Abstract
Enterohaemorrhagic Escherichia coli (EHEC) O157:H7 infections are considered a public health problem in both developed and developing countries because of their increasing incidence and the severity of clinical presentation. Approximately 10% of infected patients develop complications such as haemolytic uraemic syndrome (HUS) characterized by acute renal failure, thrombocytopenia and haemolytic anaemia. The precise sequence of events leading to HUS is still understood incompletely. Because of the lack of a reproducible small animal model for EHEC infections, in vivo studies examining EHEC–host early interactions are limited and insufficient. The aim of this study was to characterize the weaned BALB/c mouse as a model of E. coli O157:H7 infection. In this paper we report that human Shiga toxin 2 (Stx2)-producing EHEC strains can adhere to the intestinal epithelium of weaned BALB/c mice, and produce local damage which leads to systemic disease and death in a percentage of infected mice. The lethality of the EHEC strain is closely age-dependent, and is related to the bacterial ability to colonize intestine and to produce Stx2. It can be concluded that the weaned BALB/c mouse can be used as a small animal model to study host early responses, and the role of bacterial pathogenic factors in the induction of systemic disease, thus providing a useful tool for the evaluation of therapeutic or vaccine approaches.
Keywords: HUS mouse model, oral infection, renal damage, Stx2
Introduction
Shiga toxin (Stx) producing Escherichia coli (STEC) strains can cause a broad spectrum of human diseases, including diarrhoea, haemorrhagic colitis and the life-threatening haemolytic uraemic syndrome (HUS) [1]. Since its first documented outbreak in 1982 [2], enterohaemorrhagic E. coli (EHEC) has been recognized as an emerging food-borne pathogen, and E. coli O157:H7 has been the most frequent serotype associated with large outbreaks or sporadic cases of haemorrhagic colitis and HUS in many countries [3].
Although the main virulence factor of EHEC is the production of one or more type of Stx (Stx1, Stx2 or Stx variants), adherence to intestinal epithelium and colonization of the gut are also important components of the pathogenesis. Most pathogenic EHEC strains harbour a large pathogenicity island, termed the locus for enterocyte effacement, and a large 95-kb plasmid, which encodes a haemolysin (EHEC-Hly) [4,5].
Enterohaemorrhagic E. coli is usually acquired by consuming contaminated food or water, although person-to-person transmission has not been ruled out [6]. Most individuals infected with EHEC recover from the infection without further complications. However, 8–10% of patients, primarily children and elderly people, may go on to develop complications such as HUS, characterized by acute renal failure, thrombocytopenia and haemolytic anaemia [6,7].
Although EHEC is not invasive and is restricted to the lumen of the gut [6,8], in some circumstances Stx produced within the intestinal tract is able to cross the epithelial barrier and enter the bloodstream. Stx targets the endothelium of susceptible tissues, resulting in intestinal as well as systemic dysfunction [9]. Despite progress made during recent years regarding the involvement of inflammatory response in HUS pathogenesis, relatively little is known about EHEC-induced local changes in the gut tract and its association with systemic disease. In order to define these changes adequately, an animal model of EHEC oral infection is needed. EHEC induces in rabbits gastrointestinal symptoms similar to humans [10]; however, the absence of renal injury by Stx and the paucity of genetic and immunological resources are important limitations for the use of this animal model.
On the other hand, mouse models have the advantage of allowing the use of genetically modified animals for further studies. In addition, intravenous injection of Stx leads to acute renal failure, with the increase of plasmatic parameters of renal damage such as urea and creatinine, together with a strong inflammatory response, similar to that observed in humans [11]. Because adult mice are resistant to EHEC infection, different approaches have been developed to render mice susceptible for EHEC colonization and systemic disease. One of the first reported mouse models was the pretreatment with streptomycin to reduce the normal level of facultative intestinal flora [12–14]. However, exposure of E. coli O157:H7 to antibiotics has been reported to affect Stx excretion by the pathogen [15] and can induce abnormal immune responses in mice [16]. Another attempt included mice maintained under protein calorie malnutrition after weaning [17,18]. The higher sensitivity of malnourished mice to EHEC has been associated, at least in part, to the impairment of the non-specific defence system. Fujii et al. also used mitomycin (MMC) for mouse infection with Stx-producing E. coli O157:H7 [19]. However, MMC not only activates temperate phages encoding Stx, but also induces microangiopathy by itself [20]. The specific pathogen-free [21] and gnotobiotic mice [22] are also susceptible because of the absence of commensal bacteria that otherwise prevents EHEC colonization [23]. Finally, age-related resistance to disease is characteristic of infections by E. coli pathotypes in several species [24]. In this regard, the immature mouse model has been proposed previously to be a good model to study gastrointestinal infections [25] and E. coli O157:H7 oral infection [26].
The aim of this study was to characterize the weaned BALB/c mouse as a model of EHEC infection. In this report we demonstrate that human Stx2-producing EHEC strains can damage the intestinal epithelium of weaned BALB/c mice, and following local injury lead to renal disease and death in 50% of infected mice.
Materials and methods
Bacterial strains
Bacterial strains used in this study included two reference strains, EDL933 [27] and 1271–84 American Type Cell Culture (ATCC 43889) and four strains isolated from faecal specimens of children with non-bloody diarrhoea (NBD) (n = 2) and HUS (n = 2). These strains belonged to seropathotype A, according to Karmali et al.[28]. The E. coli O157:H7 strains, their origin, toxin type, eae-variant, EHEC-Hly production and CD50 on Vero cell assay, characterized as described previously [29], are listed in Table 1. The non-toxigenic 605/03 strain was isolated from a NBD case, harboured the eae and ehxA genes and was used as a non-Stx-producing control. The strains were maintained at −70°C in tryptic soy broth (TSB) (Difco, Le Point de Claix, France) supplemented with 20% glycerol.
