Abstract
We studied simultaneously Epstein–Barr virus (EBV)-specific CD4+ and CD8+ T cell responses during and after infectious mononucleosis (IM), using a previously described 12-day stimulation protocol with EBNA1 or BZLF1 peptide pools. Effector function of EBV-specific T cells was determined after restimulation by measuring intracellular interferon-γ production. During IM, BZLF1-specifc CD4+ T cell responses were dominant compared with CD8+ T cell responses. EBNA1-specific CD4+ and CD8+ T cell responses were low and remained similar for 6 months. However, 6 months after IM, BZLF1-specific CD4+ T cell responses had declined, but CD8+ T cell responses had increased. At diagnosis, EBV-specific CD8+ T cells as studied by human leucocyte antigen class I tetramer staining comprised a tetramerbrightCD8bright population consisting mainly of CD27+ memory T cells and a tetramerdimCD8dim population consisting primarily of CD27- effector T cells. The remaining EBV-specific CD8+ T cell population 6 months after the diagnosis of IM consisted mainly of tetramerbrightCD8bright CD27+ T cells, suggesting preferential preservation of memory T cells after contraction of the EBV-specific T cell pool.
Keywords: EBV, infectious mononucleosis, T cells
Introduction
Epstein–Barr virus (EBV) is a widespread human gamma herpesvirus. Primary EBV infection occurs generally during childhood and is usually asymptomatic. However, when infected during adolescence or adulthood, infectious mononucleosis (IM) can develop. Transmission of EBV between individuals occurs via saliva and leads to infection of oropharyngeal epithelial cells, after which the virus is transmitted to the B cells. IM is characterized by the presence of large numbers of mononuclear cells, which consist mainly of activated EBV-specific CD8+ T cells [1–7]. In patients with IM, responses rising to 40% of the CD8+ T cells against lytic protein epitopes have been observed by human leucocyte antigen (HLA) class I tetramer staining, although expanded responses against latent protein epitopes have also been described [4,5,8]. This is in contrast with healthy EBV carriers, in whom T cell responses are directed mainly against latent protein epitopes [5,8–10]. However, others have reported that there is no difference in breadth of the EBV-specific CD4+ and CD8+ T cell responses between IM patients and subjects with persistent infection [11].
Although multiple laboratories have reported on CD8+ T cell responses, CD4+ T cell responses have not been studied to the same extent. One of the few studies investigating EBV-specific CD4+ T cells was performed by Precopio et al.[10], who studied EBV-specific CD4+ T cell responses from acute infection to latency, using intracellular cytokine staining after 6 h stimulation with interferon (IFN)-γ production as a read-out. Whereas EBV-specific CD4+ T cells could be detected during the early phase of infection, numbers decreased to undetectable levels 6 months after acute infection. The aim of this study is to measure EBV-specific CD4+ T cell memory responses from acute infection towards latency. Secondly, we were able to measure EBV-specific CD8+ T cell memory responses in parallel. In order to enable measurement of EBV-specific CD4+ T cell memory responses in IM as well as in healthy EBV carriers, we used a previously developed assay in which EBV-specific T cells are expanded by stimulation with overlapping peptide pools for 12 days. After restimulation intracellular cytokine staining is performed to determine IFN-γ production by EBV-specific CD4+ and CD8+ T cells [12]. This assay enabled us to measure EBV-specific CD4+ T cell responses in IM patients during acute infection as well as after resolving EBV infection. The use of peptide pools enabled us to measure CD8+ T cell responses in parallel to the CD4+ T cell responses and to investigate whether there are differences between the EBV-specific CD4+ and CD8+ T cell responses against a latent (EBNA1) and lytic (BZLF1) protein.
To understand how EBV-specific CD8+ T cells control viral infection, insight into EBV-specific CD8+ T cell differentiation is essential. It has been shown that EBV-specific CD8+ T cells are mainly of the memory CD27+ phenotype in healthy latently infected EBV carriers [9,13]. Furthermore, it has been suggested that in human immunodeficiency virus (HIV)–EBV co-infection increased lytic replication of EBV occurs [14], leading to more effector CD27– T cells. Interestingly, in a study focusing on HIV- and EBV-specific CD8+ T cells in HIV-infected individuals, it was observed that individuals who did not develop EBV-related disease indeed had high numbers of EBV-specific effector CD27–CD8+ T cells, whereas those who did develop EBV-related disease did not [15]. This suggests that effector EBV-specific CD27–CD8+ T cells are important for the control of viral infection in case of frequent reactivation. In the present study the expression of CD27 on EBV-specific CD8+ T cells was followed in IM patients from time of diagnosis until 6 months afterwards, to obtain more insight into EBV-specific CD8+ T cell differentiation.
Patients and methods
Study subjects
Infections mononucleosis patients were traced through laboratory assistants of general physicians during a 2-year time-span. Individuals with clinical IM diagnosis were asked to participate in this study. Only individuals who gave their consent participated. Five IM patients diagnosed by a positive reaction in the Paul–Burnell test and marked lymphocytosis, that consisted of > 10% atypical lymphocytes, were included in this study. Blood samples were drawn when possible at diagnosis, 2 weeks after diagnosis and 6 months after diagnosis. In addition, a renal transplant patient with an acute EBV infection was enrolled. From this patient samples were available at several time-points before and after seroconversion. This enabled us to gain insight into EBV-specific T cell responses directly after infection. Patient characteristics of the IM patients and the renal transplant patient are shown in Table 1. For comparison, chronic EBV carriers (n = 11), consisting of anonymous blood bank donors screened for EBV positivity, were also included. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density centrifugation and cryopreserved.
Table 1.
