Abstract
The deposition of immune complexes (IC) induces an acute inflammatory response with tissue injury, for which the involvement of nitric oxide (NO) and carbon monoxide (CO) has been suggested. NO is induced by NO synthase (NOS) and CO is generated by haeme oxygenase (HO). Among HO isoenzymes, HO-1 is an induced type. To assess the role of NO and CO in the pathogenic process, the cutaneous reverse passive Arthus reaction was examined using NOS inhibitor, HO-1 stimulator and HO-1 inhibitor. To evaluate the reaction we considered oedema, tumour necrosis factor-α, interleukin-6, and neutrophil number. The values of these four parameters were significantly reduced in mice treated with HO-1 stimulator as compared with the positive control mice. Quite the reverse was observed in mice treated with HO-1 inhibitor. These results suggest that the HO-1/CO signalling pathway is a therapeutic target for human IC-mediated disease.
Keywords: Arthus reaction, carbon monoxide, haeme oxygenase-1, nitric oxide
Introduction
The formation and local deposition of immune complexes (IC) are thought to be important factors that induce acute inflammatory responses with significant tissue injury. IC injury has been implicated in a variety of human diseases, including vasculitis syndrome, systemic lupus erythematosus, rheumatoid arthritis and glomerulonephritis [1]. The classical and standard animal model for the inflammatory response in these IC-mediated diseases is the Arthus reaction, which results in oedema, haemorrhage and neutrophil recruitment in the skin by the intradermal injection of horse serum repeatedly into a rabbit [2]. Because of its ease and reproducibility, the experimental model most commonly used is the reverse passive Arthus reaction, in which an antibody (Ab) is injected at the site where the investigator wants the inflammatory response to develop and the antigen is applied intravenously immediately before or after the Ab injection. As the accumulations of neutrophils, nuclear dusts, fibrin deposits, haemorrhage and oedema are recognized in this model, this reverse passive Arthus reaction is thought to be a very good model for vasculitis in the field of dermatology [3,4].
On the other hand, the involvement of reactive oxygen species (ROSs) has been reported in the pathogenesis of tissue injury in the inflammation [5]. Among ROSs, nitric oxide (NO) is a gaseous radical whose role has been important in the aetiology of many diseases [6], ever since NO was found to be an endothelium-derived relaxing factor in 1991 [7]. Furthermore, NO reacts with superoxide, which is also one of the ROSs, and immediately produces peroxynitrite, which can cause severe cytotoxicity [8]. NO is produced from L-arginine by NO synthase (NOS), and three isoforms have been identified. Meanwhile, carbon monoxide (CO) is a gaseous molecule, like NO, and is induced by haeme oxygenase (HO) in the process of haeme metabolism [9]. Recently, CO has been highlighted because of its involvement especially in inflammatory diseases [10]. Both NO and CO are low molecular monoxides and have actions of vasodilatation. In addition, they can activate soluble guanylate cyclase and an increase in the level of cyclic GMP. However, NO is a radical and reported to show severe cytotoxicity, whereas CO is stable and thought to be cytoprotective [11].
With regard to NO, the possibility was reported that endothelial damage was reduced by the inhalation of NO gas during lung transplantation [12], but it was also reported that the clinical application of an NOS inhibitor could control the drop in blood pressure of a patient with septic shock [13]. Regarding CO, Giannini et al. reported the protection from cardiac injury by induction of HO-1 in a focal ischemia-reperfusion model [14]. Furthermore, the exposure to CO gas was reported to increase the survival rate in mice that suffered from a septic shock induced by lipopolysaccharide [15]. In short, NO has been implicated in the pathogenesis of diseases and the application of NOS inhibitor has been reported to demonstrate the promise to be able to relieve some conditions of diseases. Furthermore, some papers reported similar promise for HO-1 stimulator. Such evidence leads us to consider that NO seems to be cytodestrctive and CO seems to be cytoprotective in most cases [16].
There are several stimulators and inhibitors available with regard to NOS and HO-1, which is an induced type of HO. Therefore, we conducted the present study in order to examine the roles of NO and CO in the reverse passive Arthus reaction using NOS inhibitor, HO-1 stimulator and HO-1 inhibitor.
Materials and methods
Chemicals
NG-nitro-L-arginine methyl ester (L-NAME) was adopted as a NOS inhibitor, hemin as a chemical HO-1 stimulator and Zinc protoporphyrin-IX (Znpp-IX) as a competitive HO-1 inhibitor (Sigma-Aldrich, St Louis, MO, USA). Ten or 30 mg L-NAME were dissolved in 100 ml phosphate buffered saline (PBS) [17]; 65·2 mg hemin or 62·2 mg Znpp-IX were dissolved in 4 ml of 0·1 mol/l NaOH, followed by titration with 0·1 mol/l HCl, and the total volumes were raised to 10 ml by adding sterile distilled water [18].
Mice
The mice used were 12- to 16-week-old female C57BL/6, purchased from the Jackson Laboratory (Bar Harbor, ME, USA), and were healthy, fertile, and did not display evidence of infection or disease. They were housed in a pathogen-free barrier facility and screened regularly for pathogens. All studies and procedures were approved by the Committee on Animal Experimentation of Nagasaki University Graduate School of Biomedical Sciences.