Table 1.
Bacterial stains used in this study.
| Strain | Source | Serotype | Sorbitol | Type of Stx | eae | CD50/ml* ± standard deviation (×1011) |
|---|---|---|---|---|---|---|
| EDL933 | Reference strain | O157:H7 | – | 1/2 | γ | 8·5 ± 2·9 |
| 1271–84 | Reference strain | O157:H7 | – | 2/2c | γ | 2·6 ± 0·5 |
| 125/99 | HUS | O157:H7 | – | 2 | γ | 17·0 ± 6·2 |
| 119/01 | NBD | O157:H7 | – | 2 | γ | 40·9 ± 4·1 |
| 108/01 | HUS | O157:H7 | – | 2 | γ | 17·1 ± 5·9 |
| 605/03 | NBD | O157:H7 | – | – | γ | – |
CD50/ml was determined on Vero cells by triplicate, as described in Materials and methods. The cytotoxic effect was neutralized using Shiga toxin 1 (Stx1)- and Stx2-specific monoclonal antibodies (mAb 13C4 and BC5BB12 respectively), provided by Dr N. A. Strockbine, Centers for Disease Control and Prevention, Atlanta, GA, USA (CDC). NBD, non-bloody diarrhoea; HUS, haemolytic uraemic syndrome.
Bacterial growth and media
The strains were grown in TSB to mid-exponential phase. The cultures were centrifuged and the bacterial pellets were washed twice in phosphate-buffered saline (PBS), diluted (102–104) and plated onto plate count agar. The overnight cultures reached a final concentration of 1 × 1010–1·8 × 1010 colony-forming units (CFU)/ml.
Mice
Immature male and female BALB/c mice immediately after weaning (17–21 days of age, approximately 8–11 g of body weight) were used. BALB/c mice were bred in the animal facility at the Department of Experimental Medicine, Academia Nacional de Medicina, Buenos Aires. The experiments performed herein were approved by the Academia Nacional de Medicina Animal Care Committee in accordance with the principles set forth in the Guide for the Care and Use of Laboratory Animals (National Institute of Health, 1985). The weaned mice were divided randomly into experimental groups.
Oral infection model
The bacterial suspension (0·1 ml) was diluted to appropriate concentration and delivered directly into the stomach of mice after 8 h of starvation for food [21,25], via a 5-French paediatric feeding tube. Animals received a total inoculum of 6 × 109 CFU/kg weight of each E. coli strain. Control animals received 0·1 ml of sterile PBS. After 4 h of ingestion of the bacterial suspension, both food and water were provided to the mice ad libitum. Animals were observed daily for activity level and water intake up to 10 days, when survivors were euthanized. At 48, 72 and 96 h after infection blood and stools were also examined. Some animals per group, chosen randomly, were killed and intestinal and renal tissues were processed for further analysis.
Bacterial shedding
Rectal swabs were taken at 48 and 72 h after infection to determine the excretion of E. coli O157:H7 organisms. These samples were cultured onto sorbitol MacConkey agar (SMAC) (Difco) at 37°C during 18 h. The non-fermenting sorbitol colonies in confluent growth zones were screened for stx1, stx2 and rfbO157 genes by a multiplex polymerase chain reaction (PCR) using the primers described by Pollard et al. [30], Ziebell et al.[31] and Paton and Paton [32] respectively. The reference E. coli strains EDL933 O157:H7 (stx1 and stx2), and ATCC 25922 were used as positive and negative controls of gene expression respectively. From each positive faecal sample, one stx-colony was selected for further characterization.
Colonization: isolation and enumeration of EHEC strains from mouse intestine
Mice were killed at 48 and 72 h and 7 days after bacterial inoculation to determine intestinal colonization. The small and large intestines were excised and the stool was removed, diluted to 0·1 g/ml and plated onto SMAC agar to determine the number of CFU per gram of faeces. Each part of the intestine (5 cm each) was washed vigorously with PBS and homogenized in 0·5 ml PBS. Aliquots of homogenized intestinal tissues were plated on SMAC agar and incubated overnight at 37°C. The non-sorbitol-fermenting colonies were counted and selected randomly for confirmation by multiplex PCR for the presence of stx1, stx2 and stx2/rfbO157 genes and by serotyping methods with somatic and flagellar antisera of Instituto Nacional de Producción de Biológicos-ANLIS ‘Dr Carlos G. Malbrán’[33]. The number of CFU per intestinal section was calculated multiplying the CFU per ml by the total volume of each sample.
Haematological and histological studies
Blood samples were obtained by puncture of the retroorbital plexus at 48, 72 and 96 h after bacterial feeding for laboratory analyses that included total and differential blood cell count in Neubauer chamber, blood smears and blood urea nitrogen determination. Kidneys and intestinal samples, in which stool was removed, were excised and put immediately into formalin from euthanized mice at 48 and 72 h for histological evaluation. Each sample was placed in 5 ml of fixing solution containing formol/PBS 10% and processed routinely. Sections of paraffin-embedded tissue were stained with haematoxylin and eosin (H&E) and examined by light microscopy.
Plasma determination of urea
Biochemical determinations of urea in mouse plasma were performed in an autoanalyser CCX spectrum (Abbot Diagnostics Systems, Buenos Aires, Argentina) following standardized instructions. Values higher than the mean ± 2 standard deviations of age-matched normal mice were considered increased.