Patient characteristics of infectious mononucleosis (IM) patients and renal transplant patient.
| Load | CD8+ T cell activation | T cell numbers (109/l) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EBV DNA copies/106 PBMC | % HLA-DR+/CD38+ | Diagnosis | 2 weeks | 26 weeks | ||||||||||
| Donor | Age (years) | HLA | Diagnosis | 2 weeks | 26 weeks | Diagnosis | 2 weeks | 26 weeks | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 |
| IM 1 | 17 | B8 | 1 500 | > 50 | 200 | 81 | 76 | 20 | 0·68 | 2·45 | 0·52 | 0·94 | 0·78 | 0·76 |
| IM 2 | 16 | A11 | 78 300 | 400 | > 50 | 84 | 63 | 11 | 0·45 | 2·35 | 0·64 | 0·75 | 0·60 | 0·48 |
| IM 3 | n.a. | A2, B8 | 273 300 | 15 600 | 1700 | 93 | 11 | n.d. | 0·42 | 2·01 | 0·46 | 0·77 | 0·42 | 0·46 |
| IM 4 | 52 | A2 | 33 800 | 500 | n.a. | 89 | n.d. | n.a. | n.d. | n.d. | n.d. | n.d. | n.a. | n.a. |
| IM 5 | 25 | A2 | 51 800 | 200 | n.a. | 21·88 | 39·13 | n.a. | 0·60 | 4·38 | 0·56 | 3·21 | n.a. | n.a. |
| Load | CD8+ T cell activation | T cell numbers (109/l) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EBV DNA copies/ml | % HLA-DR+/CD38+ | s.c. | 1 week | 30 weeks | ||||||||||
| Donor | Age (years) | HLA | s.c. | 1 week | 30 weeks | s.c. | 1 week | 30 weeks | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 |
| Renal | 19 | A1, A29, | 33 900 | 29 000 | 11 900 | 66·25 | 32·29 | 0·75 | 0·59 | 0·46 | 0·56 | 0·66 | 0·37 | 0·29 |
| Tx | B8, B44 | |||||||||||||
n.d., not determined; n.a., not available; s.c., seroconversion; EBV, Epstein–Barr virus; HLA-DR, human leucocyte antigen D-related; PBMC, peripheral blood mononuclear cells; Tx, transplant.
Real-time quantitative polymerase chain reaction assay for measurement of EBV load in PBMC
Epstein–Barr virus DNA was measured in duplicate from 2 × 106 cells. Polymerase chain reaction (PCR) primers specific for the non-glycosylated membrane protein p143 were used for real-time PCR amplification, as has been described previously [16–18]. To detect the 74 base-pair product a fluorogenic probe was used (PE Biosystems, Nieuwekerk a/d IJssel, the Netherlands). The amount of β-albumin, a household gene present at two copies/cell, was measured as a control, as described previously [19].
T cell stimulation
Peripheral blood mononuclear cells were stimulated with overlapping peptide pools consisting of 15-mer peptides with 11 amino acid overlap. Peptide pools spanned the immunogenic C-terminal region of EBNA1 (57 peptides) and the entire BZLF1 protein (59 peptides) (JPT Peptide Technologies GmbH, Berlin, Germany). Purity and sequences were verified by high-performance liquid chromatography and mass spectrometry. Peptides were dissolved in dimethylsulphoxide and pooled at a final concentration of 1 mg/ml of each peptide.
Expansion of EBV-specific T cells and measuring IFN-γ production by EBV-specific T cells
To expand the number of EBV-specific T cells, PBMC were cultured for 12 days in the presence of EBV overlapping peptide pools, as described previously [12]. PBMC were thawed and incubated at 37°C, 5% CO2 in U-shaped 96-well plates (2 × 105 PBMC/well, 3·0 × 106 PBMC total) together with peptide pool of EBNA1 or BZLF1. Culture medium consisted of RPMI-1640 (Gibco life Technologies, Breda, the Netherlands) supplemented with 10% human pool serum and 1% penicillin/streptomycin. On days 0 and 6 peptide pool was added (2 and 14 µg/ml respectively) and at days 3, 6 and 9 PBMC were supplemented with 10 U/ml (∼180 IU/ml) interleukin (IL)-2. After 12 days PBMC were pooled and rested overnight without peptide pool or IL-2. On day 13, 1–2 × 106 PMBC were restimulated with peptide pool (2 µg/ml). CD28 (1 µg/ml) and CD49d (1 µg/ml) were added for co-stimulation. After 1 h, 1 : 1000 Brefeldin A was added (Golgiplug, BD Biosciences, Breda, the Netherlands) to allow accumulation of cytokines in the cytosol, and PBMC were incubated for another 5 h. As a negative control PBMC were stimulated with medium and co-stimulation alone. As a positive control PBMC were stimulated with phorbol myristate acetate (10 ng/ml) and ionomycin (2 µg/ml). Afterwards PBMC were washed with PBS + 0·5% bovine serum albumin, permeabilized [fluorescence activated cell sorter (FACS) permeabilizing solution; BD Biosciences], washed again and stained with specific antibodies for CD3-peridinin chlorophyll (PerCP), CD4-allophycocyanin (APC), CD8-phycoerythrin (PE) and IFN-γ–fluorescein isothiocyanate (FITC) (Becton Dickinson, San José, CA, USA). Cells were washed, fixed (Cellfix, BD Biosciences) and 200 000 cells were acquired by the FACSCalibur flow cytometer (BD Biosciences). Based on forward- and side-scatter, lymphocytes were gated and data were analysed by CellQuest software (BD Biosciences).
