbach, Germany), according to the manufacturer's instructions. CD14+ monocytes were isolated by positive selection using MACS CD14 microbeads (Miltenyi Biotech). PBMC depleted of CD14+ cells were also collected. The purity of isolated NK cells, T cells and CD14+ cells was checked for each preparation using flow cytometry and found to be ≥ 90% CD16/56+CD3-, CD3+ and CD14+ respectively.
Isolation of CD4+ and CD8+ T cells was performed by cell sorting using the Aria fluorescence activated cell sorter (BD Biosciences, San Diego, CA, USA). T cells were labelled with anti-CD4-fluorescein isothiocyanate (FITC) and anti-CD8-allophycocyanin (APC) (BD Biosciences). The purity of the isolated populations was > 99%.
Parasites and antigens
The wild-type (wt) L. major strain NIH S (MHOM/SN/74/Seidman) clone A2 (A2WF), the gp63 ko and the gp63ko retransfected with L. major gp63 gene 1 [10] were propagated as described previously [23]. In contrast to other reported gp63ko variants which excluded the gp63 gene 7, this gp63ko strain has a complete deletion of gp63. Live promastigotes were harvested at stationary growth phase. Recombinant gp63 was produced at the Institute Pasteur as described previously [24] and was used at 5 μg/well, resulting in a final concentration of 25 μg/ml.
For the assessment of IFN-γ induction in Iranian patients and controls, NK cells were stimulated with L. major vaccine strain MRHO/IR/76/ER.
Parasite-to-cell ratio
The parasite-to-cell ratio used for each of the assays were determined following dose–response tests. The optimal ratio and cell numbers varied from assay to assay.
Surface marker expression
Purified NK cells (3 × 104) or bulk PBMC (1 × 105) were incubated in medium or in the presence of 5 × 104 wt, gp63ko and gp63ko retransfected L. major for 24 h and subsequently single-, double- or triple-stained using anti-human CD16/CD56, CD94 (BD Biosciences), NKp30, NKp44 and NKp46 (Beckman-Coulter, Fullertown, CA, USA).
Assessment of proliferation
Peripheral blood mononuclear cells were labelled with PKH-67 green dye (Sigma-Aldrich, Stockholm, Sweden) according to the manufacturer's instructions. Afterwards, NK cells were purified and 2 × 105 bulk PBMC or 3 × 104 NK cells were incubated with 5 × 104L. major wt and the gp63ko for 5 days in the presence or absence of 100 units/ml IL-2 using RPMI-1640 medium supplemented with 10% human antibody-positive serum respectively. Cells were washed once with phosphate-buffered saline (PBS) supplemented with 1% fetal calf serum (FCS), stained for NK surface markers CD56 and CD16, washed once again in PBS and 1% FCS and fixed with 4% paraformaldehyde (PFA).
In some experiments T cells were isolated from bulk PBMC and 1 × 105 T cells were incubated with purified NK cells and L. major promastigotes. Furthermore, PBMC were depleted of CD14+ monocytes and 5 × 104 of the isolated CD14+ monocytes were added to the purified NK cells or recombinant gp63 were added to bulk PBMC or purified NK cells respectively.
Assessment of IFN-γ secretion by enzyme-linked immunospot assay
Enzyme-linked immunospot assays were performed as described previously [19] using commercial IFN-γ enzyme-linked immunospot (ELISpot) kits (Mabtech, Stockholm, Sweden) and filter-bottomed plates (MAHA S45 or ELIIP; Millipore, Molsheim, France). Spot-forming units were counted using a computerized ELISpot counting system (AID, Strassberg, Germany).
Assessment of intracellular IFN-γ by flow cytometry
Purified NK cells (2–3 × 105) were incubated with 5 × 104 parasites in 96-well round-bottomed plates. Intracellular IFN-γ/IL-10 was analysed after 48 h and 5 days incubation using the BD Cytofix-Cytoperm kit, according to the manufacturer's instructions. To allow maximum accumulation of cytokine, monensin (GolgiStopTM; BD Biosciences) was added to the cultures for the last 8 h of culture. Production of IFN-γ was assessed using anti-human IFN-γ antibodies and negative immunoglobulin (Ig)G–APC control (BD Biosciences).
Biotinylation of recombinant gp63
Four hundred μg of recombinant (r)-gp63 in 1 ml PBS were incubated for 2 h on ice with 20 μl EZ-link sulpho–NHS–biotin reagent solution, prepared according to the manufacturer's instructions (Pierce, Rockford, USA). Afterwards, non-labelled biotin was removed with a dialysis chamber (Pierce) in PBS overnight.
For detection of gp63 binding on NK cells, 8 μg biotin-labelled r-gp63 were incubated with purified NK cells for 30 min on ice. Cells were washed subsequently in PBS and 1% FCS and incubated with streptavidin–FITC (BD Bioscience) for another 30 min on ice and washed once again in PBS and 1% FCS and fixed with 4% PFA.
Statistical analysis
Samples were analysed with SigmaStat version 2·03 (San Rafael, CA, USA) using the two-way anovaF-test.