Reverse passive Arthus reaction
For cutaneous reverse passive Arthus reactions, the mice were anaesthetized by inhalation of diethyl ether, shaved on their dorsal skins, and wiped with 70% alcohol. Rabbit immunoglobulin G (IgG) anti-chicken egg albumin Abs (60 mg/30 ml; Cappel, Aurora, OH, USA) were injected intradermally with a 29-gauge needle, followed immediately by an intravenous injection of chicken egg albumin (20 mg/Kg; Sigma-Aldrich), which was meant to create a positive IC challenge [19]. The intradermal injection of purified polyclonal rabbit IgG (60 mg/30 ml, Sigma-Aldrich) followed by intravenous introduction of chicken egg albumin served as a negative control. The solution of chicken egg albumin contained 0·4% Evans blue dye (Sigma-Aldrich). A zero control group was established by sacrificing some of the positive control group immediately after positive IC challenge. Besides these negative, positive and zero control groups, four additional groups were created in the present study: (i) 1 mg/kg L-NAME treated group, mice injected intravenously with 1 mg/kg L-NAME just after positive IC challenge; (ii) 3 mg/kg L-NAME treated group, mice injected intravenously with 3 mg/kg L-NAME just after positive IC challenge [17]; (iii) hemin treated group, mice injected intravenously with 80 mmol/kg hemin just after positive IC challenge; and (iv) Znpp-IX treated group, mice injected intraperitoneally with 100 mmol/Kg Znpp-IX, 48 h, 24 h and 1 h before positive IC challenge [20]. The Znpp-IX treated group of mice was sacrificed at 4 h after IC challenge and other groups at either 4 h or 8 h. The zero control group consisted of 5 mice while each other group consisted of 7 to 10 mice.
Quantification of vasculitis
The degrees of vasculitis were assessed by the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, oedema and neutrophil recruitment in the lesional skins [4]. The levels of TNF-α and IL-6 were measured using enzyme-linked immunosorbent assay (ELISA) kits. Oedema was evaluated by measuring the vascular leak. Evans blue dye binds to serum proteins and thereby can be used to quantify alterations in vascular permeability [21]. One hundred mg/ml Evans blue solution was made and then standard series were produced by two-fold dilution from 50 mg/ml to 0·4 mg/ml. The absorbances of samples and standard series were measured at 620 nm using a spectrophotometer (Shimadzu Scientific Instruments, Kyoto, Japan), and the concentration of Evans blue dye in each sample was calculated against the calibration curve of the standard Evans blue solution. The measured values were divided by respective sample weights and corrected values were used for statistical analysis.
Histological examination
Tissues were harvested 0, 4 or 8 h after IC challenge using a disposable sterile 6-mm biopsy (Maruho, Osaka, Japan). The excised tissues were cut into halves and one half was fixed in 4% paraformaldehyde, and then paraffin embedded. The remainder was immediately immersed in a cold PBS, quickly cut into small pieces, snap frozen in liquid nitrogen and stored at −70°C until use. Paraffin sections (6 mm) were stained with haematoxylin and eosin (H&E) for neutrophil evaluation and with toluidine blue for mast cell staining. Neutrophil and mast cell infiltration was evaluated by counting extravascular neutrophils and mast cells in the entire section and averaging the numbers present in 10 serial skin sections from the injection site. Each section was examined independently by three investigators in a blind manner, and the mean was used for analysis.
Enzyme-linked immunosorbent assay for cytokines
Minced samples were weighed and homogenized in 1 ml of Passive Lysis Buffer (Promega, Madison, NJ, USA). The supernatants were collected from homogenized samples and used for the measurement. Levels of murine TNF-α (BIOSOURCE, Camarillo, CA, USA), IL-6 (PIERCE ENDOGEN, Rockford, IL, USA) and IL-10 (BIOSOURCE) in the inflammatory site were determined by ELISA kits or immunoassay kits respectively. The measured values were divided by respective sample weights and calculated values were used for statistical analysis.
Measurement of nitrite
The concentration of nitrite was measured as a stable metabolite reflecting NO by a fluorometric method (NO2/NO3 Assay Kit-FX (Fluorometric), 2,3-Diaminonaphthalene Kit, DOJINDO, Kumamoto, Japan). According to the manufacturer's instructions, homogenized solutions were spun at 5000 g at 4 °C for 1 h using Centricon-10 (Millipore) in order to remove protein. Eighty ml of centrifuged filtrate was applied as a sample. Measured values were divided by respective sample weights and calculated values were used for statistical analysis.
Immunohistochemistry for HO-1
The labelled streptavidin biotin (LSAB) method was adopted (DAKO LSAB 2 System, DAKO, Carpinteria, CA, USA), and anti-mouse HO-1 Ab, which was polyclonal (rabbit Ig), was purchased from StressGen (San Diego, CA, USA). Tissue sections (6 mm) were deparaffinized in xylene. After soaking in 0·05 M Tris-buffered saline (TBS), endogenous peroxidase was blocked with 3% hydrogen peroxide in distilled water for 5 min at room temperature (RT).
After washing in 0·05 M TBS for 5 min, each tissue section was immersed in 0·05 M (TBS) containing 10% normal goat serum for 20 min at RT to diminish the non-specific binding of the secondary Ab. Then the sections were treated with anti-mouse HO-1 Ab (1:250) at 4 °C overnight. The sections were washed and treated with biotinated secondary Ab at RT for 10 min and rinsed. Then, the sections were treated with streptavidin-horse raddish peroxidase at RT for 10 min. After washing, the slides were treated with substrate-chromogen solution (DAB chromogen) for about 10 min at RT. In the end, the slides were counterstained with haematoxylin solution. Negative control staining was obtained by the replacement of the primary Ab with normal rabbit Ig (DAKO).
Statistical analysis
The Mann–Whitney U-test was used for determining the level of significance of differences in sample means, and Bonferroni's test was used for multiple comparisons. A P value < 0·05 was considered statistically significant. All data are shown as means ± standard error of the mean.
Results
Oedema in the cutaneous reverse Arthus reaction
Oedema was assessed by measuring the concentration of Evans blue dye using a spectrophotometer (Fig. 1). Although both positive and negative control mice developed oedema significantly 4 and 8 h after IC formation compared with the levels at 0 h after IC challenge, oedema formation was significantly greater in the positive control mice than in the negative control mice (P < 0·05). Remarkably, 4 h after IC challenge, the corrected values of Evans blue dye absorbance in mice treated with hemin, a HO-1 stimulator, were 67% lower than the positive control mice (P < 0·05) and at a level similar to the negative control mice. By contrast, the mice treated with Znpp-IX, a HO-1 inhibitor, exhibited a significant increase (83%, P < 0·05) 4 h after IC challenge. Eight hours after IC challenge, similar results were obtained, although Znpp-IX treatment was not performed. Significantly lower levels of oedema were confirmed in the hemin treated mice (52%) and in the negative control mice (68%). Treatment with L-NAME, a NOS inhibitor, did not result in any significant change even at a higher concentration 4 or 8 h after IC challenge. Thus, hemin treatment inhibited oedema, while Znpp-IX treatment augmented it.