Statistics
Survival and frequency data were analysed for significance using log-rank and χ2 tests. All other data correspond to the mean ± standard error of the mean of individual mice. Statistical differences were determined using one-way analysis of variance. Comparisons a posteriori between two groups were performed using the Student–Newman–Keuls test.
Results
Dose- and age-dependent studies in vivo: survival curves
BALB/c mice at weaning were infected orally with 6 × 109 CFU/kg. Higher concentrations of bacterial inoculum led mice to die before 24 h, and lower concentrations did not induce any pathological effect up to 10 days. Experimental mice were observed daily and samples of blood and stool collected until death. As can be seen in Fig. 1, the non-Stx-producing control strain (605/03) was not lethal, while bacterial strains Stx2+ showed lethal effects, independently of whether they were isolated from NBD or HUS children.
Fig. 1.
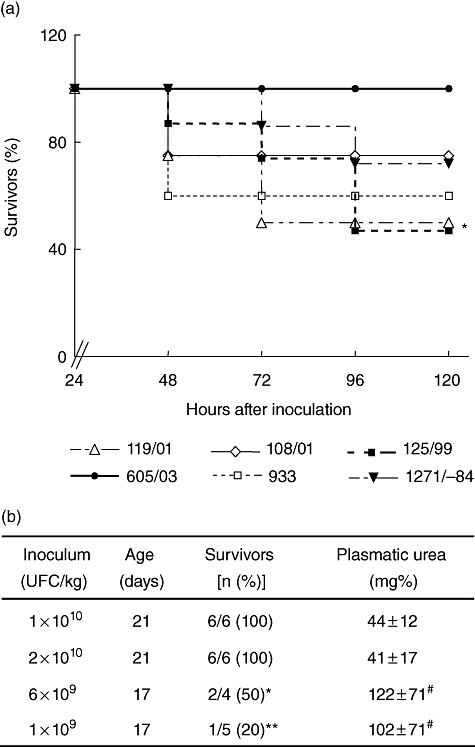
Effect of enterohaemorrhagic Escherichia coli (EHEC) strains on mice survival. (a) Seventeen–19-day-old mice were inoculated orally with 6 × 109 colony-forming units/kg of the indicated Shiga toxin 2 (Stx2)-producing EHEC strains (119/01; 108/01; 125/99; 1271−84; EDL933) and non-Stx2-producing EHEC (605/03) strains. Survival of mice was monitored every 6 h after bacterial inoculation. Survival percentages corresponding to at least two experiments are shown (n = 10–30 mice by bacterial strain). *P< 0·05, 125/99 compared with 605/03 by log-rank test. (b) Influence of age on mice survival after EHEC oral infection. Values of plasmatic urea are expressed as mean ± standard error of the mean; #P < 0·05, *P < 0·01 and **P < 0·001 compared with 21 days old.
To continue the characterization of the weaning HUS model we selected the 125/99 strain, isolated from a HUS patient, which produced both high levels of Stx2 in vitro (Table 1) and high mortality (Fig. 1).
In order to analyse the pathogenic age-dependence, strain 125/99 was inoculated in mice immediately after weaning, at 17 or 21 days after birth. As can be observed in Fig. 1b, toxicity was related clearly to the age of the mice, and even higher concentrations by kg of body weight did not induce any pathological effect in more mature mice, as evaluated by mortality rate and plasmatic urea levels.
Clinical parameters in weaning BALB/c mice infected with EHEC
To assess the systemic effects of EHEC infection, mice were observed closely and blood samples were obtained daily. Mice that died during the first day were not considered for statistical analysis, as they probably did not die as a consequence of Stx2-specific damage. Plasmatic urea was determined daily. Figure 2a shows that among animals infected with the Stx2-producing EHEC in which it was possible to evaluate urea, between 22 and 27% presented a high concentration of urea at 48 and 72 h, respectively, after bacterial feeding, while none of the mice infected with a non-Stx-producing strain showed a rise in this parameter at any time after a period of 10 days. It is important to note that some animals fed with 125/99 strain and recorded in survival curves died before they were bled. Most importantly, all mice with high levels of urea at 72 h died. In contrast, only 8% of mice with normal levels of urea at 72 h died. When the data were analysed retrospectively, we observed that mice that died had urea levels significantly higher than mice that remained alive (Fig. 2b).
Fig. 2.