Results
Leishmania major promastigotes do not activate NK cells to IFN-γ secretion
While our previous report showed that several Leishmania spp. were potent inducers of contact-dependent IFN-γ secretion [19], subsequent studies using L. major (MRHO/IR/76/ER) vaccine strain parasites indicated that this species failed to induce an IFN-γ response in purified NK cells from both patients and controls. More interestingly NK cells from CL patients tended to produce less IFN-γ, seen both as spontaneous and after exposure to stimuli (phytohaemagglutinin and Leishmania promastigotes) compared with controls (Fig. 1). We thus hypothesized that in addition to the possible IFN-γ activating ability of some strains, other species, e.g. L. major, may be able to down-modulate NK cell responses/function.
Fig. 1.
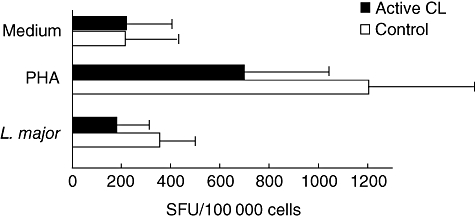
Leishmania major suppresses interferon (IFN)-γ response of purified natural killer (NK) cells. NK cells from active cutaneous leishmaniasis (CL) patients (n = 8; black bars) and healthy endemic controls (n = 8; open bars) were restimulated with non-specific stimulator phytohaemagglutinin or with live promastigotes of L. major and the frequency of IFN-γ spot-forming units (SFU) was assessed after 48 h. All NK cells were isolated by negative selection. Results shown are mean SFU + standard deviation.
This hypothesis is in line with results using a second L. major strain (Seidman A2WF) to assess IFN-γ production in healthy Swedish donors. However, again there was no induction of IFN-γ in naive purified NK cells (mean ± standard deviation: medium 4.7%; A2WF 1.1% IFN-γ-producing NK cells).
Interleukin-2-induced NK IFN-γ secretion can be suppressed by L. major
It is well known that NK cells respond with proliferation and IFN-γ production when stimulated with IL-2. Thus, we tested the effect of L. major (Seidman A2WF) promastigotes on IL-2-induced IFN-γ production by NK cells. We found that addition of L. major to IL-2 treated NK cells reduced the frequency of IFN-γ-producing cells in four of six donors (medium 30·0 ± 13·7%; A2WF 7·8 ± 3·2% IFN-γ-producing NK cells).
The variation in responses between individuals was extremely large; thus, to achieve significant differences between groups the sample sizes also need to be extremely large, with previous reports supporting the results reported here implicating an inhibition of NK cells during CL [21]. We followed-up this trend and tested if the L. major parasite may have an inhibitory effect on NK cells and mechanisms that may lie behind inhibition.
Promastigotes modulated NK cell phenotype by gp63
Although the function is unknown the abundant molecule gp63, found on the surface of most Leishmania spp., has interesting chemical properties [2], which we speculated could have an effect on NK cells. Thus, we assessed if gp63 affects NK cells by exposing bulk PBMC or purified NK cells to L. major wt (A2WF) or promastigotes where the gp63 had been ko. When the gp63 gene had been deleted, we found that inhibition of IL-2 induced the IFN-γ response, seen using wt parasites, was not particularly pronounced (A2WF 7·8 ± 3·2%; gp63ko 17·4 ± 4·7% IFN-γ-producing NK cells), although light microscopic investigation of interactions between NK cells with L. major revealed contact formation with both wt A2WF and gp63ko promastigotes (data not shown).
Because changes of NK cell IFN-γ production tended to be fewer with gp63ko promastigotes compared with the A2WF, we analysed more effects on NK cells after exposure to both forms of promastigotes.
Following 24 h exposure to purified NK cells, L. major wt promastigotes induced an appreciable reduction of expression of CD16, CD56, NKp30 and, to a certain degree, some reduction of NKp44, whereas the expression of CD94 and NKp46 remained unchanged. In contrast, incubation of NK cells with the gp63ko promastigotes did not affect significantly the expression levels of any of the NK cell surface markers assessed. To verify gp63 as a key responsible agent for receptor expression modulation, NK cells were exposed to L. major ko promastigotes retransfected with gp63 gene and again found a down-modulation of receptors (Fig. 2a).
Fig. 2.
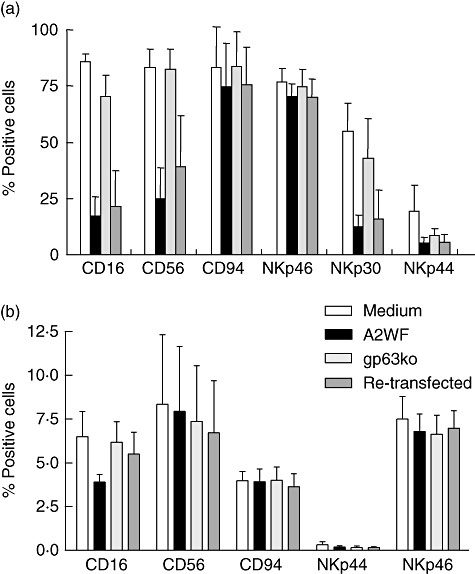
Natural killer (NK) receptor expression is reduced after co-culture with promastigotes. Purified NK cells (3 × 104) (a) or bulk peripheral blood mononuclear cells (1 × 105) (b) from healthy Swedish donors exposed to 5 × 104Leishmania major promastigotes of wild-type (wt) and gp63ko for 24 h were stained for the indicated NK receptors. To confirm gp63 as an agent of the down-regulation of the receptors, NK cells were incubated with gp63ko promastigotes which were retransfected with the gp63 gene. Results are shown as percentage of stained cells, mean ± standard deviation of three independent experiments.