Tetramer staining
Major histocompatibility complex (MHC) class I tetramers complexed with specific peptides were produced as described elsewhere [20]. The HLA-A2 tetramer was loaded with the EBV BMLF1-derived peptide GLCTLVAML (GLC), the HLA-A11 tetramer was loaded with the EBV EBNA3B-derived peptide AVFDRKSDAK (AVF) and the HLA-B8 tetramer was loaded with the EBV BZLF1-derived peptide RAKFKQLL (RAK). All tetramer complexes were APC-labelled. Thawed PBMC (1–1·5 × 106) were washed with PBS + 0·5% bovine serum albumin and stained with a combination of the following monoclonal antibodies: one of the tetramer complexes APC-labelled, PerCP-conjugated monoclonal antibody (mAb) CD8, CD8-APC Cy7, CD103-PE, CCR7-PE Cy7 (BD Biosciences) and CD27-FITC (Sanquin Reagents, Amsterdam, the Netherlands). Cells were fixed (Cellfix, BD Biosciences) and at least 200 000 events were acquired by the either the FACSCalibur or the LSR II flow cytometer (BD Biosciences) and analysed by CellQuest software (BD Biosciences) or BD FACSDiva software (BD Biosciences) respectively.
Results
Characteristics of the study population
Five acute IM patients, identified based on clinical symptoms and diagnosed by a positive reaction in the Paul–Burnell test, were studied at time of diagnosis, 2 weeks and 6 months after diagnosis. At time of diagnosis, all patients had high viral DNA loads (range 1500–273 300 EBV DNA copies/106 PBMC), which decreased during the following 6 months (range > 50–1700 EBV DNA copies/106 PBMC). In a renal transplant patient with an acute EBV infection viral load decreased from 33 900 EBV DNA copies/ml at time of seroconversion to 11 900 EBV DNA copies/ml 30 weeks after seroconversion. Whereas the CD4+ T cell counts of the IM patients remained reasonably stable over time [range 0·42–0·68 × 109/l (diagnosis) to 0·42–0·72 × 109/l (6 months after diagnosis)], the CD8+ T cell numbers were high at time of diagnosis (range 2·01–4·38 × 109/l), but had dropped 6 months afterwards (range 0·46–0·76 × 109/l). In the renal transplant patient variation in CD8+ T cell numbers was less pronounced.
CD4+ T cell responses against BZLF1 dominate during acute infection
To study EBV-specific CD4+ and CD8+ T cell responses during and after IM, PBMC were stimulated with overlapping peptide pools derived from the latent protein EBNA1 and the lytic protein BZLF1 for 12 days, followed by an intracellular IFN-γ staining to assess the presence of effector T cells. This prolonged stimulation is required, because in particular CD4+ T cell responses against EBV-specific proteins are very low after short-term stimulation (EBNA1: range 0·03–0·13% CD4+ T cells; BZLF1: range 0·03–0·36% of CD4+ T cells) [12]. In addition, EBNA1- and BZLF1-specific CD8+ T cell responses directly ex vivo varied between 0·04–0·25% and 0·04–0·61% of CD8+ T cells respectively (unpublished data).
The latent protein EBNA1 is known to be expressed during almost all latency phases [21]. BZLF1 is also an important CTL target in healthy EBV seropositive individuals [22]. A representative example of IFN-γ-producing T cells present after 12 days of stimulation with EBV peptide pools is shown in Fig. 1a. Particularly in response to the EBV peptide pool BZLF1, production of IFN-γ was observed in both CD4+ and CD8+ T cells (7·34% and 16·52% respectively). In contrast, IFN-γ was produced only by CD4+ T cell in response to EBNA1 (1·10%), whereas the response was lacking in the CD8+ T cells. To indicate EBV-specific T cell responses in the chronic phase, healthy EBV seropositive individuals were included.
Fig. 1.
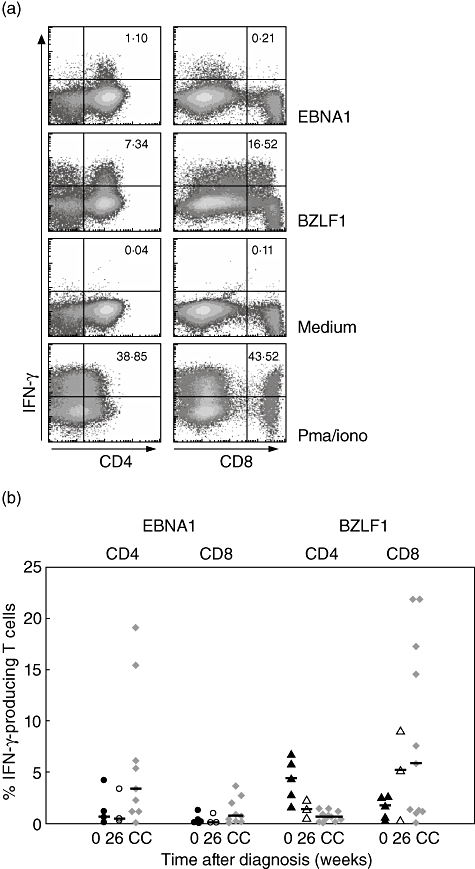
CD4+ T cell responses against BZLF1 dominate during acute infectious mononucleosis (IM). (a) A representative example of interferon (IFN)-γ production of CD4+ and CD8+ T cells after 12 days’ expansion (IM patient, 2 weeks after diagnosis). Cells were stimulated with a peptide pool of EBNA1 or BZLF1, with phorbol myristate acetate/ionomycin (positive control) or medium (negative control). The percentages of IFN-γ-producing CD4+ (left panels) and CD8+ T cells (right panels) are shown in the upper right corners. (b) CD4+ and CD8+ T cell responses, as measured by the amount of IFN-γ produced, of all IM patients against EBNA1 (○, •) or BZLF1 (▵, ▴). Closed circles or triangles indicate the responses at time of diagnosis (n = 5), whereas the responses 6 months after diagnosis are indicated by open circles or triangles (n = 3). Epstein–Barr virus-specific T cell responses by the chronic carriers (CC) are indicated by grey diamonds (n = 11).