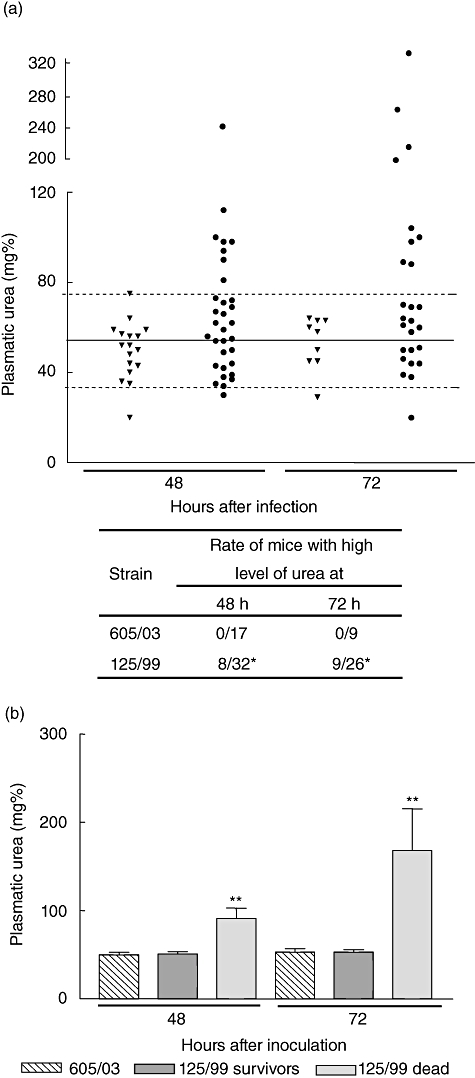
Plasmatic urea concentration after bacterial inoculation. Mice were bled at 48 and 72 h after oral inoculation and urea concentration was measured in plasma. (a) Mice were inoculated with (▾) 605/03 control strain or (•) 125/99 strain. Each point represents individual urea levels of experimental mice. Solid and broken lines represent the mean ± 2 standard deviations of normal plasmatic urea. The number of mice with high levels of plasmatic urea in the groups of mice inoculated with 605/03 and 125/99 were compared: *P < 0·05 versus 605/03 at the same time. (b) Mean ± standard error of the mean of plasmatic urea values from at least 10 mice/group. Mice inoculated with 125/99 strain were classified retrospectively in two groups according their evolution into survivors or dead mice. Urea values from mice inoculated with 605/03 control strain are also shown. **P < 0·01 compared with controls and 125/99 survivors at the same time.
Alterations in circulating leucocytes were evaluated and were analysed retrospectively according to their evolution. Interestingly, we observed a significant increase in the percentage of circulating neutrophils as soon as 48 h after bacterial administration in those mice which died after 72 h ( Fig. 3a). On the contrary, the survivors did not show a relative enhancement of neutrophils, although the total white cell count was increased 48 and 72 h after bacterial feeding (Fig. 3a and b). Meanwhile, mice that died did not show an increase in total circulating leucocytes but a significant decrease was observed shortly before death (Fig. 3b).
Fig. 3.
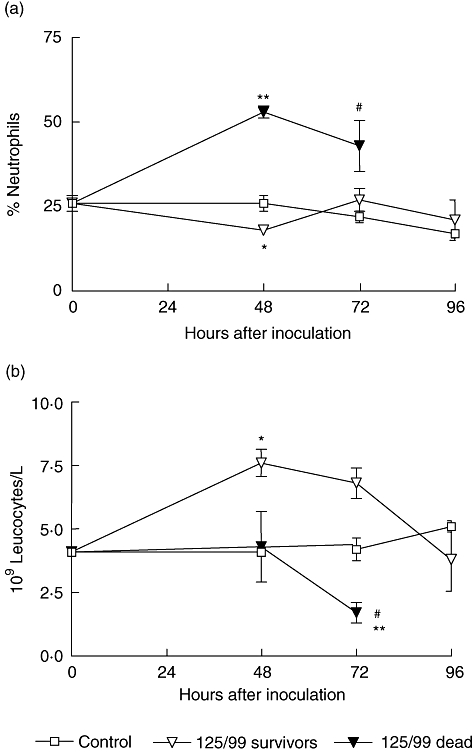
Peripheral white cell count after bacterial inoculation. Mice were bled at 48, 72 and 96 h after oral inoculation and total and differential leucocyte counts were evaluated. In the figure, each point represents the mean ± standard error of the mean of three to nine mice. Infected mice (125/99) were classified retrospectively according to their evolution into survivors or dead. (a) Percentage of neutrophils. All data were analysed by analysis of variance (anova) multiple comparison test (P < 0·0001) and a posteriori Student–Newman–Keuls test. *P < 0·05 versus control; **P < 0·001 versus control and 125/99 survivors; #P < 0·05 versus control and 125/99 survivors. (b) Absolute count of total white cells. All data were analysed by anova multiple comparison test (P < 0·02), and a posteriori Student–Newman–Keuls test. *P < 0·05 versus control and dead mice; **P < 0·001 versus survivors; #P < 0·05 versus control mice.
Shedding of bacteria
The presence of bacteria was tested in rectal swabs taken from the 125/99 infected groups at 48 and 72 h after infection. At 48 h 60% of infected mice shed bacteria in stool, independently of their evolution. However, at 72 h 100% of mice that died continued shedding bacteria in stools, while only 16% of survivors showed a positive result. Bloody diarrhoea is a common feature in EHEC infection but it was not observed in faeces of mice; therefore, we determined hidden blood in stool at 48 and 72 h using the benzidine method [34]. A positive reaction was obtained only in mice that died (data not shown).
Effect of EHEC infection on the mouse intestine
To define host responses to EHEC infection, the mouse intestine was examined macroscopically. The colon of control or non-Stx2-producing infected mice formed pellets of stool beginning just distal to the caecum, and the small intestine was occupied fully. The level and extent of intestinal alterations varied among animals infected with Stx2-producing EHEC. However, the proximal colon of these animals after 48 and 72 h contained semisolid stool, and formed stool pellets were seen only in the distal colon (Fig. 4). Also, the small intestine appeared to be slightly sticky, considerably empty, irrigated, flimsy and breakable.
Fig. 4.
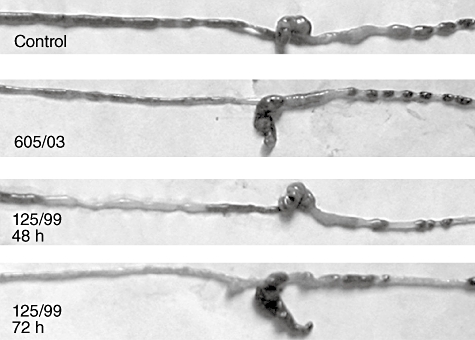
Macroscopic changes in the intestines of enterohaemorrhagic Escherichia coli (EHEC)-infected mice. Whole intestines of control, non-Shiga toxin 2 (Stx2)-producing EHEC (605/03) and Stx2-producing EHEC (125/99) infected mice at 48 and 72 h. One representative animal of each group is shown. Pictures were taken with a Nikon Coolpix 4500 digital camera (Tokyo, Japan).