However, assessment of characteristic NK receptors in bulk PBMC revealed changes in expression only for CD16, whereas all other tested receptors remained similar after exposure to L. major wt or the gp63ko promastigotes (Fig. 2b).
Proliferation of activated NK cells is abrogated by gp63
To investigate further the effect of L. major on NK cell function, we induced proliferation of isolated NK cells (Fig. 3) or bulk PBMC (Fig. 4) by co-culture with promastigotes alone or in combination with IL-2 and monitored proliferation by flow cytometry by staining lymphocytes with the fluorescent dye PKH-67. In these experiments freshly purified NK cells (purity: CD56+/CD16+: 78·8%; CD56+: 10·4%; CD16+: 5·0%; in total: 94·2%. Fig. 3a) were exposed to L. major for 5 days, which is different to the assessment of NK receptor expression shown in Fig. 2. This prolonged incubation leads to considerable numbers of NK cells that seemed to be CD56-negative even in the absence of L. major, which might be due to spontaneous loss of this receptor (Fig. 3b, upper row). However, in contrast to the findings after 24 h, in 5 days' exposure of naive NK cells to L. major promastigotes, not only did the wt lead to reduced CD56 expression to almost zero, but the gp63ko promastigotes also diminished CD56 expression significantly. CD16 expression was also reduced using both the wt and the gp63ko promastigotes (medium 26·1 ± 6·2%; A2WF 3·4 ± 1·5% and gp63ko 7·1 ± 3·4% CD16-positive cells).
Fig. 3.
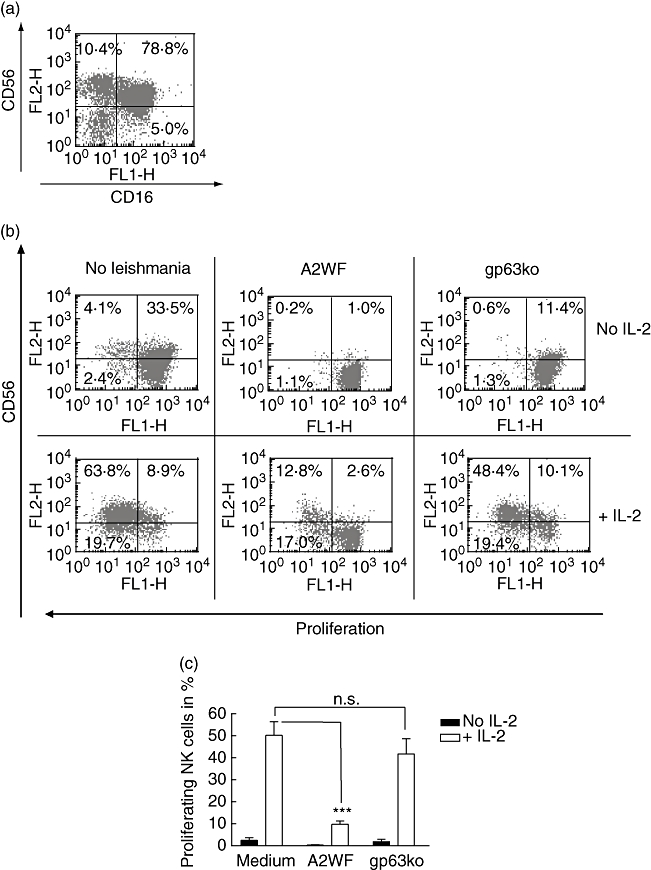
Leishmania major wild-type (wt) but not gp63ko promastigotes inhibit proliferation of purified natural killer (NK) cells; 3 × 104 PKH-labelled purified NK cells were incubated with 5 × 104L. major wt and the gp63ko for 5 days with or without 100 units/ml interleukin (IL)-2 respectively. CD56 expression decreased during incubation significantly even if incubated in medium alone (b, upper row, left scattergram), although freshly isolated NK cells (CD16+/CD56+ cells) showed a purity of > 90% (a). However, in presence of L. major wt CD56 expression was abrogated or decreased in the presence of L. major gp63ko. Only weak spontaneous proliferation is detectable in the absence of IL-2. Addition of exogenous IL-2 enhanced the CD56 expression and stimulated cell division in medium and in presence of gp63ko but not in the presence of L. major wt (b). Proliferation of NK cells is indicated in a representative scattergram by decreasing fluorescence of CD56+ cells (b) or as graphs summarizing three independent experiments mean + standard deviation (c); n.s.: not significant; *P-value ≤ 0·05; **P-value ≤ 0·01; ***P-value ≤ 0005.
Fig. 4.