Overall, CD4+ T cell responses directed against BZLF1 dominated in IM patients at time of diagnosis (n = 5; median 4·35%; range 2·86–6·71%) (Fig. 1b). CD8+ T cells were also able to proliferate and differentiate to IFN-γ-producing cells in response to BZLF1 (median:1·74%; range 0·65–2·61%). Six months after the diagnosis of IM (n = 3), BZLF1-specific CD4+ T cell responses had declined from a median of 4·35% to a median of 1·35% (range 0·59–2·32%). CD8+ T cell responses had increased from a median of 1·74% to a median of 5·23% (range:0·31–9·02%), reaching levels comparable to chronic EBV carriers (median 0·65%; range 0·05–1·48% and median 5·81%; 0·07–21·80% BZLF1-specific CD4+ and CD8+ T cell responses respectively).
Epstein–Barr virus-specific CD4+ and CD8+ T cell responses directed against EBNA1 were also detectable during acute infection, albeit at a lower level than BZLF1-specific responses (median 0·68%; range 0·46–4·23%, median 0·16%; range 0·1–0·39% respectively). CD4+ as well as CD8+ EBV-specific T cell responses directed against EBNA1 remained low (median 0·42%; range 0·25–3·37% and median 0·12%; range 0·11–0·97% respectively) but stable during the first 6 months after diagnosis of IM. Interestingly, in chronic carriers, EBNA1-specific CD4+ T cell responses were higher (median 3·40%; range 0·07–19·06%) compared with IM patients.
Contraction of EBV-specific CD8+ T cells after resolving IM in HLA-B8 patients
In order to study EBV-specific CD8+ T cells in more detail, the frequency of these cells was also determined directly by HLA class I tetramer staining. The percentage of EBV-specific CD8+ T cells varied enormously between the five IM patients at time of diagnosis (n = 5, range 0·1–22·1%). In healthy chronic carriers 0·10–1·85% EBV-specific CD8+ T cells were observed. In two of five IM patients high numbers of EBV-specific CD8+ T cells were detectable, all recognizing the immunodominant HLA-B8-restricted RAK epitope, which is characteristic for acute IM (Fig. 2). Similarly, we measured high frequencies of EBV-specific CD8+ T cells in a HLA-B8-positive renal transplant patient who we could follow from onset of infection [23]. In contrast, EBV-specific CD8+ T cells numbers were lower in two HLA-A2 positive patients, recognizing the HLA-A2 restricted GLC epitope. Similarly, low numbers of HLA-A11 restricted AVF-specific CD8+ T cells were found in the HLA-A11-positive patient. Six months after diagnosis of IM only a relatively small number of EBV-specific CD8+ T cells was left in all patients (n = 4, range 0·24–2·20%). In the HLA-B8 individuals, in whom high numbers of EBV-specific CD8+ T cells were detected at time of diagnosis, a clear decrease in the frequency of EBV-specific CD8+ T cells was observed in time. In patient 4 (HLA-A2) the frequency of EBV-specific CD8+ T cells remained below 0·1%. In patient 5 (HLA-A2) EBV-specific CD8+ T cells increased a further 2 weeks after the diagnosis. In the HLA-A11 patient the percentage of EBV-specific CD8+ T cells seemed to be stable from diagnosis until 6 months post-diagnosis.
Fig. 2.
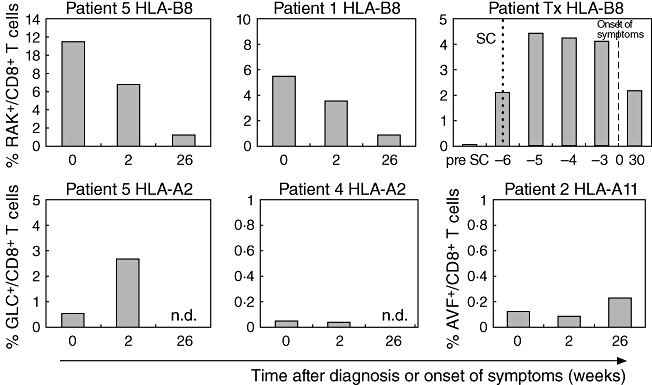
The percentage of Epstein–Barr virus (EBV)-specific CD8+ T cells in infectious mononucleosis (IM) patients and a renal transplant patient. The bars indicate the percentages of EBV-specific CD8+ T cells within the lymphocytes at time of diagnosis of IM, 2 weeks after diagnosis and 26 weeks after diagnosis or time (weeks) before and after onset of clinical symptoms in case of the renal transplant patient. In the renal transplant patient seroconversion is indicated by a dotted line and the onset of clinical symptoms by a dashed line. Cells were stained with tetramer B8-RAK (BZLF1, RAKFKQLL), A2-GLC (BMLF1, GLCTLVAML) or A11-AVF (EBNA3B, AVFDRKSDAK); n.d., not determined; SC, seroconversion.
In the renal transplant patient (HLA-B8) seroconversion was indicated by the appearance of IgM antibodies against the viral capsid antigen [23]. At time of seroconversion, EBV-specific CD8+ T cells were already present (Fig. 2), whereas the percentage of EBV-specific CD8+ T cells reached its peak 1 week after seroconversion, which was 5 weeks before the onset of clinical symptoms. EBV-specific CD8+ T cells had already started to decrease before the onset of clinical symptoms.