Fig. 1.
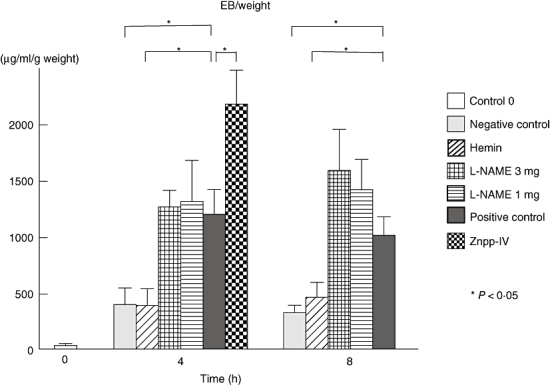
Oedema in the cutaneous reverse passive Arthus reaction. Mice were injected intradermally with rabbit IgG anti-chicken egg albumin Ab, followed by intravenous injection of chicken egg albumin and 0·4% Evans blue dye, which served as positive controls. Dorsal skins were harvested form mice 0, 4 and 8 h after IC challenge. Mice that received an intradermal injection of polyclonal rabbit IgG followed by intravenous installation of chicken egg albumin served as negative controls. The zero control group meant a positive control group sacrificed immediately after positive IC challenge. L-NAME was adopted as a NOS inhibitor, hemin as a chemical HO-1 stimulator and Znpp-IX as a competitive HO-1 inhibitor. The absorbances of samples and standard series were measured at 620 nm using a spectrophotometer, and the concentration of Evans blue dye in each sample was calculated against the calibration curve of the standard Evans blue solution. IgG, immunoglobulin G; IC, immune complexes; NOS, nitric oxide synthase; HO, haeme oxygenase; Znpp, zinc protoporphyrin; L-NAME, NG-nitro-L-arginine methyl ester.
Leukocyte infiltration in the cutaneous reverse Arthus reaction
Extravascular neutrophils were assessed in skin tissue sections after 4 and 8 h of IC formation in the negative control mice, mice treated with 3 mg/kg L-NAME, and mice treated with hemin and compared with the positive control mice (Figs 2a and 3a). In mice treated with Znpp-IX, skin sections were assessed only after 4 h of IC formation. Compared with the positive controls, at 4 h after IC challenge, neutrophil numbers were significantly lower in the mice treated with hemin (65% lower, P < 0·01) and near the level of the negative controls, while they were significantly higher in mice treated with Znpp-IX (86% higher, P < 0·05). In contrast, neutrophil accumulation was not significantly lower in mice treated with 3 mg/kg L-NAME. Similar results were obtained after 8 h, although Znpp-IX treatment was not performed. Mast cell numbers and localization were also assessed in skin tissue sections stained with toluidine blue (Figs 2b and 3b). Compared with the positive controls, at 4 h after IC challenge, mast cell numbers were significantly lower only in the negative control mice (40% lower, P < 0·05). Meanwhile, 8 h after IC challenge, no significant differences in mast cell number were observed among any of the groups. None of the mice treated with hemin, L-NAME or Znpp-IX exhibited significant changes in mast cell accumulation in the lesional skin. Mast cells were recognized beneath the epidermis, around the perivascular area, in the dermis, and in the subcutaneous fat tissue, as well as in and around the muscular area, in all groups. On the localization of mast cells, no specific tendencies could be noticed in the present study. Thus, neutrophil infiltration was reduced by hemin treatment, whereas it was augmented by Znpp-IX treatment.
Fig. 2.
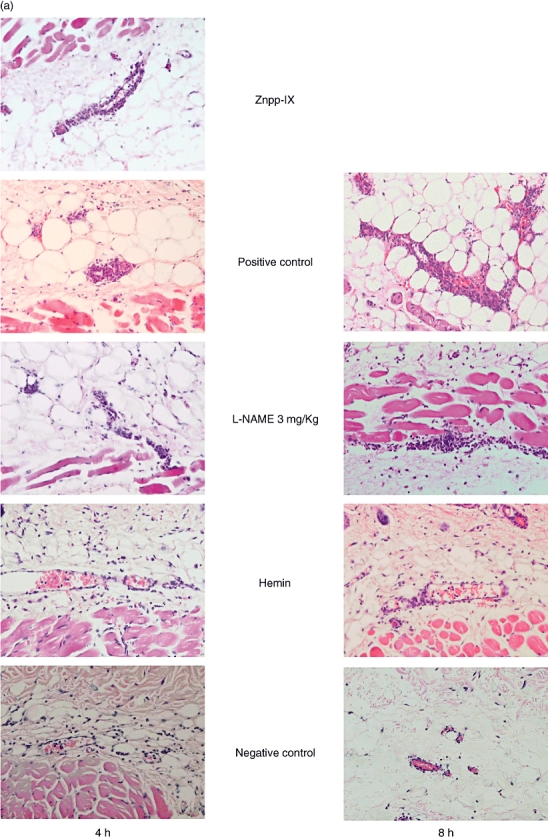
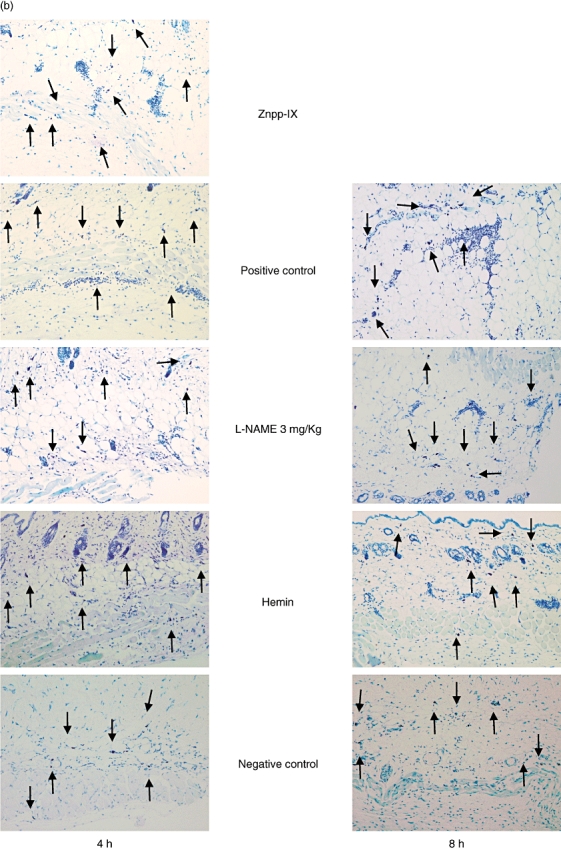
Histological tissue sections showing neutrophil infiltration (a) and mast cell accumulation (b) in the lesional skin at 4 and 8 h after IC challenge. Neutrophils were revealed by H&E staining and mast cells (arrow) were detected as cells with metachromatic staining of granules in toluidine blue-staining. Original magnifications were ×100 (a) and ×50 (b) respectively. With regard to Znpp-IX, positive control, L-NAME and hemin, refer to the legends in Fig. 1. Znpp, Zinc protoporphyrin; L-NAME, NG-nitro-L-arginine methyl ester; IC, immune complexes; H&E, haematoxylin and eosin.