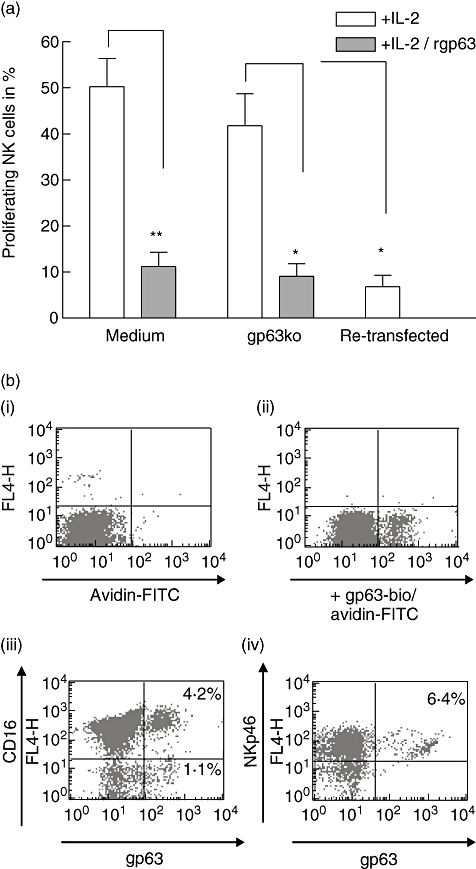
gp63 binds directly on natural killer (NK) cells and inhibits proliferation of purified NK cells; 3 × 104 PKH-labelled purified NK cells were stimulated with 100 units/ml interleukin (IL)-2 and incubated in medium alone (medium) or exposed to 5 × 104Leishmania major gp63ko promastigotes (gp63ko) and gp63ko L. major retransfected with gp63 (retransfected) for 5 days respectively. Cultures with medium and gp63ko parasites were either untreated or supplemented with 25 μg/ml recombinant gp63 (a). To confirm direct binding of gp63 to NK cells, recombinant gp63 was biotinylated (gp63-bio) and detected on NK cells with streptavidin/fluorescein isothiocyanate (b). Binding of gp63-bio (b, ii) was significant over background (b, i). Double staining with anti-CD-16 (b, iii) and NKp46 (b, iv) revealed that NK cells bind gp63. Results are shown as graphs summarizing four independent experiments (a) or as a representative scattergram of three independent experiments (b); n.s.: not significant; *P-value ≤ 0·05; **P-value ≤ 0·01; ***P-value ≤ 0005.
Natural killer cells incubated with IL-2 in the absence of L. major parasites proliferate strongly. Interestingly, in the presence of L. major wt the IL-2-induced proliferation of NK cells was blocked almost completely (P ≤ 0·0073). This inhibition correlated with gp63 expression on the promastigotes, as IL-2-induced proliferation of NK cells was only slightly less in the presence of gp63ko promastigotes in comparison with the medium control (P= 0·15) (Fig. 3b and c).
Glycoprotein 63 binds directly on NK cells
To determine whether gp63 alone is sufficient to inhibit proliferation of NK cells or whether bystander molecules on the surface of L. major promastigotes are necessary for suppression, we analysed the proliferation of NK cells after incubation with rgp63. In the presence of recombinant (r)-gp63, IL-2-induced NK cell proliferation was abrogated in the presence of gp63ko promastigotes (P ≤ 0·0424) or in medium alone (P ≤ 0·0113). Furthermore, retransfection of the gp63 gene to L. major gp63ko shows that suppression of proliferation is gp63-dependent, as these promastigotes triggered inhibition similar to that seen using A2WF or adding rgp63 to L. major gp63ko (P ≤ 0·0429) (Fig. 4a).
Biotinylation of recombinant gp63 and detection with fluorochrome-labelled streptavidin revealed direct binding of gp63 to NKp46+ NK cells (Fig. 4b). Interestingly, just a subpopulation of NK cells bound gp63, and not all of these were CD16+.
Proliferation of NK cells among bulk PBMC is abrogated even in the presence of gp63ko promastigotes
Surprisingly for us, 5-day incubation of bulk PBMC with L. major wt A2WF and gp63ko promastigotes revealed different results compared with purified NK cells. First, in the absence of IL-2, naive PBMC express normal/high levels of CD56, which is still down-regulated significantly by L. major wt but not by gp63ko. In contrast, the expression of CD56 is increased slightly in comparison with medium control (Fig. 5a, upper row). In the presence of IL-2, CD56 expression in medium control is increased (35% in comparison with 28% in absence of IL-2), whereas expression of CD56 in the presence of L. major wt is significantly lower, albeit with increased levels in comparison with PBMC exposed to the wt without IL-2. However, in the case of gp63ko L. major there is less CD56 expression detectable after IL-2 stimulation in comparison with non-exposed PBMC but still significantly more in comparison with the wt (Fig. 5a, lower row).
Fig. 5.