Colonization of intestinal epithelium
The number of adherent EHEC organisms was quantified in intestines of weaning BALB/c mice 48 and 72 h post-infection. The results are summarized in Fig. 5 and show that EHEC colonized similarly the small and large intestine but only transiently, because no significant amounts of CFU were counted at 72 h post-infection. Bacteria had been cleared completely in all surviving mice by day 7 (data not shown). No colonies were recovered at any time after infection from stool or tissues of mice fed with non-Stx2-producing strain (605/03), except in one animal.
Fig. 5.
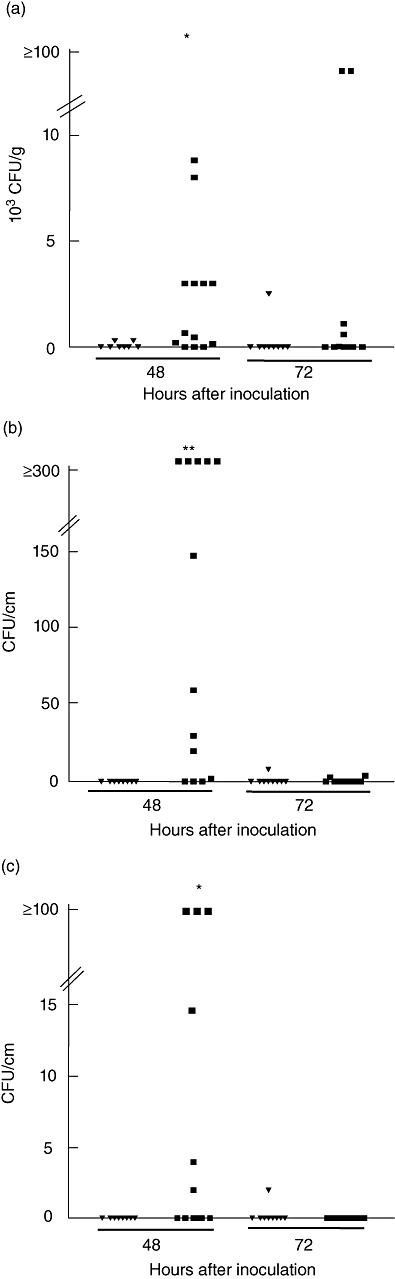
Bacterial recovery in faeces (a), small (b) and large (c) intestine. Mice were killed at 48 and 72 h after intragastric inoculation of 605/03 (▾) and 125/99 (▪) bacterial strains and the number of colony-forming units sorbitol MacConkey agar-negative were determined in stools and intestines as described in Materials and methods. Data represent individual mice. *P < 0·05 and **P < 0·001, compared with 605/03 at 48 h.
To confirm that the colonies counted were indeed STEC, randomly selected non-sorbitol-fermenting colonies were examined for the presence of stx1, stx2 and rfbO157 genes by multiplex PCR. All colonies tested were positive for rfbO157 and stx2 genes, while the non-Stx-producing control strain yielded only rfbO157 PCR product (data not shown). These data demonstrate that the human enteric pathogen EHEC can adhere to and colonize transiently the small and large intestine in weaning BALB/c mice.
Histological studies in EHEC-infected weaning mice
We also studied the effect of EHEC infection on their main target organs, intestine and kidney by examining H&E-stained sections of control and EHEC-infected mice. Histological examination of small intestines of EHEC-infected mice showed multi-focal areas of villous or surface epithelium degeneration in which detached and sloughing epithelial cells were observed. Congestion and absence of goblet cells were associated commonly with the foci of epithelial degeneration. The intestinal wall showed a moderate to intense thinning (Fig. 6). In addition, diffuse leucocyte and neutrophil infiltration was prominent in the large intestine or ileum 72 h after inoculation with EHEC strains. Although some mice fed with non-Stx2-producing EHEC showed intestinal alterations, the frequency and intensity were much less than those observed with Stx2-producing EHEC. As shown in Fig. 6, the Stx2-producing E. coli-infected group showed the typical Stx2-associated focal cortical necrosis involving tubular epithelial swelling and disrupted basement membrane. Some glomeruli showed different degrees of hypercellularity and retraction. On the contrary, control group of mice infected with the non-Stx-producing E. coli strain showed only minimal shape alterations of glomeruli while the architecture of the kidney was conserved, confirming Stx2-induced renal damage after 125/99 administration.
Fig. 6.