Fig. 3.
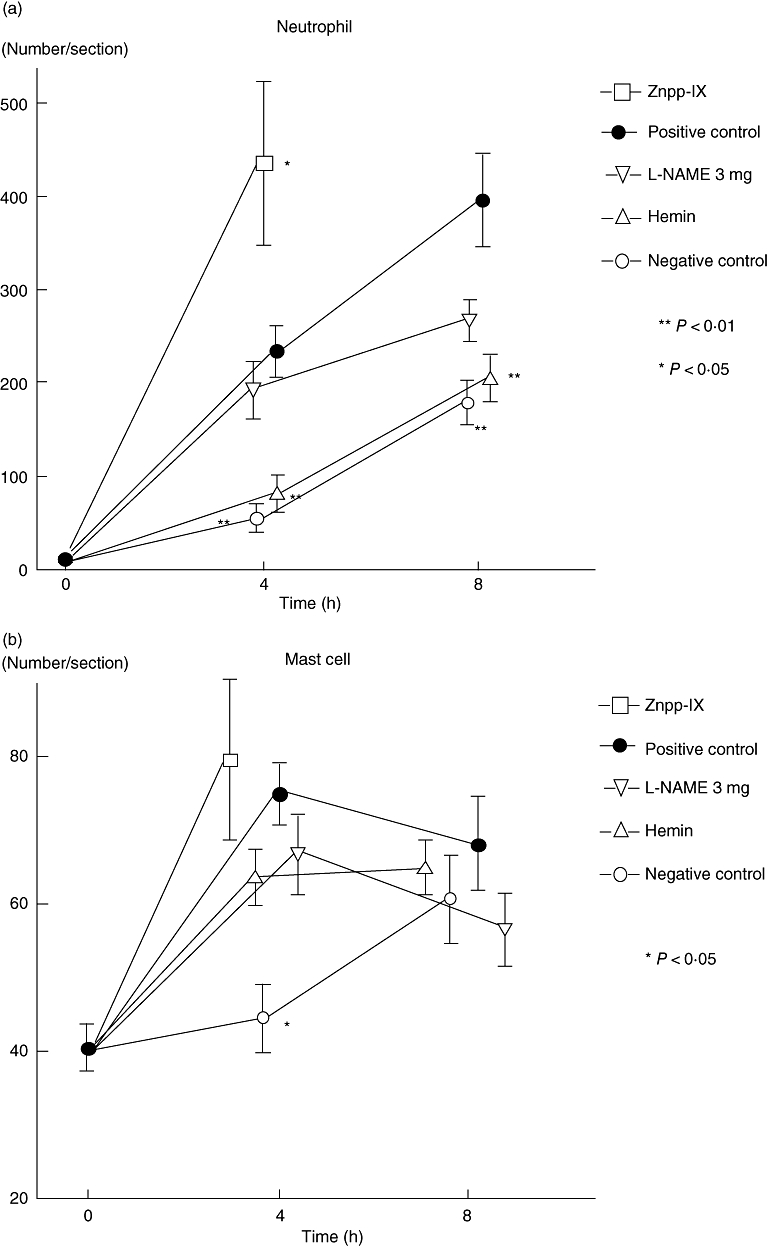
Arthus reaction-induced recruitment of neutrophils (a) and mast cells (b) in the lesional skins. Numbers of neutrophils and mast cells per skin section were determined by counting in H&E- and toluidine blue-stained skin sections. All values represent the mean ± SEM of results obtained from five to 10 mice in each group. Statistical analysis is provided in the Results. With regard to Znpp-IX, positive control, L-NAME, hemin and negative control, refer to the legends in Fig. 1. Znpp, zinc protoporphyrin; L-NAME, NG-nitro-L-arginine methyl ester; H&E, haematoxylin and eosin; SEM, standard error of the mean.
Cytokine levels in the cutaneous reverse Arthus reaction
Immune complex-induced inflammation in the skin is associated with the production and release of proinflammatory cytokines, including TNF-α and IL-6, by infiltrating leukocytes. To assess the degrees of vasculitis in the lesional skin, TNF-α and IL-6 levels were measured in the homogenized samples after 4 and 8 h of IC formation. Compared with the positive controls, at 4 after IC challenge TNF-α levels in the hemin-treated mice (P < 0·05) were 32% lower and were much closer to the levels of the negative controls. By contrast, Znpp-IX-treated mice showed a 44% higher level of TNF-α than the positive controls. Even at the 8-h point similar TNF-α levels were detected in hemin-treated and negative control mice (P < 0·05) (Fig. 4).