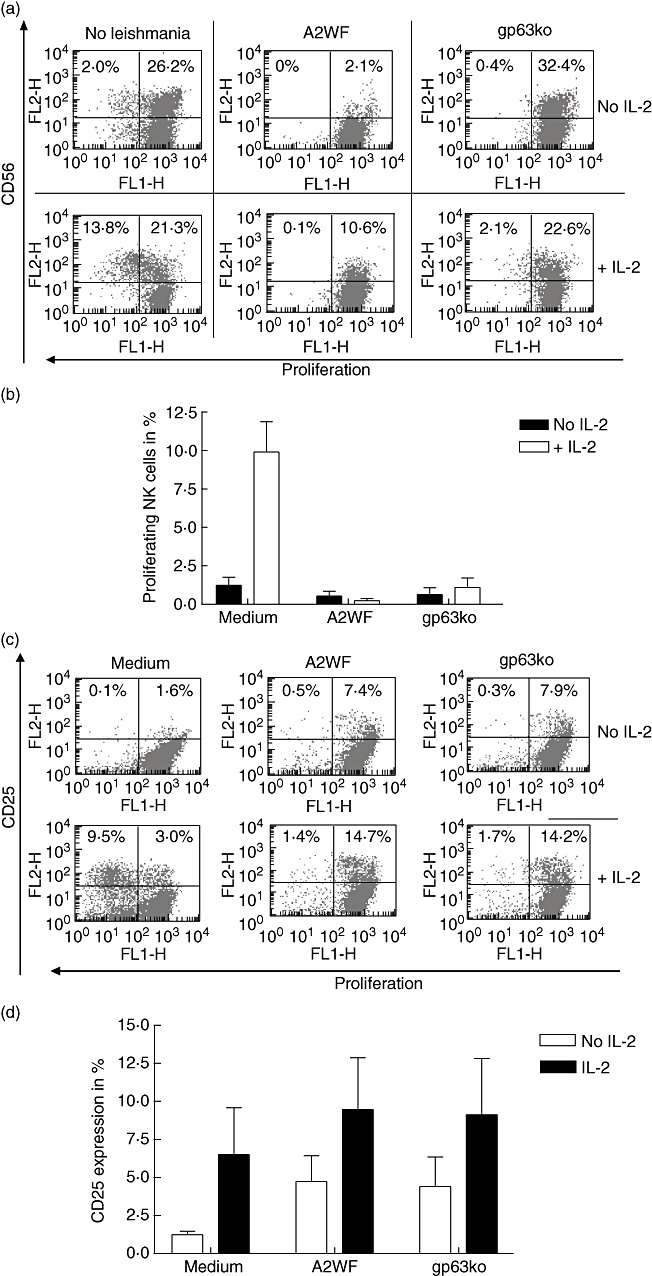
Bulk peripheral blood mononuclear cells (PBMC) show no signs of proliferation even after exposure to gp63ko Leishmania major; 2 × 105 PKH-labelled bulk PBMC were incubated with 5 × 104L. major wild-type (wt) and the gp63ko for 5 days with or without 100 units/ml interleukin (IL)-2 respectively. In contrast to isolated natural killer (NK) cells, CD56 expression in medium alone or in presence of gp63ko is not diminished, whereas L. major wt reduced the levels of expression almost completely. Proliferation of NK cells is indicated in a representative scattergram by decreasing fluorescence of CD56+-positive cells (a) or as graphs summarizing three independent experiments mean ± standard deviation (b). To evaluate if inhibition of proliferation is caused by blockage of IL-2 receptor, expression of CD25 in the absence and presence of IL-2 was assessed (c + d). Exposing bulk PBMC to L. major promastigotes led to increased CD25 expression, although lymphocytes did not proliferate. Results are shown as a representative scattergram (c) or as graphs summarizing three independent experiments (d).
None the less, the most important difference to purified NK cells is suppression of NK cell proliferation, even after exposure of bulk PBMC to gp63ko L. major in the presence of IL-2 (Fig. 5a and b).
One possibility for the inhibition of proliferation is that exogenous IL-2 does not have an impact on the NK cells because the IL-2 receptor expression is blocked by L. major. To exclude this possibility, we assessed the expression of CD25 in the presence or absence of IL-2 after exposure to promastigotes. Surprisingly, the CD25 expression was higher by incubation of the PBMC with L. major (both wt and gp63ko) compared with the medium control, although L. major did not induce proliferation in the absence of IL-2 and blocked it after IL-2 stimulation (Fig. 5c and d). This lack of proliferation in bulk PBMC incubated with L. major with or without IL-2 could not be attributed to cell death because of exhaustion of the nutrients in the culture, as the medium did not show signs of pH changes and cells showed normal expression of surface receptors, such as CD3.
T cells inhibit NK cell proliferation
Inhibition of NK cells in bulk PBMC, even with the gp63ko promastigotes, could indicate an interference of NK cells with other cells. Such cells would most probably be the ones able to process and present Leishmania antigens which react with bystander effects, abrogating proliferation through a mechanism which is as yet undefined. To address this we purified CD14+ monocytes from bulk PBMC, as these cells are the main host cells for L. major, and added them to purified NK cells. The co-incubation of NK cells and monocytes with gp63ko promastigotes caused a significant decrease of proliferation (data not shown). Paradoxically, the depletion of CD14+ from bulk PBMC also did not induce proliferation of NK cells (Fig. 6a). Thus, additionally to monocytes, other cell types appear to interact with NK cells to block their proliferation after activation. We thus isolated CD3+ lymphocytes and incubated them together with gp63ko L. major and purified NK cells and observed complete inhibition of NK cell proliferation. Furthermore, we separated CD4+ and CD8+ T cells by cell sorting with a purity of 99·5% and added them to the NK cells exposed to gp63ko L. major. Both subpopulations are able to suppress NK cell proliferation (Fig. 6b).
Fig. 6.