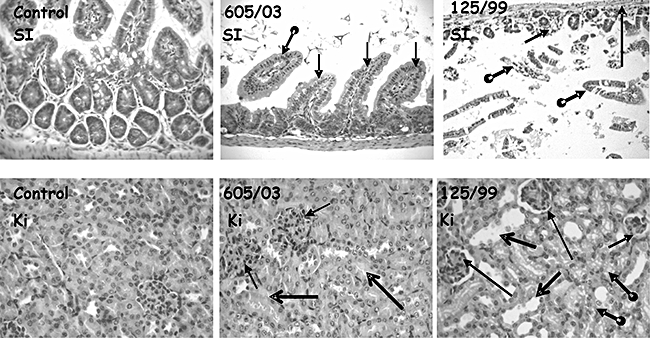
Histological studies of small intestines and kidneys. Tissue samples from experimental mice were fixed and stained with haematoxylin and eosin. Images were acquired using a C. Zeiss III photomicroscope (Oberkochen, Germany). Original magnification ×400. Small intestines (SI) and cortical renal tissue (Ki) of mice inoculated with phosphate-buffered saline (control), 605/03 non-Shiga toxin 2 (Stx2)-producing or 125/99 Stx-producing Escherichia coli strains are shown. Control SI shows normal-thickness mucosa with longitudinal and transversal-cut sections of intestinal villous, and epithelia with basal nuclei and cytoplasm with scanty small mucous vacuoles. SI from 605/03-inoculated mouse shows slender intestinal wall with thin muscular layers, and slime mucosa with few, different shapes, longitudinal-cut intestine villous and epithelium with clear cytoplasm cells and small non-tight basal disposed nuclei. SI from 125/99-inoculated mouse shows dilated intestinal lumen with loose intestinal oedematous, histological ill-defined recognizable mucosa (round – posterior extreme thick arrows); periphery, low – height, transversal-cut glands (short full thin arrow), a preserved interglandular lamina propria and smooth muscular layers (long full thin arrow). Kidney parenchyma (Ki) from control mouse shows two glomeruli and proximal tubules with normal histology. Kidney from 605/03-inoculated mouse shows two enlarged glomeruli with increased lobulation, diffuse mesangial hypercellularity and Bowman's space obliterated (full thin arrows). Representative kidney from 125/99-inoculated mouse shows three glomeruli with size reduction (full thin short arrow) by fibrosis retraction phenomena, irregular shape, mesangial hypocellularity and pseudo-Bowman's space persistence (full thin large arrow). Variable size and shape of tubular cross-sections (full thick open-head arrows) with microvacuolar cytoplasm and ill-defined luminal edge cells in lining epithelium (round – posterior extreme thick arrows).
Discussion
The data presented herein demonstrate that weaned BALB/c mice show enhanced susceptibility to EHEC colonization and Stx2-mediated systemic toxicity as assessed by renal damage. Moreover, lethality of EHEC strain is dependent closely upon the age of the mice, and is related to the bacterial ability to colonize intestine and to produce Stx2.
In our experimental model, E. coli O157:H7 colonization was transient, and close to 50% of infected mice evolved to systemic complications including a marked neutrophilia, renal dysfunction and death (Fig. 1a). Variable data have been reported previously concerning intestinal adherence and colonization, as well as clinical evolution after E. coli O157:H7 intragastrical infection in mice. Conlan et al. have also reported transient colonization of adult mice by E. coli O157:H7 [35], and only one of nine different bacterial isolates resulted lethal [36]. On the other hand, Nagano et al. reported effective intestinal colonization up to 28 days after oral infection with an E. coli strain isolated from a patient with haemorrhagic colitis [21], although no systemic symptoms or death were registered.
The high toxicity of Argentinian strains recovered from patients is related to the expression of Stx2, more than Stx1 or Stx1 plus Stx2. However, the mortality rate induced by the strains tested in weaned mice did not correlate with the severity of the illness induced in children. This fact probably suggests that the pathogenic potential of bacterial strain is an important factor to induce HUS, but that the host response is also very important.
Our findings indicate that immaturity of weaned mice is one of the predisposing factors to infection with EHEC and of systemic complications. Immaturity includes a non-specific defence system along with a mucosal adaptive immune system not fully developed, so that the physical barrier of intestinal epithelia has an increased permeability in comparison with adult mice. Similarly, Kurioka et al. suggested that, in malnourished adult mice, permeability of epithelia is increased, and therefore it is possible that not only Stx but also lipopolysaccharide (LPS) O157 is absorbed through the intestinal epithelia in both models [18]. It has been largely reported that LPS increases the in vitro and in vivo cytotoxic effects of Stx. In addition, drastic age-dependent changes occur in commensal bacteria composition in the intestine of mice between 10 and 18 days of age [37]. One major effector system for mucosal immunity is the secretory immunoglobulin A antibody, which functions by inhibiting pathogen adherence to the epithelial layer and colonization [38,39]. Interestingly, the mucosal adaptive immune system of suckling rodents begins to mature during this phase of development, when lymphocytes begin populating the lamina propria and intraepithelial compartments [40]. Taking these considerations into account, it can be postulated that children are specifically prone to E. coli infection and Stx-derived complications, at least in part because of their immature immune local response and to the structure of the intestine, which usually attains the adult shape at about 10 years of age.
This study shows not only that weaned mice present enhanced susceptibility to oral infection with E. coli O157:H7, but also demonstrates that Stx production by E. coli O157:H7 enhances bacterial colonization and induces systemic damage. In agreement with our data, Mundy et al. have reported recently that adult mice infected orally with a non-Stx-producing EHEC strain did not show any evidence of bacteria colonization or systemic disease [41]. Moreover, other reports have revealed that Stx2 enhances the grade and duration of STEC intestine colonization in mice [42], and increases diarrhoea and intestinal inflammation in rabbits [10,42]. However, the involvement of alternative pathogenic factors in such efficient colonization should be further determined in the weaned mouse model.
A high percentage of infected mice shed Stx2-producing bacteria in their stools 2 days after infection independently of their clinical evolution. However, at the third day, the percentage of mice with stools positive for EHEC culture was lower, but the majority of these mice died with clinical symptoms of renal failure. We suggest that bacterial shedding after 2 days is the result of an effective bacterial colonization, which leads to the intestinal damage necessary to permit the passage of Stx to the bloodstream.