Fig. 4.
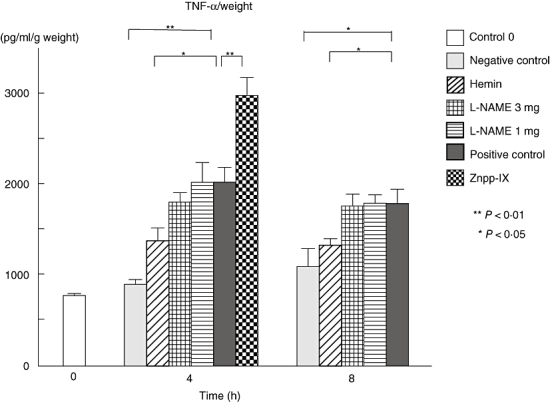
Arthus reaction-induced TNF-α production in the homogenized, lesional skin from each group at 0, 4 and 8 h after IC challenge. TNF-α levels in the lesional skins were determined by ELISA. Measured values were corrected by respective sample weights. All corrected values represent the mean ± standard error of the mean of results obtained from 5 to 10 mice in each group. *P < 0·05 and **P < 0·01 versus levels of positive control group at 4 and 8 h after IC challenge. TNF, tumour necrosis factor; L-NAME, NG-nitro-L-arginine metyl ester; Znpp, zinc protoporphyrin; IC, immune complexes; ELISA, enzyme-linked immunosorbent assay.
As for IL-6 (Fig. 5), at 4 h the hemin-treated mice were at a 35% lower level than the positive controls (P < 0·05), but remained high relative to the negative controls. By contrast, Znpp-IX-treated mice showed a 68% higher level of IL-6 (P < 0·01) compared with the positive controls.
Fig. 5.
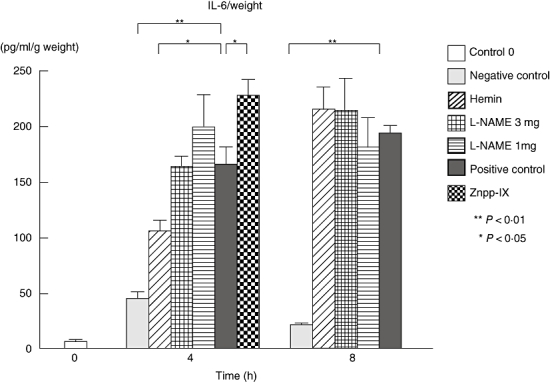
Arthus reaction-induced IL-6 production in the homogenized, lesional skin from each group at 0, 4 and 8 h after IC challenge. IL-6 levels in the lesional skins were determined by ELISA. Measured values were corrected by respective sample weights. All corrected values represent the mean ± SEM of results obtained from 5 to 10 mice in each group. *P < 0·05 and **P < 0·01 versus levels of positive control group at 4 and 8 h after IC challenge. IL, interleukin; L-NAME, NG-nitro-L-arginine methyl ester; Znpp, Zinc protoporphyrin; IC, immune complexes; ELISA, enzyme-linked immunosorbent assay; SEM, standard error of the mean.
At 8 h the only significant decrease relative to the positive controls was in the negative control group (P < 0·01). Thus, hemin treatment displayed significantly lower TNF-α and IL-6 levels, but Znpp-IX treatment displayed significantly higher levels. By contrast, the L-NAME treatments did not show any significant difference in TNF-α or IL-6 levels when compared with the positive controls at either 4 or 8 h.
Interleukin-10 levels were significantly higher in hemin-treated mice compared with the positive controls, both 4 and 8 h after IC challenge (62% and 35% higher respectively, P < 0·01) (Fig. 6). Neither L-NAME nor Znpp-IX treatments displayed any significant differences to the positive controls either 4 or 8 h after IC formation.
Fig. 6.
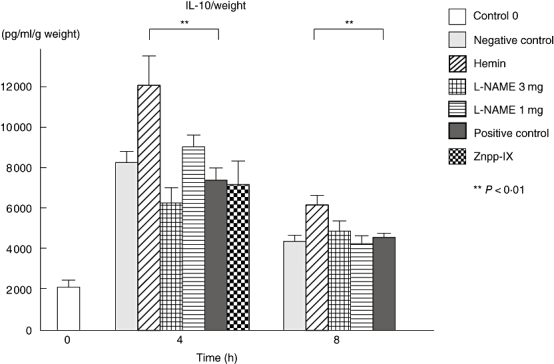
IL-10 production in the homogenized, lesional skin from each group at 0, 4 and 8 h after IC challenge. IL-10 levels in the lesional skins were determined by ELISA. Measured values were corrected by respective sample weights. All corrected values represent the mean ± SEM of results obtained from 5 to 10 mice in each group. *P < 0·05 and **P < 0·01 versus levels of positive control group at 4 and 8 h after IC challenge. IL, interleukin; L-NAME, NG-nitro-L-arginine methyl ester; Znpp, zinc protoporphyrin; IC, immune complexes; ELISA, enzyme-linked immunosorbent assay; SEM, standard error of the mean.
Nitrite levels in the cutaneous reverse Arthus reaction
Nitrite levels of the mice treated with 3 mg/Kg L-NAME were significantly lower (45%) than those of the positive controls at 4 h (P < 0·05) (Fig. 7). Significantly lower levels of nitrite were recognized in the negative controls (P < 0·05) and the mice treated with 3 mg/kg (60%, P < 0·01) and 1 mg/kg L-NAME (63%, P < 0·01) when compared with the positive controls at 8 h. Although neither hemin nor Znpp-IX treatments caused any effect, L-NAME treatments actually suppressed nitrite formation in a dose-dependent manner.