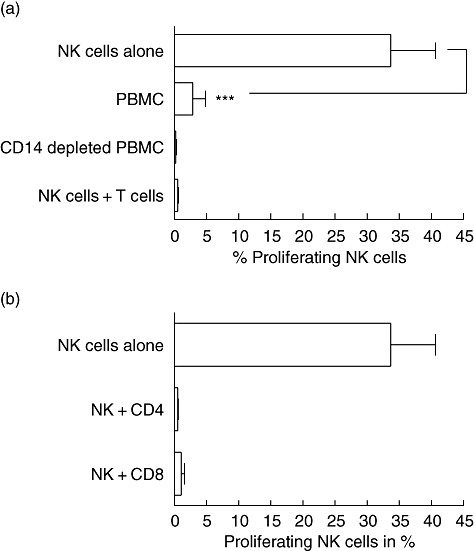
Natural killer (NK) cell proliferation is regulated by different cell types. Leishmania major gp63ko promastigotes allow proliferation of isolated NK cells but not among bulk peripheral blood mononuclear cells (PBMC), thus other cell populations must participate in blocking NK division in the presence of Leishmania; 3 × 104 PKH-labelled purified NK cells and 2 × 105 bulk PBMC or PBMC depleted of CD14+ monocytes were incubated with 5 × 104L. major gp63ko promastigotes for 5 days in the presence of 100 units/ml interleukin (IL)-2 respectively. As no changes in proliferation of CD14+ depleted PBMC could be observed, NK cells were supplemented with 1 × 105 purified CD3+ lymphocytes and incubated as described above (a). Addition of T cells to purified NK cells suppresses NK proliferation. To assess which T cell population cause this inhibition, we isolated CD4+ and CD8+ T cells by cell sorting with highest purity and added 1 × 105 cells to the purified NK cells, as described above. Both T cell populations are able to block NK cell proliferation (b). All lymphocytes were PKH-labelled and proliferation was analysed using flow cytometry. Graphs represent three independent experiments mean ± standard deviation; n.s.: not significant; *P-value ≤ 0·05; **P-value ≤ 0·01; ***P-value ≤ 0005.
Discussion
Natural killer cells are important players in providing protective immunity in L. major disease, as has been described in mouse infection models [18]. However, the role of NK cells in human infection is yet to be elucidated, but there are signs of suppression of NK cells and their release of cytokines such as IFN-γ in patients infected with L. aethiopica, causing CL [21] and L. major. The decrease in numbers of NK cells in the blood of patients with active disease reported by Maasho et al. [21] may be explained in several ways, including: (i) NK cells die to a higher extent or (ii) the receptors characteristic for NK cells are down-regulated, thus they cannot be identified during active infection. The in vitro results following exposure of L. major promastigotes to NK cells, which showed significantly lower expression levels of certain receptors, is consistent with the second possibility. However, incubation of purified NK cells with L. major gp63ko promastigotes did not change the receptor expression significantly. This L. major gp63ko strain has all seven gp63 genes deleted [10]. In general these ko parasites show a decreased virulence, but are still able to cause significant footpad swelling. Importantly, retransfection of gp63 gene 1 led to Leishmania-induced NK receptor down-regulation similar to L. major wt promastigotes. As gp63 is a metalloprotease the loss of the receptors may be due to cleavage, as has been reported for CD16 receptors on NK cells [25], and not by down-regulation of protein expression. However, the selective loss of some receptors including CD16, CD56 and NKp30 and the unchanged expression of other receptors such as CD94 and NKp46 indicate other mechanisms for the changes in receptor expression than non-specific protease activity. This is in line with the results using bulk PBMC exposed to L. major, where almost no changes in receptor expression of NK cells could be observed.
Effects on NK receptor expression caused by pathogens has also been described in other infections. Following human immunodeficiency virus (HIV) infection, NK cells change their expression of receptors including NKp30, NKp44 and NKp46, whereas NKG2D remained unchanged. Furthermore, NK cells from HIV patients undergo a selective loss of the cytolytic, CD16high/CD56low NK cell subset and an expansion of CD16high/CD56– which secrete lower amounts of cytokines and chemokines, and respond less to cytokines than the CD16high/CD56low subset [26].
Activation of NK cells was also detected through increased IL-2 receptor expression of PBMC and NK cells (not shown) after exposure to L. major. However, despite the enhanced CD25 expression NK cells were inhibited in proliferation even in the presence of a high dose of exogenous IL-2. This inhibition of cell division was caused by L. major, and in purified NK cells gp63 could be defined as (one) ligand for as yet unknown inhibitory receptor(s), as addition of recombinant gp63 to NK cells also led to inhibition of proliferation.
The limitation of extrapolation of the results of in vitro methods using promastigotes for understanding of in vivo findings is acknowledged, particularly as long-lasting exposure of NK cells to the infectious promastigote form is unlikely. However, expression of gp63 has been described for amastigotes even though at lower levels [27], and even more interestingly a proteolytic active form of gp63 is released from the surface of the parasites as well as secreted from the cytoplasm [28]. Thus, while there might be rare contacts between NK cells and promastigotes in the mammalian host there might still be potential of exposure of NK cells to gp63. We plan to assess the possible in vivo relevance of our initial findings with promastigotes in in vitro studies using intracellular amastigote forms.