Epstein–Barr virus-specific CD8dim and CD8bright T cells differ in expression of CD27
Epstein–Barr virus-specific CD8+ T cell populations as detected using B8–RAKFKQLL tetramer complexes could be dissected in a tetramerbrightCD8bright and a tetramerdimCD8dim population. To exclude the possibility that the tetramerdimCD8dim population consisted of natural killer (NK) cells, an additional staining was performed including CD3. Because the tetramerdimCD8dim population consisted of CD3+ cells, a possible contribution of NK cells to the tetramerdimCD8dim population was excluded (data not shown).
The two IM patients with the highest frequency of EBV-specific CD8+ cells (patients 1 and 3) and renal transplant patient are depicted in Fig. 3. The phenotype of these EBV-specific T cell populations was identified by CD27 staining. Using CD27, T cells can be separated into memory (CD27+) and effector (CD27–) T cells. Interestingly, the tetramerbrightCD8bright T cell population consisted mainly of CD27+ T cells (median at time of diagnosis 88·2%), whereas the tetramerdimCD8dim population consisted of CD27– T cells (median at time of diagnosis 88·2%). After resolution of IM, the number of tetramer-specific T cells decreased (median from time of diagnosis until 6 months after diagnosis: 5·45%–1·2% respectively). The remaining tetramer-specific CD8+ T cell memory population consisted primarily of tetramerbrightCD8bright CD27+ T cells, suggesting a specific depletion of CD27– T cells or survival of CD27+ T cells. In the chronic EBV carriers only tetramerbrightCD8bright T cells were detected, which were primarily CD27+. Representative data are shown in Fig. 3. CD8+ T cell activation in general was high at time of diagnosis of IM and decreased in time (see Table 1).
Fig. 3.
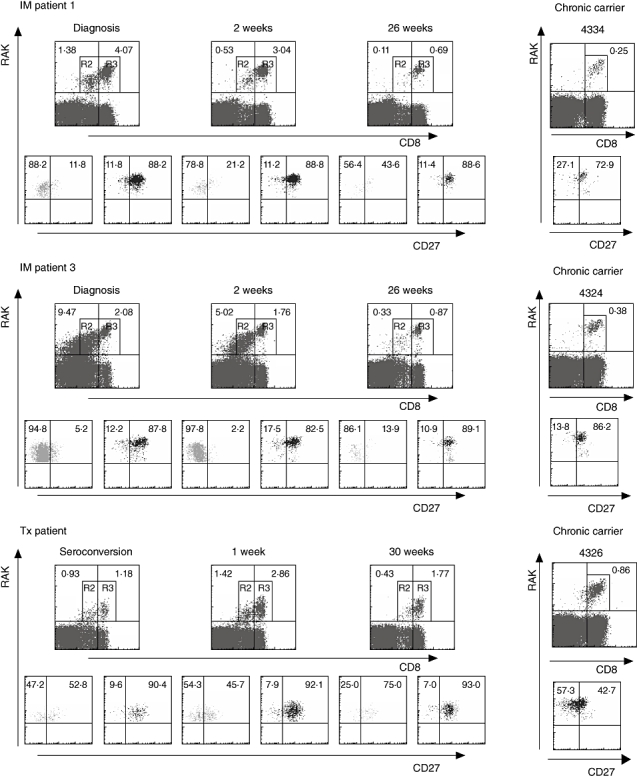
The tetramer-positive CD8+ T cells could be dissected in a tetramerdim/CD8dim and a tetramerbright/CD8bright population. The figures demonstrate the results at time of diagnosis of infectious mononucleosis (IM), 2 weeks after diagnosis and 6 months after diagnosis (n = 2) or time of seroconversion and 1 and 30 weeks after seroconversion in the case of the renal transplant patient (n = 1). Patient numbers are shown in the upper left corners. In addition, three Epstein–Barr virus chronic carriers are added in the right panels. The upper panels of each donor show the RAK+CD8+ T cells; the T cells were first gated on the lymphocytes. The percentages in the corners represent the percentages of R2 and R3. Within the tetramerdimCD8dim and tetramerbrightCD8bright populations the expression of CD27 was determined, which is shown in the lower panels (tetramerdim/CD8dim: R2, light grey) (tetramerbright/CD8bright: R3, dark grey). The percentages in the corners indicate the percentage of CD27+ or CD27- T cells, within the tetramer+CD8+ T cells.
In three of the IM patients we aimed to determine the characteristics of the EBV-specific T cells in more detail by measuring the expression of CD103, which is a marker of retention at mucosal sites, and the lymph node homing marker CCR7. However, these markers were not detected on EBV-specific T cells of the IM patients and chronic carriers at any time-point (data not shown).
Discussion
This is the first study in which EBV-specific CD4+ T cell responses were measured in parallel to CD8+ T cell responses in IM patients using the same assay, from diagnosis to latency. Because of a limited number of PBMC, the antigens tested were restricted to peptide pools of one latent protein (EBNA1) and one lytic protein (BZLF1). Previously, we have shown that it is possible to determine EBV-specific CD4+ as well as CD8+ T cell responses specific for peptides in the overlapping peptide pools of EBNA1 and BZLF1 after 12-day culturing followed by restimulation, even in healthy EBV-positive donors [12].