Fig. 7.
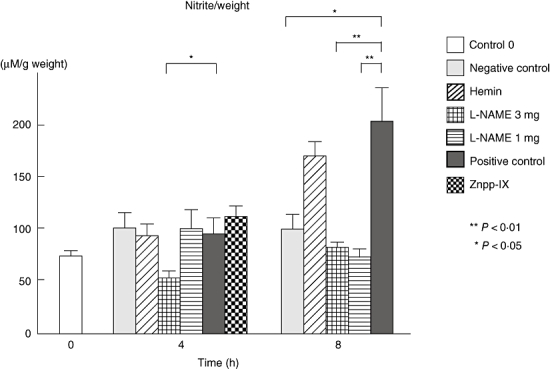
Nitrite was measured as a stable metabolite reflecting NO in the homogenized samples. Measured values were corrected by respective sample weights. All corrected values represent the mean ± SEM of results obtained from 5 to 10 mice in each group. *P < 0·05 and **P < 0·01 versus levels of positive control group at 4 and 8 h after IC challenge. L-NAME, NG-nitro-L-arginine methyl ester; Znpp, zinc protoporphyrin; NO, nitric oxide; SEM, standard error of the mean; IC, immune complexes.
Immunohistochemical analysis of HO-1 protein
Haeme oxygenase-1 expression was reported to be confirmed in keratinocytes, endothelial cells, fibroblasts and macrophages mainly in in vitro studies [22–[25]. To assess the cell sources of HO-1, cutaneous HO-1 expression during the reverse passive Arthus reaction was examined immunohistochemically. In the immunohistochemical examination, HO-1 expression was predominantly expressed in the vesicular cytoplasm of infiltrating macrophages, especially in the lesional skins of hemin-treated mice (Fig. 8). They were mainly scattered in the submuscular area in the lesional skins of hemin-treated mice. Thus, HO-1 was predominantly expressed in infiltrating macrophages.
Fig. 8.
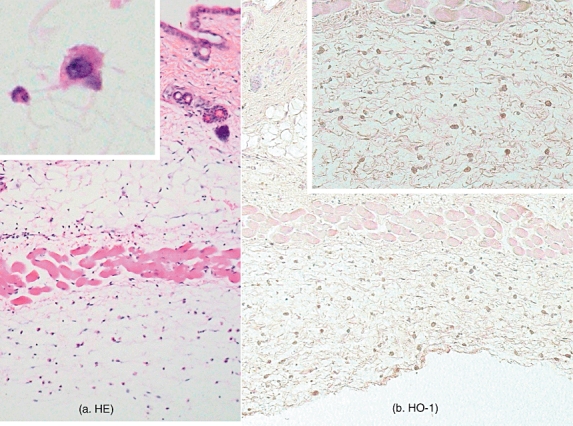
Histological tissue sections showing macrophage infiltration in the skin of hemin-treated mice at 4 h after IC challenge. (a) Sections were stained with H&E staining. Original magnification, ×25. High magnification of infiltrating macrophage, the cytoplasm of which was vesicular (×100, insert). (b) HO-1 expression in skin from hemin-treated mice during cutaneous passive Arthus reaction. HO-1 expression in the lesional skin from hemin-treated mice after 4 h of IC challenge was assessed by immunohistochemistry using anti-HO-1 Ab. Sections were counterstained with haematoxyline. Original magnification, ×25, ×50 (insert). HO, haeme oxygenase; IC, immune complexes; H&E, haematoxylin and eosin.
Discussion
Both NO and CO are gaseous molecules. NO is produced by inducible NOS and CO by HO-1, especially in the lesions of inflammation, because they are both induced types. We selected hemin as a chemical HO-1 inducer, Znpp-IX as a competitive HO-1 inhibitor and L-NAME as a NOS inhibitor in the present study. The levels of TNF-α, IL-6 and Evans blue dye, and the numbers of infiltrating neutrophils at the lesions were significantly higher in the positive control mice than those in the negative control mice, both 4 and 8 h after IC formation. Therefore, these four parameters were thought to be good markers for the evaluation of this cutaneous reverse Arthus reaction. The increase in the number of neutrophils and mast cells in the negative control may be due to the weak inflammatory response against rabbit IgG Ab. A zero control group showed a relatively high amount of TNF-α and low concentration of IL-6, although these were both proinflammatory cytokines. They have been reported to be produced and released by cells such as neutrophils, monocytes and mast cells [26,27]. It was also reported that a low expression level of IL-6 was recognized, although an increase of TNF-α was very prominent at 1 h after stimulation in the in vitro experiment using mouse monocytes [28]. Therefore, it seemed to be possible that the response of TNF-α was more prompt than that of IL-6 during the very early stage of inflammation, although crucial stimuli could be uncertain in the present study.
Meanwhile, a significant decrease in the value of IL-6 was not recognized at 8 h among four parameters, although the decreases in the other four parameters were significant both at 4 and 8 h after IC formation in hemin-treated mice when compared with positive control mice. HO-1 has been reported to suppress TNF-α, IL-6 and IL-8 [29]. Although we did not examine IL-8 levels, we did confirm a significant decrease in the number of neutrophils infiltrating into inflammatory sites in the hemin-treated mice. Actually, we did not confirm a significant decrease in the value of IL-6 at 8 h, but we did recognize a significant decrease in the value of IL-6 at 4 h after IC formation in hemin-treated mice when compared with positive control mice. Therefore, we believe that our results support their findings. Some papers reported the involvement of TNF-α in the pathogenesis of oedema formation [30]. Our results made us consider the possibility that IL-6 was not mainly involved in the pathogenesis of oedema formation 8 h after IC formation in the present study. In order to confirm whether or not this improvement was caused via the HO-1/CO signalling pathway, we additionally conducted Znpp-IX treatment, which was a competitive HO-1 inhibitor at 4 h in the present study. As expected, quite the reverse reactions were observed in Znpp-IX-treated mice 4 h after IC challenge. These results indicate that the HO-1/CO signalling pathway could be involved in the improvement of the cutaneous reverse Arthus reaction.