Human NK cells have been reported to express sialic acid binding Ig-like lectins (Siglec)-7 which have inhibitoryfunctions on NK cell activity [29]. Numerous cell–cellinteractions are based on sialic acids connected to gp and specific lectins. gp63 is highly glycosylated with mannose and N-acetylglucosamine and N-acetylgalactosamine [2], thus sialization of gp63 might be possible. To assess if Siglec–gp63 interactions are responsible for the suppression of proliferation we treated both with sialidase from Clostridium perfringens. However, the effect was very weak, thus we assume that Siglec-7 is not involved in inhibition of proliferation (data not shown).
This is supported by the finding that upon exposure to L. major only a subset of NK cells is able to bind gp63. Interestingly, this subset cannot be assigned to the general subpopulation described as CD16high/CD56low and CD16low/CD56high[30], as recombinant and biotinylated gp63 binds to NKp46+/CD16+ NK cells as well as to NKp46+/CD16– NK cells. Thus, one might consider the possibility of another unique subset of NK cells that bind gp63. Interestingly, although only this subset of NK cells was able to bind gp63, even recombinant gp63 itself inhibited proliferation, indicating possible regulatory functions of this subset on all NK cells.
Regulatory function of NK cells on other cell population has become increasingly within the focus of NK research. As well as the strong contact of NK cells with immature and mature DC mediated through NKp30 [31], there is evidence of direct antigen non-specific interactions of NK cells with T cells in multiple sclerosis. Here again, only a certain subset of NK cells has a regulatory function on autoimmune T cells to suppress their IFN-γ response [32]. This is in line with our performed in vitro experiments, where the IFN-γ response of NK cells after exposure to promastigotes revealed a suppressed cytokine release by IL-2-activated NK in contrast to naive NK cells, which are activated by extracellular promastigotes [19].
However, regulation/inhibition of NK cell proliferation in L. major infection is not only driven by gp63. To our surprise, we found suppression of NK proliferation among other cell populations of bulk PBMC, even when gp63ko parasites were used. To identify the cell population mediating this general inhibition we incubated NK cells with purified CD14+ monocytes and still found proliferation, although to a considerably reduced degree. Contact of NK cells with macrophages and DC presenting Leishmania antigen has been shown for murine cells in vitro and in situ in draining lymph nodes [33,34]. However, antigen-presenting monocytes seem not to be the cell type responsible for the inhibition of proliferation and cytokine production among bulk PBMC. Purified T cells abrogated NK cell proliferation completely. Interestingly, both CD4 and CD8 T cells are able to act as suppressors for NK cells. The understanding of interactions of NK cells with T cells is still at a preliminary stage and restricted to activatory actions [34]. In contrast, little is known about possible influence of T cells on NK cell activity.
This study concurs with previous indications of NK cell suppression during CL. The consequences of inhibited NK cell response in the early phase of infection in humans needs to be elucidated. However, not only proliferation and receptor expression of NK cells are affected by L. major; IFN-γ release is also suppressed during active CL, which might have an important impact by directing the immune response away from a Th1 response crucial to control the parasites. This finding differs from our previous findings that Leishmania promastigotes induce IFN-γ from NK cells. However, the species tested in those studies ranged from L. aethiopica and L. donovani to L. mexicana, although L. major was not analysed and it might be reasonable to suppose that agents of different diseases interact differently with the immune system. Such comparisons will need to be conducted. Furthermore, the link between these findings and the in vivo situation has to be evaluated further using amastigote-infected monocytes and closer evaluation of ex-vivo cells from patients.
Obstruction of NK cells by Leishmania or even by Leishmania-derived molecules might cause diminished ability to induce a strong immune response against Leishmania. To create efficient vaccines against Leishmania disease it is fundamental to understand the initial interplay between Leishmania and the cells relevant for first-line defence.