The results indicate that EBV-specific CD4+ T cell responses against the lytic protein BZLF1 decrease after retention of IM. Furthermore, the EBV-specific CD4+ T cell responses against the latent protein EBNA1 remained stable until 6 months after acute IM, whereas the responses were higher in chronic carriers. CD4+ T cell responses against latent antigens have been observed in healthy carriers in several studies [11,24–26]. In a previous report by Precopio et al.[10], CD4+ T cell responses were detected readily during acute IM against lytic (BZLF1 and BMLF1) and latent (EBNA1 and EBNA3a) proteins, but were shown to decrease over time. Woodberry et al.[11] showed an increased percentage of individuals with a persistent infection whose immunodominant HLA class II-restricted epitopes reside in EBNA1 compared with IM patients. This suggests that EBNA1-specific CD4+ T cell responses continue to arise during the latent phase, which fits with the higher EBNA1-specific CD4+ T cell responses that we observed. Interestingly, EBNA1-specific IgG levels increase similarly after resolution of acute IM [27]. The protocol used for expansion of EBV-specific CD4+ T cells has been based on the method used by Reese et al.[28], which has been used for the detection of Plasmodium falciparum-specific CD4+ T cells. They report that particularly central memory T cell responses were measured using this assay. This is in accordance with our preliminary data (not shown), which also indicate a preferential outgrowth of memory T cells. Therefore, we assume that the measured CD4+ T cell responses in our assay also represent mainly the memory CD4+ T cell pool. This in contrast to the usually used short-term stimulation assays, which in general represent both memory and effector T cell responses.
The EBNA1-specific CD8+ T cell responses were low in IM patients from diagnosis until 6 months thereafter, as well as in chronic carriers. As endogenous EBNA1 is protected from proteasomal cleavage and therefore cannot be presented to CD8+ T cells [29], this might explain the low CD8+ T cell responses against EBNA1. Although low in frequency, some CD8+ T cell responses directed against EBNA1 were present and have also been described previously [12,30–33]. The EBNA1-specific CD8+ T cell responses might be a consequence of the presence of defective ribosomal products (DRiPs), a major source of MHC class I-restricted EBNA1 epitopes [34]. However, the role of EBNA1-specific CD8+ T cell responses in the control of EBV-infected B cells is still unknown.
In contrast, the CD8+ T cell response after 12 days of expansion in IM patients seemed to increase over time, reaching levels comparable to chronic carriers after 6 months. In previous reports, using direct ex-vivo assays measuring mainly effector responses, a decrease in lytic responses has been observed [5,11,35], which is also what we found using tetramer staining in the two patients recognizing the BZLF1-derived epitope RAK. This discrepancy may be explained by the fact that recently activated T cells in vivo are known to be sensitive for activation-induced cell death. Because, after 12 days’ expansion, specifically memory T cells able to survive and proliferate are detected, in-vivo-activated T cells may be lost in the early phase of expansion and stimulation and therefore cannot contribute to the percentage of IFN-γ-producing T cells measured after 12 days. In contrast, directly ex vivo these cells may still be able to produce cytokines.
Preliminary data in healthy EBV carriers using single peptides instead of peptide pool as stimulation after the expansion period also showed mainly CD4+ T cell responses against peptides derived from EBNA1, whereas CD8+ T cell responses against EBNA-1 were lacking. Both CD4+ and CD8+ T cell responses were measurable in response to single peptides derived from BZLF1 (data not shown). As responses were directed towards several different peptides of EBNA1 and BZLF1, responses between donors are thought to be quite diffuse. However, further research is required to confirm these findings.
High percentages of RAK-specific CD8+ T cells during diagnosis of IM and the subsequent decrease afterwards, as measured by tetramer staining, is in accordance with previous reports [5,35]. In contrast, the number of GLC+/CD8+ T cells remained undetectable in one of the patients. In accordance with the literature [5], the frequency of EBV-specific CD8+ T cells against a latent epitope (in this case AVF, derived from EBNA 3B) increased from time of diagnosis until 6 months after diagnosis.
As well as differences in HLA type and antigen target, the main problem in these IM patients is that time of diagnosis most probably does not correspond with the actual time of infection. Furthermore, the time between acute infection and time of diagnosis will be different between patients. Therefore, a renal transplant patient with known time of infection was included. This patient has been described previously by Piriou et al.[23]. Interestingly, no increased CD8+ T cells numbers were observed in the renal transplant patient, although this patient had a persistently high viral load, in contrast to the IM patients in whom increased CD8+ T cell numbers were observed. This lack of increased CD8+ T cell numbers in the renal transplant patient might be related to immunosuppressive therapy. Shortly after seroconversion a sharp increase in RAK+CD8+ T cells was observed in this patient. In the first week of follow-up the percentage of RAK+CD8+ T cells reached a maximum, which fits with other viral infections such as influenza and lymphocytic choriomeningitis in mice [36,37]. Six weeks after seroconversion, clinical symptoms occurred. This indicates that in IM patients seroconversion had already occurred some weeks before diagnosis. In patient 5 T cell responses increased from week 0 to week 2, suggesting that patient 5 might have been included soon after acute infection.
Phenotypic analysis of the EBV-specific CD8+ T cells showed a tetramerdimCD8dimCD27- and a tetramerbrightCD8brightCD27+ population. The tetramer+CD27+ population represents the memory T cells. Because EBV-specific T cells are hardly developing to a terminally differentiated CD28-CD27-CD45RA+ phenotype [9,38], we considered the tetramer+CD27- population to consist of effector T cells. After contraction of the EBV-specific T cell pool, the remaining tetramerbrightCD8bright population consisted mainly of CD27+ T cells, indicating that in particular the CD27+ memory population survives. In addition, in chronic carriers the tetramerbright/CD8bright population also consisted mainly of CD27+ T cells. It is known that T cells down-regulate their T cell receptor during a phase of high antigen triggering, which is accompanied by the loss of CD27 expression. Therefore, the CD8dim/tetramerdim population observed in IM patients consist probably of CD8 T cells with down-regulated T cell receptors. After resolving the acute infection most of the CD27- effector T cells die, leading to a preferential survival of CD27+ memory T cells. These memory T cells are important to control the virus during reactivation or reinfection with EBV.