Inflammatory sites contained oedematous stromas where many cells were recruited, including macrophages. We confirmed HO-1 expression immunohistologically in the cytoplasm of macrophages infiltrating into the inflammatory sites, especially in hemin-treated mice. As previous studies have shown that the cell source of HO-1 was macrophages [31], it was recognized that the supply of HO-1 was thought to be mainly derived from macrophages in the stromas of inflammatory sites. Therefore, it could be recognized that the improvement of vasculitis in hemin-treated mice and the exacerbation in Znpp-IX-treated mice depended on the HO-1/CO signalling pathway. Actually, we injected rabbit IgG antibodies intradermally with a 29-gauge needle, according to the original method. However, we were not able to confirm capillary vessels damaged beneath the epidermis like an anaphylactoid purpura, although we have reported some papers using this model [3,4]. Namely, damaged vessels usually exist deep around muscular layer and subcutaneous fat tissue in this model (Fig. 2a). We thought that the levels of damaged vessels depended on the types of injected Ig. Therefore, we tried the intradermal injection of IgA antibodies, which were confirmed in the lesional skins of anaphylactoid purpura, in this model, but we could not recognize damaged vessels even in the superficial dermis (data not shown). In the end, we cannot explain the discrepancy in this model. It is, however, a fact that diseased vessels exist deep around muscular layer and subcutaneous fat tissue, although we actually injected rabbit IgG antibodies intradermally in the cutaneous passive reverse Athus reaction. We now consider that diseased vessels exist deep around muscular layer and subcutaneous fat tissue in this model, so macrophage infiltration is mainly located in deep subcutaneous areas, as shown in Fig. 8.
Meanwhile, L-NAME was selected as a NOS inhibitor in the present study. Although the actual decrease of nitrite in the inflammatory lesions, which was one of the stable metabolites of NO, was confirmed in a dose-dependent manner, neither significant improvement nor exacerbation was recognized in mice treated with 1 mg/kg and 3 mg/kg L-NAME, at both 4 and 8 h after IC formation. Some reports have mentioned that NO acts as a cytodestructive molecule in some pathological conditions [32], but the inhibition of NOS did not show any improvement in the cutaneous reverse passive Arthus reaction. Namely, none of four markers of vasculitis showed any improvement in the mice treated with L-NAME. Although CO was reported to selectively suppress the expression of inducible NOS [33], hemin treatment, which showed remarkable improvements of four markers of vasculitis, did not suppress lesional nitrite. Therefore, it can be considered that for the improvement of this cutaneous reverse Arthus reaction, a requisite is an increase of HO-1 but not a decrease of nitrite. Therefore, it was speculated that hemin did improve vasculitis independent of the NOS/ NO pathway.
We confirmed significant increases of IL-10 in mice treated with hemin, both 4 and 8 h after IC formation. It has been reported that IL-10 was an anti-inflammatory cytokine and HO-1 could induce IL-10 [29,34]. Thus, the possibility was recognized that HO-1 could improve inflammation via the IL-10 signalling pathway in the cutaneous reverse passive Arthus reaction. However, the suppression of IL-10 was not observed in mice treated with Znpp-IX, which was a competitive inhibitor of HO-1. We also confirmed that the zero control group contained a relatively high amount of IL-10, which was reported to be produced by some cells including monocytes, keratinocytes and mast cells [35]. It was reported that IL-10 was constitutively expressed and secreted by the human normal colonic mucosa [36]. Furthermore, TNF-α can also induce IL-10 because of negative feedback mechanism. Therefore, a relatively high amount of IL-10 was detected during the very early stage of cutaneous inflammation in the present study.
Mast cell recruitment into tissue is thought to occur by release of immature mast cell precursors from the bone marrow into the peripheral blood, followed by migration of these precursors into tissues and their subsequent differentiation into mature mast cells [37]. Increased numbers of mast cells are noticed at the sites of inflammation [38]. Indeed, we confirmed a significant increase of mast cell numbers in the positive control mice compared with the negative controls only 4 h after IC formation, and no significant differences were seen between the L-NAME-treated, hemin-treated, positive control and negative control mice 8 h after IC challenge. As L-NAME, hemin and Znpp-IX treatment did not affect mast cell numbers, it was considered that the improvement or exacerbation of vasculitis occurred without relation to mast cell function in the present study. Further experiments should be conducted in order to clarify the exact role of mast cells in the inflammation associated with the Arthus reaction.
In conclusion, the results of this study indicate that the HO-1/CO signalling pathway plays a critical role in the improvement of the cutaneous passive reverse Arthus reaction. Furthermore, our results may provide important basic information for the therapy of IC-mediated human diseases by the development of drugs that can increase the HO-1/CO signalling pathway.