References
- 1.Descoteaux A, Turco SJ. Glycoconjugates in Leishmania infectivity. Biochim Biophys Acta. 1999;1455:341–52. doi: 10.1016/s0925-4439(99)00065-4. [DOI] [PubMed] [Google Scholar]
- 2.Wright EP, el Amin ER. Leishmania infection: surfaces and immunity. Biochem Cell Biol. 1989;67:525–36. doi: 10.1139/o89-084. [DOI] [PubMed] [Google Scholar]
- 3.Brittingham A, Morrison CJ, McMaster WR, McGwire BS, Chang KP, Mosser DM. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155:3102–11. [PubMed] [Google Scholar]
- 4.Russell DG. The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. Eur J Biochem. 1987;164:213–21. doi: 10.1111/j.1432-1033.1987.tb11013.x. [DOI] [PubMed] [Google Scholar]
- 5.Joshi PB, Kelly BL, Kamhawi S, Sacks DL, McMaster WR. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol Biochem Parasitol. 2002;120:33–40. doi: 10.1016/s0166-6851(01)00432-7. [DOI] [PubMed] [Google Scholar]
- 6.Elkington PT, O'Kane CM, Friedland JS. The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol. 2005;142:12–20. doi: 10.1111/j.1365-2249.2005.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handman E, Button LL, McMaster RW. Leishmania major: production of recombinant gp63, its antigenicity and immunogenicity in mice. Exp Parasitol. 1990;70:427–35. doi: 10.1016/0014-4894(90)90127-x. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento E, Mayrink W, da Costa CA, et al. Vaccination of humans against cutaneous leishmaniasis: cellular and humoral immune responses. Infect Immun. 1990;58:2198–203. doi: 10.1128/iai.58.7.2198-2203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berberich C, Ramirez-Pineda JR, Hambrecht C, Alber G, Skeiky YA, Moll H. Dendritic cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J Immunol. 2003;170:3171–9. doi: 10.4049/jimmunol.170.6.3171. [DOI] [PubMed] [Google Scholar]
- 10.Joshi PB, Sacks DL, Modi G, McMaster WR. Targeted gene deletion of Leishmania major genes encoding developmental stage-specific leishmanolysin (GP63) Mol Microbiol. 1998;27:519–30. doi: 10.1046/j.1365-2958.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 11.Tosi MF. Innate immune responses to infection. J Allergy Clin Immunol. 2005;116:241–9. doi: 10.1016/j.jaci.2005.05.036. quiz 50. [DOI] [PubMed] [Google Scholar]
- 12.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 13.Biassoni R, Cantoni C, Falco M, et al. Human natural killer cell activating receptors. Mol Immunol. 2000;37:1015–24. doi: 10.1016/s0161-5890(01)00018-9. [DOI] [PubMed] [Google Scholar]
- 14.Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Human natural killer cells: molecular mechanisms controlling NK cell activation and tumor cell lysis. Immunol Lett. 2005;100:7–13. doi: 10.1016/j.imlet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–8. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 16.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–77. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belosevic M, Finbloom DS, Van Der Meide PH, Slayter MV, Nacy CA. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–74. [PubMed] [Google Scholar]
- 18.Laskay T, Diefenbach A, Rollinghoff M, Solbach W. Early parasite containment is decisive for resistance to Leishmania major infection. Eur J Immunol. 1995;25:2220–7. doi: 10.1002/eji.1830250816. [DOI] [PubMed] [Google Scholar]
- 19.Nylen S, Maasho K, Soderstrom K, Ilg T, Akuffo H. Live Leishmania promastigotes can directly activate primary human natural killer cells to produce interferon-gamma. Clin Exp Immunol. 2003;131:457–67. doi: 10.1046/j.1365-2249.2003.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrera L, Gazzinelli RT, Badolato R, et al. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–26. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maasho K, Sanchez F, Schurr E, Hailu A, Akuffo H. Indications of the protective role of natural killer cells in human cutaneous leishmaniasis in an area of endemicity. Infect Immun. 1998;66:2698–704. doi: 10.1128/iai.66.6.2698-2704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7. [PubMed] [Google Scholar]
- 23.Maasho K, Akuffo HO. Cells from healthy non-exposed individuals produce cytokines to selected fractions of Leishmania promastigotes. Scand J Immunol Suppl. 1992;11:179–84. doi: 10.1111/j.1365-3083.1992.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 24.Habibi GR, Khamesipour A, McMaster WR, Mahboudi F. Cytokine gene expression in healing and non-healing cases of cutaneous leishmaniasis in response to in vitro stimulation with recombinant gp63 using semi-quantitative RT–PCR. Scand J Immunol. 2001;54:414–20. doi: 10.1046/j.1365-3083.2001.00990.x. [DOI] [PubMed] [Google Scholar]
- 25.Harrison D, Phillips JH, Lanier LL. Involvement of a metalloprotease in spontaneous and phorbol ester-induced release of natural killer cell-associated Fc gamma RIII (CD16-II) J Immunol. 1991;147:3459–65. [PubMed] [Google Scholar]
- 26.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–43. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 27.Leifso K, Cohen-Freue G, Dogra N, Murray A, McMaster WR. Genomic and proteomic expression analysis of Leishmania promastigote and amastigote life stages: the Leishmania genome is constitutively expressed. Mol Biochem Parasitol. 2007;152:35–46. doi: 10.1016/j.molbiopara.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.McGwire BS, O'Connell WA, Chang KP, Engman DM. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J Biol Chem. 2002;277:8802–9. doi: 10.1074/jbc.M109072200. [DOI] [PubMed] [Google Scholar]
- 29.Yamaji T, Teranishi T, Alphey MS, Crocker PR, Hashimoto Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J Biol Chem. 2002;277:6324–32. doi: 10.1074/jbc.M110146200. [DOI] [PubMed] [Google Scholar]
- 30.Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56 (bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 31.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi K, Aranami T, Endoh M, Miyake S, Yamamura T. The regulatory role of natural killer cells in multiple sclerosis. Brain. 2004;127:1917–27. doi: 10.1093/brain/awh219. [DOI] [PubMed] [Google Scholar]
- 33.Aranha FC, Ribeiro U, Basse P, Corbett Jr, Laurenti MD. Interleukin-2-activated natural killer cells may have a direct role in the control of Leishmania (Leishmania) amazonensis promastigote and macrophage infection. Scand J Immunol. 2005;62:334–41. doi: 10.1111/j.1365-3083.2005.01681.x. [DOI] [PubMed] [Google Scholar]
- 34.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. NK cell regulation of T cell-mediated responses. Mol Immunol. 2005;42:451–4. doi: 10.1016/j.molimm.2004.07.025. [DOI] [PubMed] [Google Scholar]