In some of the patients we were also able to determine the characteristics of the EBV-specific CD8+ T cells in more detail by studying the expression of CD103 and CCR7. However, these markers were not up-regulated on the EBV-specific T cells of the IM patients and chronic carriers at any time-point. The lack of expression of CD103 and CCR7 is in accordance with data reported previously [39,40]. As CD103 is a tonsil retention marker, it not surprising that this marker is lacking on EBV-specific T cells derived from blood. The lack of CCR7 indicates that the EBV-specific T cells have already passed the early memory phase, suggesting that at the time of diagnosis, infection has already been occurring for some time. This is in accordance with the appearance of symptoms 6 weeks after seroconversion in the renal transplant patient.
In conclusion, during the acute phase of infection EBV-specific CD4+ and CD8+ T cell responses are directed mainly against the lytic proteins, which are expressed abundantly during this phase of infection. In healthy chronic EBV carriers, in particular the CD4+ T cell responses against the latent protein EBNA1 were increased, although CD8+ T cell responses against the lytic protein BZLF1 were also present. As EBV reactivates frequently, EBV-specific CD8+ T cells remain important to control the virus. Furthermore, after the acute phase the memory CD27+ T cells remain present, whereas the percentage of effector CD27– T cells decreased, suggesting that memory CD8+ T cells are most important to keep the virus under control whenever EBV becomes reactivated.
Acknowledgments
We are very grateful to Marijke Th. L. Roos for providing PBMCs from IM patients and Laila Gamadia for providing PBMCs from the renal transplant patient.
References
- 1.Callan MF, Steven N, Krausa P, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–11. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 2.Annels NE, Callan MF, Tan L, Rickinson AB. Changing patterns of dominant TCR usage with maturation of an EBV-specific cytotoxic T cell response. J Immunol. 2000;165:4831–41. doi: 10.4049/jimmunol.165.9.4831. [DOI] [PubMed] [Google Scholar]
- 3.Callan MF, Fazou C, Yang H, et al. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J Clin Invest. 2000;106:1251–61. doi: 10.1172/JCI10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callan MFC, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein–Barr virus in vivo. J Exp Med. 1999;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hislop AD, Annels NA, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein–Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino Y, Morishima T, Kimura H, Nishikawa K, Tsurumi T, Kuzushima K. Antigen-driven expansion and contraction of CD8+-activated T cells in primary EBV infection. J Immunol. 1999;163:5735–40. [PubMed] [Google Scholar]
- 7.Roos MTL, Van Lier RAW, Hamann D, et al. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein–Barr virus and human immunodeficiency virus infection in man. J Infect Dis. 2000;182:451–8. doi: 10.1086/315737. [DOI] [PubMed] [Google Scholar]
- 8.Catalina MD, Sullivan JL, Bak KR, Luzuriaga K. Differential evolution and stability of epitope-specific CD8(+) T cell responses in EBV infection. J Immunol. 2001;167:4450–7. doi: 10.4049/jimmunol.167.8.4450. [DOI] [PubMed] [Google Scholar]
- 9.Appay V, Dunbar PR, Callan MFC, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med; 2002. pp. 379–85. 8. [DOI] [PubMed] [Google Scholar]
- 10.Precopio ML, Sullivan JL, Willard C, Somasundaran M, Luzuriaga K. Differential kinetics and specificity of EBV-specific CD4(+) and CD8(+) T cells during primary infection. J Immunol. 2003;170:2590–8. doi: 10.4049/jimmunol.170.5.2590. [DOI] [PubMed] [Google Scholar]
- 11.Woodberry T, Suscovich TJ, Henry LM, et al. Differential targeting and shifts in the immunodominance of Epstein–Barr virus-specific CD8 and CD4 T cell responses during acute and persistent infection. J Infect Dis. 2005;192:1513–24. doi: 10.1086/491741. [DOI] [PubMed] [Google Scholar]
- 12.Piriou ER, van Dort K, Nanlohy NM, van Oers MH, Miedema F, van Baarle D. Novel method for detection of virus-specific CD4(+) T cells indicates a decreased EBV-specific CD4(+) T cell response in untreated HIV-infected subjects. Eur J Immunol. 2005;35:796–805. doi: 10.1002/eji.200425792. [DOI] [PubMed] [Google Scholar]
- 13.Hoji A, Connolly NC, Buchanan WG, Rinaldo CR., Jr CD27 and CD57 expression reveals atypical differentiation of human immunodeficiency virus type 1-specific memory CD8+ T cells. Clin Vaccine Immunol. 2007;14:74–80. doi: 10.1128/CVI.00250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piriou ER, van Dort K, Nanlohy NM, Miedema F, van Oers MH, van Baarle D. Altered EBV viral load setpoint after HIV seroconversion is in accordance with lack of predictive value of EBV load for the occurrence of AIDS-related non-Hodgkin lymphoma. J Immunol. 2004;172:6931–7. doi: 10.4049/jimmunol.172.11.6931. [DOI] [PubMed] [Google Scholar]
- 15.van Baarle D, Kostense S, Hovenkamp E, et al. Lack of Epstein–Barr virus- and HIV-specific CD27– CD8+ T cells is associated with progression to viral disease in HIV-infection. AIDS. 2002;16:1–11. doi: 10.1097/00002030-200210180-00004. [DOI] [PubMed] [Google Scholar]