Age-related resistance to intestinal injury is characteristic of infection by other E. coli pathotypes in several animal species [24], and it has been reported that EHEC induces A/E lesions in several neonatal or infant animals, such as rabbits, calves, piglets, goats and lambs [10,24,43]. Although EHEC-infected weaned mice did not present diarrhoea or visible faecal blood, the macroscopic and microscopic features of intestines, as well as the positive reaction for occult blood in faeces, are strong indicators of intestinal injury.
Renal damage in weaned mice fed with Stx-producing bacteria would indicate that the toxin has reached systemic circulation, and the close association between high urea levels, prompt neutrophilia and death suggests that kidney is one of the Stx targets in weaned mice and that both phenomena, strong inflammatory response and renal damage, are associated.
On the other hand, in the malnourished model mice died after 6 days of oral E. coli O157:H7 infection with signs of neurological but not renal injury [17,18]. Similarities between both, malnourished and weaned mouse models, such as the incomplete development of intestinal epithelia and mucosal immune response, can account for the Stx passage to circulation, and consequently systemic complications. However, low protein consumption in diet and high levels of circulating tumour necrosis factor may contribute to the absence of renal damage and/or alterations in the blood–brain barrier that could render mice more susceptible to neurological than to renal damage [17].
Histological changes in the kidney of Stx2-producing EHEC infected and ill mice showed tubular necrosis and glomerular alterations compatible with Stx2 effects [11,44]. It is important to point out that toxic effects of Stx2 in mice, and also in humans, are systemic and that the glomerular endothelial injury can be secondary to tubular necrosis. On the contrary, weaned mice orally infected with non-Stx2-producing EHEC show normal kidneys.
In conclusion, our data provide evidence that human Stx2-producing EHEC colonizes weaned mice intestine and induces intestinal and renal epithelial damage, confirming that the weaned BALB/c mouse can be used as a small animal model to study host early responses. This model may also be useful to study the role of bacterial pathogenic factors in the induction of systemic disease, providing a useful tool for the evaluation of therapeutic or vaccine approaches.
Acknowledgments
The authors thank Héctor Costa and Antonio Morales for their excellent technical assistance. This work was supported by grants from the Alberto J. Roemmers Foundation, CONICET and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
References
- 1.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley LW, Remis RS, Helgerson SD, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–5. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 3.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 4.Gal-Mor O, Finlay BB. Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell Microbiol. 2006;8:1707–19. doi: 10.1111/j.1462-5822.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 5.Garmendia J, Frankel G, Crepin VF. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect Immun. 2005;73:2573–85. doi: 10.1128/IAI.73.5.2573-2585.2005. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–9. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–86. doi: 10.1016/S0140-6736(05)71144-2. Review. [DOI] [PubMed] [Google Scholar]
- 8.Acheson DW, Moore R, De Breucker S, et al. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1996;64:3294–300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochoa TJ, Cleary TG. Epidemiology and spectrum of disease of Escherichia coli O157. Curr Opin Infect Dis. 2003;16:259–63. doi: 10.1097/00001432-200306000-00013. Review. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie JM, Thorpe CM, Rogers AB, Waldor MK. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect Immun. 2003;71:7129–39. doi: 10.1128/IAI.71.12.7129-7139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez GC, Lopez MF, Gomez SA, et al. Relevance of neutrophils in the murine model of haemolytic uraemic syndrome: mechanisms involved in Shiga toxin type 2-induced neutrophilia. Clin Exp Immunol. 2006;146:76–84. doi: 10.1111/j.1365-2249.2006.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindgren SW, Melton AR, O'Brien AD. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect Immun. 1993;61:3832–42. doi: 10.1128/iai.61.9.3832-3842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadolkowski EA, Burris JA, O'Brien AD. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58:2438–45. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadolkowski EA, Sung LM, Burris JA, Samuel JE, O'Brien AD. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–65. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grif K, Dierich MP, Karch H, Allerberger F. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1998;17:761–66. doi: 10.1007/s100960050181. [DOI] [PubMed] [Google Scholar]