References
- 1.Kohl J, Gessner JE. On the role of complement and Fc gamma-receptors in the Arthus reaction. Mol Immunol. 1999;36:893–903. doi: 10.1016/s0161-5890(99)00111-x. [DOI] [PubMed] [Google Scholar]
- 2.Arthus MF. Injections répetées de serum de cheval chez ie lapin. V R Soc Biol. 1903;55:817–20. [Google Scholar]
- 3.Kaburagi Y, Hasegawa M, Nagaoka T, et al. The cutaneous reverse Arthus reaction requires intercellular adhesion molecule 1 and L-selectin expression. J Immunol. 2002;168:2970–8. doi: 10.4049/jimmunol.168.6.2970. [DOI] [PubMed] [Google Scholar]
- 4.Yanaba K, Kaburagi Y, Takehara K, Steeber DA, Tedder TF, Sato S. Relative contributions of selectins and intercellular adhesion molecule-1 to tissue injury induced by immune complex deposition. Am J Pathol. 2003;162:1463–73. doi: 10.1016/S0002-9440(10)64279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashenko Y, Ilan N, Krausz MM, Vlodavsky I, Hirsh MI. Heparanase pretreatment attenuates endotoxin-induced acute lung injury in rats. Shock. 2007;28:207–12. doi: 10.1097/shk.0b013e3180311d84. [DOI] [PubMed] [Google Scholar]
- 6.Matucci Cerinic M, Kahaleh MB. Beauty and the beast. The nitric oxide paradox in systemic sclerosis. Rheumatology (Oxford) 2002;41:843–7. doi: 10.1093/rheumatology/41.8.843. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 8.Good PF, Werner P, Hsu A, Olanow CW, Perl DP. Evidence of neuronal oxidative damage in Alzheimer's disease. Am J Pathol. 1996;149:21–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida T, Noguchi M, Kikuchi G. The step of carbon monoxide liberation in the sequence of heme degradation catalyzed by the reconstituted microsomal heme oxygenase system. J Biol Chem. 1982;257:9345–8. [PubMed] [Google Scholar]
- 10.Hoetzel A, Dolinay T, Schmidt R, Choi AM, Ryter SW. Carbon monoxide in sepsis. Antioxid Redox Signal. 2007;19:2013–26. doi: 10.1089/ars.2007.1762. [DOI] [PubMed] [Google Scholar]
- 11.Tamion F, Richard V, Renet S, Thuillez C. Protective effects of heme-oxygenase expression against endotoxic shock: inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10. J Trauma. 2006;61:1078–84. doi: 10.1097/01.ta.0000239359.41464.ef. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg L, Kimblad PO, Sjoberg T, Ingemansson R, Steen S. Inhaled nitric oxide reveals and attenuates endothelial dysfunction after lung transplantation. Ann Thorac Surg. 1996;62:1639–43. doi: 10.1016/s0003-4975(96)00744-8. [DOI] [PubMed] [Google Scholar]
- 13.Miller MJ, Sadowska-Krowicka H, Chotinaruemol S, Kakkis JL, Clark DA. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993;264:11–16. [PubMed] [Google Scholar]
- 14.Giannini L, Vannacci A, Fabrizi F, et al. Protection from cardiac injury by induction of heme oxygenase-1 and nitric oxide synthase in a focal ischaemia-reperfusion model. Cell Mol Biol (Noisy-le-grand) 2005;51:393–401. [PubMed] [Google Scholar]
- 15.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–6. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi H, Takeno M, Saito T, et al. Regulatory role of heme oxygenase 1 in inflammation of rheumatoid arthritis. Arthritis Rheum. 2006;54:1132–42. doi: 10.1002/art.21754. [DOI] [PubMed] [Google Scholar]
- 17.Szabo C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993;108:786–92. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henningsson R, Alm P, Ekstrom P, Lundquist I. Heme oxygenase and carbon monoxide: regulatory roles in islet hormone release: a biochemical, immunohistochemical, and confocal microscopic study. Diabetes. 1999;48:66–76. doi: 10.2337/diabetes.48.1.66. [DOI] [PubMed] [Google Scholar]
- 19.Hopken UE, Lu B, Gerard NP, Gerard C. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J Exp Med. 1997;186:749–56. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue S, Suzuki M, Nagashima Y, et al. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum Gene Ther. 2001;12:967–79. doi: 10.1089/104303401750195926. [DOI] [PubMed] [Google Scholar]
- 21.Harada M, Takeuchi M, Fukao T, Katagiri K. A simple method for the quantitative extraction of dye extravasated into the skin. J Pharm Pharmacol. 1971;23:218–19. doi: 10.1111/j.2042-7158.1971.tb08647.x. [DOI] [PubMed] [Google Scholar]
- 22.Clark JE, Green CJ, Motterlini R. Involvement of the heme oxygenase-carbon monoxide pathway in keratinocyte proliferation. Biochem Biophys Res Commun. 1997;241:215–20. doi: 10.1006/bbrc.1997.7742. [DOI] [PubMed] [Google Scholar]
- 23.Yee EL, Pitt BR, Billiar TR, Kim YM. Effect of nitric oxide on heme metabolism in pulmonary artery endothelial cells. Am J Physiol. 1996;271(4 Pt 1):L512–18. doi: 10.1152/ajplung.1996.271.4.L512. [DOI] [PubMed] [Google Scholar]
- 24.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvath I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax. 1998;53:668–72. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Schutte RJ, Abu-Shakra A, Reichert WM. Protein array method for assessing in vitro biomaterial-induced cytokine expression. Biomaterials. 2005;26:1081–5. doi: 10.1016/j.biomaterials.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Takeda Y, Takeno M, Iwasaki M, et al. Chemical induction of HO-1 suppresses lupus nephritis by reducing local iNOS expression and synthesis of anti-dsDNA antibody. Clin Exp Immunol. 2004;138:237–44. doi: 10.1111/j.1365-2249.2004.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falk S, Goggel R, Heydasch U, et al. Quinolines attenuate PAF-induced pulmonary pressor responses and edema formation. Am J Respir Crit Care Med. 1999;160:1734–42. doi: 10.1164/ajrccm.160.5.9902033. [DOI] [PubMed] [Google Scholar]
- 31.Ma JL, Yang PY, Rui YC, Lu L, Kang H, Zhang J. Hemin modulates cytokine expressions in macrophage-derived foam cells via heme oxygenase-1 induction. J Pharmacol Sci. 2007;103:261–6. doi: 10.1254/jphs.fp0060270. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt HH, Walter U. NO at work. Cell. 1994;78:919–25. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 33.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–55. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 34.Kapturczak MH, Wasserfall C, Brusko T, et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–53. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 36.Jarry A, Bossard C, Bou-Hanna C, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–42. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–15. [PubMed] [Google Scholar]
- 38.Ishizaka T, Ishizaka K. Biology of immunoglobulin E. Molecular basis of reaginic hypersensitivity. Prog Allergy. 1975;19:60–121. [PubMed] [Google Scholar]