- 16.Cameron KR, Stamminger T, Craxton M, Bodemer W, Honess RW, Fleckenstein B. The 160,000-Mr virion protein encoded at the right end of the herpesvirus saimiri genome is homologous to the 140,000-Mr membrane antigen encoded at the left end of the Epstein–Barr virus genome. J Virol. 1987;61:2063–70. doi: 10.1128/jvi.61.7.2063-2070.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niesters HGM, van Esser J, Fries E, Wolthers KC, Cornelissen J, Osterhaus ADME. The development of a high-throughput real-time quantitative assay for Epstein–Barr virus detection. J Clin Microbiol. 2000;38:712–5. doi: 10.1128/jcm.38.2.712-715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Baarle D, Wolthers KC, Hovenkamp E, et al. Absolute level of Epstein–Barr virus DNA in human immunodeficiency virus type 1 infection is not predictive of AIDS-related non-Hodgkin lymphoma. J Infect Dis. 2002;186:405–9. doi: 10.1086/341460. [DOI] [PubMed] [Google Scholar]
- 19.Pongers-Willemse MJ, Verhagen OJ, Tibbe GJ, et al. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia. 1998;12:2006–14. doi: 10.1038/sj.leu.2401246. [DOI] [PubMed] [Google Scholar]
- 20.Kostense S, Ogg GS, Manting EH, et al. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired T cell effector function. Eur J Immunol. 2001;31:677–86. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt's lymphoma Epstein–Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA. 2004;101:239–44. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott SL, Pye SJ, Schmidt C, Cross SM, Silins SL, Misko IS. Dominant cytotoxic T lymphocyte response to the immediate–early trans-activator protein, BZLF1, in persistent type A or B Epstein–Barr virus infection. J Infect Dis. 1997;176:1068–72. doi: 10.1086/516513. [DOI] [PubMed] [Google Scholar]
- 23.Piriou ER, van Dort K, Weel JF, et al. Detailed kinetics of EBV-specific CD4+ and CD8+ T cells during primary EBV infection in a kidney transplant patient. Clin Immunol. 2006;119:16–20. doi: 10.1016/j.clim.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Heller KN, Upshaw J, Seyoum B, Zebroski H, Munz C. Distinct memory CD4+ T-cell subsets mediate immune recognition of Epstein–Barr virus nuclear antigen 1 in healthy virus carriers. Blood. 2007;109:1138–46. doi: 10.1182/blood-2006-05-023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leen A, Meij P, Redchenko I, et al. Differential immunogenicity of Epstein–Barr virus latent-cycle proteins for human CD4+ T-helper responses. J Virol. 2001;75:8649–59. doi: 10.1128/JVI.75.18.8649-8659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munz C, Bickham KL, Subklewe M, et al. Human CD4(+) T lymphocytes consistently respond to the latent Epstein–Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–60. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rickinson AB, Kieff E. Epstein–Barr virus. In: Knipe DM, Howley PM, editors. Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 2575–628. [Google Scholar]
- 28.Reece WHH, Pinder M, Gothard PK, et al. A CD4+ T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–10. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 29.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein–Barr virus nuclear antigen 1. Proc Natl Acad Sci USA. 1997;94:12616–21. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blake N, Haigh T, Shakaá G, Croom-carter D, Rickinson A. The importance of exogenous antigen in priming the human CD8+ T cell response: lessons from the EBV nuclear antigen EBNA1. J Immunol. 2000;165:7078–87. doi: 10.4049/jimmunol.165.12.7078. [DOI] [PubMed] [Google Scholar]
- 31.Lee SP, Brooks JM, Al-Jarrah H, et al. CD8 T cell recognition of endogenously expressed Epstein–Barr virus nuclear antigen 1. J Exp Med. 2004;199:1409–20. doi: 10.1084/jem.20040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piriou E, Jansen CA, van Dort K, et al. Reconstitution of EBV latent but not lytic antigen-specific CD4+ and CD8+ T cells after HIV treatment with highly active antiretroviral therapy. J Immunol. 2005;175:2010–7. doi: 10.4049/jimmunol.175.3.2010. [DOI] [PubMed] [Google Scholar]
- 33.Piriou E, van Dort K, Nanlohy NM, van Oers MH, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106:3166–74. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 34.Voo KS, Fu T, Wang HY, et al. Evidence for the presentation of major histocompatibility complex class I-restricted Epstein–Barr virus nuclear antigen 1 peptides to CD8+ T lymphocytes. J Exp Med. 2004;199:459–70. doi: 10.1084/jem.20031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callan MF. The evolution of antigen-specific CD8+ T cell responses after natural primary infection of humans with Epstein–Barr virus. Viral Immunol. 2003;16:3–16. doi: 10.1089/088282403763635401. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174:5332–40. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 37.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 38.Amyes E, Hatton C, Montamat-Sicotte D, et al. Characterization of the CD4+ T cell responses to Epstein–Barr virus during primary and persistent infection. J Exp Med. 2003;198:903–11. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hislop AD, Kuo M, Drake-Lee AB, et al. Tonsillar homing of Epstein–Barr virus-specific CD8+ T cells and the virus–host balance. J Clin Invest. 2005;115:2546–55. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodberry T, Suscovich TJ, Henry LM, et al. AlphaEbeta7 (CD103) expression identifies a highly active, tonsil-resident effector-memory CTL population. J Immunol. 2005;175:4355–62. doi: 10.4049/jimmunol.175.7.4355. [DOI] [PubMed] [Google Scholar]