- 16.Domingo S, Diaz R, Gamazo C. Antibiotic treatment induces an increase of the specific antibody levels in Brucella melitensis infected mice. FEMS Immunol Med Microbiol. 1995;12:91–5. doi: 10.1111/j.1574-695X.1995.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 17.Kita E, Yunou Y, Kurioka T, et al. Pathogenic mechanism of mouse brain damage caused by oral infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun. 2000;68:1207–14. doi: 10.1128/iai.68.3.1207-1214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurioka T, Yunou Y, Kita E. Enhancement of susceptibility to Shiga toxin-producing Escherichia coli O157:H7 by protein calorie malnutrition in mice. Infect Immun. 1998;66:1726–34. doi: 10.1128/iai.66.4.1726-1734.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii J, Kita T, Yoshida S, et al. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H7 in mitomycin-treated mice. Infect Immun. 1994;62:3447–53. doi: 10.1128/iai.62.8.3447-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molyneux G, Gibson FM, Gordon-Smith EC, et al. The haemotoxicity of mitomycin in a repeat dose study in the female CD-1 mouse. Int J Exp Pathol. 2005;86:415–30. doi: 10.1111/j.0959-9673.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagano K, Taguchi K, Hara T, Yokoyama S, Kawada K, Mori H. Adhesion and colonization of enterohemorrhagic Escherichia coli O157:H7 in cecum of mice. Microbiol Immunol. 2003;47:125–32. doi: 10.1111/j.1348-0421.2003.tb02795.x. [DOI] [PubMed] [Google Scholar]
- 22.Isogai E, Isogai H, Kimura K, et al. Role of tumor necrosis factor alpha in gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Infect Immun. 1998;66:197–202. doi: 10.1128/iai.66.1.197-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperandio V, Torres AG, Giron JA, Kaper JB. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol. 2001;183:5187–97. doi: 10.1128/JB.183.17.5187-5197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean EA, Whipp SC, Moon HW. Age-specific colonization of porcine intestinal epithelium by 987P-piliated enterotoxigenic Escherichia coli. Infect Immun. 1989;57:82–7. doi: 10.1128/iai.57.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez MI, Thuizat A, Pedron T, Neutra M, Phalipon A, Sansonetti PJ. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol. 2003;5:481–91. doi: 10.1046/j.1462-5822.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohara T, Kojio S, Taneike I, et al. Effects of azithromycin on shiga toxin production by Escherichia coli and subsequent host inflammatory response. Antimicrob Agents Chemother. 2002;46:3478–83. doi: 10.1128/AAC.46.11.3478-3483.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien AD, Lively TA, Chen ME, Rothman SW, Formal SB. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (Shiga) like cytotoxin. Lancet. 1983;1:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 28.Karmali MA, Mascarenhas M, Shen S, et al. Association of genomic Oisland 122 of Escherichia coli EDL933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J Clin Microbiol. 2003;41:4930–40. doi: 10.1128/JCM.41.11.4930-4940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas M, Miliwebsky E, Chinen I, et al. Characterization and epidemiologic subtyping of Shiga toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathogens Dis. 2006;3:88–96. doi: 10.1089/fpd.2006.3.88. [DOI] [PubMed] [Google Scholar]
- 30.Leotta GA, Chinen I, Epszteyn S, et al. Validation of a multiplex PCR for detection of Shiga toxin-producing Escherichia coli. Rev Argent Microbiol. 2005;37:1–10. [PubMed] [Google Scholar]
- 31.Ziebell KA, Read SC, Johnson RP, Gyles CL. Evaluation of PCR and PCR RFLP protocols for identifying Shiga toxins. Res Microbiol. 2002;153:289–300. doi: 10.1016/s0923-2508(02)01322-0. [DOI] [PubMed] [Google Scholar]
- 32.Paton AW, Paton JC. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ørskov F, Ørskov I. Serotyping of Escherichia coli. In: Bergan T, editor. Methods in microbiology. Vol. 14. London: Academic Press; 1984. pp. 43–112. [Google Scholar]
- 34.Aiquel F. Manual de Análisis Clínicos (Faeces. Manual of Clinical Studies) 4th. Buenos Aires: Panamericana; 1977. Heces; p. 473. [Google Scholar]
- 35.Conlan JW, Perry MB. Susceptibility of three strains of conventional adult mice to intestinal colonization by an isolate of Escherichia coli O157:H7. Can J Microbiol. 1998;44:800–5. doi: 10.1139/cjm-44-8-800. [DOI] [PubMed] [Google Scholar]
- 36.Conlan JW, Bardy SL, KuoLee R, Webb A, Perry MB. Ability of Escherichia coli O157:H7 isolates to colonize the intestinal tract of conventional adult CD1 mice is transient. Can J Microbiol. 2001;47:91–5. [PubMed] [Google Scholar]
- 37.Diaz RL, Hoang L, Wang J, et al. Maternal adaptive immunity influences the intestinal microflora of suckling mice. J Nutr. 2004;134:2359–64. doi: 10.1093/jn/134.9.2359. [DOI] [PubMed] [Google Scholar]
- 38.Nagano K, Sugisaki T, Taguchi K, Hara T, Naiki M, Mori H. A murine model of enterohemorrhagic Escherichia coli O157:H7 infection to assess immunopotentiating activity of drugs on mucosal immunity: effect of drugs. J Pharmacol Sci. 2003;91:219–28. doi: 10.1254/jphs.91.219. [DOI] [PubMed] [Google Scholar]
- 39.Uren TK, Johansen FE, Wijburg OL, Koentgen F, Brandtzaeg P, Strugnell RA. Role of the polymeric Ig receptor in mucosal B cell homeostasis. J Immunol. 2003;170:2531–9. doi: 10.4049/jimmunol.170.5.2531. [DOI] [PubMed] [Google Scholar]
- 40.Cebra JJ, Bos NA, Cebra ER, et al. Development of components of the mucosal immune system in SCID recipient mice. Adv Exp Med Biol. 1994;355:255–9. doi: 10.1007/978-1-4615-2492-2_43. [DOI] [PubMed] [Google Scholar]
- 41.Mundy R, Girard F, FitzGerald AJ, Frankel G. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol Lett. 2006;265:126–32. doi: 10.1111/j.1574-6968.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 42.Sjogren R, Neill R, Rachmilewitz D, et al. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology. 1994;106:540–3. doi: 10.1016/0016-5085(94)90587-8. [DOI] [PubMed] [Google Scholar]
- 43.Dean-Nystrom EA, Bosworth BT, Cray WC, Moon HV. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect Immun. 1997;65:1842–8. doi: 10.1128/iai.65.5.1842-1848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dran GI, Fernandez GC, Rubel CJ, et al. Protective role of nitric oxide in mice with Shiga toxin-induced hemolytic uremic syndrome. Kidney Int. 2002;62:1338–48. doi: 10.1111/j.1523-1755.2002.kid554.x. [DOI] [PubMed] [Google Scholar]


